Tiberiu Emilian Sârbu, Iulian Gabur, Dănuț Petru Simioniuc, Daniela Domnica Plăcintă, Danela Murariu, Violeta Simioniuc
ABSTRACT. The intensity of selection, inadvertent pathways of domestication, and the influence of climate change collectively amplify the frequency of detrimental alleles. This phenomenon, in turn, triggers genetic drift, leading to an in-advertent decline in the diversity of plant genetic resources. Genetic variability is of utmost importance for a species’ adaptability and overall fitness. Our investigation centres on Triticum germplasm within the agroclimatic conditions of the Suceava Gene Bank, encompassing 2021–2022 field trials. Comprehensive morphophysiological characterisation was conducted across 200 cultivated varieties, spanning three Triticum species (T. aestivum, T. turgidum and T. monococcum). Initially, the data underwent meticulous processing involving the computation of amplitude of variation, variance (s2), standard deviation (√s), and coefficients of variation (s%) for three pivotal agronomical traits: plant height, spikelets per spike, and total seeds per spike. Furthermore, an extensive cluster analysis was performed, encompassing multiple vital plant descriptors. The findings unveiled a remarkable dispersion of data, with standard deviation, amplitude of variation, and coefficient of variation collectively indicating substantial variability among the cultivated varieties. Within the same population, an intriguing observation emerged; of the 200 genotypes analysed, 83 exhibited immunity to Septoria tritici. Delving deeper into the statistical analysis, we identified two primary clusters within the population. Overall, a significant proportion of this germplasm showcased elevated phenotype scores, rendering them well-suited for further exploration as foundational material in pre-breeding initiatives.
Keywords: cluster analysis; morphophysiological traits; Septoria tritici; Triticum germplasm.
Cite
ALSE and ACS Style
Sârbu T.E.; Gabur, I.; Simioniuc, D.P.; Plăcintă, D.D.; Murariu, D.; Simioniuc, V. Phenotypic variability evaluation of wheat varieties from the Suceava Gene Bank collection. Journal of Applied Life Sciences and Environment 2023, 56 (3), 289-302.
https://doi.org/10.46909/alse-563101
AMA Style
Sârbu TE, Gabur I, Simioniuc DP, Plăcintă DD, Murariu D, Simioniuc V. Phenotypic variability evaluation of wheat varieties from the Suceava Gene Bank collection. Journal of Applied Life Sciences and Environment. 2023; 56 (3): 289-302.
https://doi.org/10.46909/alse-563101
Chicago/Turabian Style
Sârbu, Tiberiu Emilian, Iulian Gabur, Dănuț Petru Simioniuc, Daniela Domnica Plăcintă, Danela Murariu, and Violeta Simioniuc. 2023. “Phenotypic variability evaluation of wheat varieties from the Suceava Gene Bank collection” Journal of Applied Life Sciences and Environment 56, no. 3: 289-302.
https://doi.org/10.46909/alse-563101
View full article (HTML)
Phenotypic Variability Evaluation of Wheat Varieties from the Suceava Gene Bank Collection
Tiberiu Emilian SÂRBU1, Iulian GABUR1*, Dănuț Petru SIMIONIUC1, Daniela Domnica PLĂCINTĂ2, Danela MURARIU2* and Violeta SIMIONIUC1
1Department of Plant Science, Faculty of Agriculture, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 3, Mihail Sadoveanu Alley, 700490, Iasi, Romania; email: tibisarbu@yahoo.com; simion@uaiasi.ro
2Vegetal Genetic Resources Bank “Mihai Cristea” Suceava, 17, 1 Mai Boulevard, 720224, Suceava, Romania; email: genebank@suceava.astral.ro
*Correspondence: gaburi@uaiasi.ro; danela.murariu@svgenebank.ro;
Received: Jul. 28, 2023. Revised: Aug. 22, 2023. Accepted: Sep. 01, 2023. Published online: Sep. 14, 2023
ABSTRACT. The intensity of selection, inadvertent pathways of domestication, and the influence of climate change collectively amplify the frequency of detrimental alleles. This phenomenon, in turn, triggers genetic drift, leading to an in-advertent decline in the diversity of plant genetic resources. Genetic variability is of utmost importance for a species’ adaptability and overall fitness. Our investigation centres on Triticum germplasm within the agroclimatic conditions of the Suceava Gene Bank, encompassing 2021–2022 field trials. Comprehensive morphophysiological characterisation was conducted across 200 cultivated varieties, spanning three Triticum species (T. aestivum, T. turgidum and T. monococcum). Initially, the data underwent meticulous processing involving the computation of amplitude of variation, variance (s2), standard deviation (√s), and coefficients of variation (s%) for three pivotal agronomical traits: plant height, spikelets per spike, and total seeds per spike. Furthermore, an extensive cluster analysis was performed, encompassing multiple vital plant descriptors. The findings unveiled a remarkable dispersion of data, with standard deviation, amplitude of variation, and coefficient of variation collectively indicating substantial variability among the cultivated varieties. Within the same population, an intriguing observation emerged; of the 200 genotypes analysed, 83 exhibited immunity to Septoria tritici. Delving deeper into the statistical analysis, we identified two primary clusters within the population. Overall, a significant proportion of this germplasm showcased elevated phenotype scores, rendering them well-suited for further exploration as foundational material in pre-breeding initiatives.
Keywords: cluster analysis; morphophysiological traits; Septoria tritici; Triticum germplasm.
INTRODUCTION
The evolution of enhanced technologies, novel high-yield cultivars, mechanisation, and heightened reliance on chemicals and fertilisers has resulted in amplified yields for key crops, with wheat notably benefiting from these advancements (Voss-Fels et al., 2019). However, this progress has not had repercussions for agroecology and labour dynamics within agriculture (Rosset et al., 2000). The emergence of advanced technologies and the advent of the Green Revolution have nearly tripled agricultural production over the past five decades. Nevertheless, the impending alteration in input availability and usage patterns raises concerns, as the prevailing state of affairs is disconcerting due to the degradation of natural resources and ecosystems.
The Green Revolution, while contributing to prolific agricultural output, has exacted a toll on the environment, public well-being and societal welfare. Contemporary agricultural practices have significantly disrupted the natural climate cycle, resulting in excessive precipitation, snowfall, drought, storms and other catastrophic events that impact human communities. In response to these socioeconomic challenges, numerous industrialised nations have instituted extensive public breeding initiatives rooted in the principles of sustainable agriculture. The evolution of plant breeding has effectively tackled numerous environmental and societal issues, offering novel and economically viable avenues for the betterment of society as a whole.
Wheat (Triticum aestivum L.), one of the oldest plant species with agronomic importance, was cultivated as early as 12,000 years before Christ. In Romania, wheat was first cultivated 2500 years ago, as evidenced by findings at archaeological sites in the south of the country. On a global level, wheat provides about 20% of the population’s food needs, and increasing the productivity of this crop is a very important task for breeders. Modern plant breeding approaches, combined with state-of-the art and efficient production systems, can be used to meet the needs of the population by 2050 (Crespo-Herrera et al., 2017).
The genus Triticum includes ancient wheat species, such as Triticum monococcum (einkorn, AA genome), T. turgidum subsp. dicoccum (emmer, AABB genome), T. turgidum subsp. turanicum (Khorasan wheat, AABB genome) and T. aestivum subsp. spelta (spelt wheat, AABBDD genome), and modern wheat varieties, such as as T. turgidum subsp. durum (durum wheat, AABB genome) and Triticum aestivum (common wheat, AABBDD genome) (Geisslitz and Scherf, 2020). All species of the genus Triticum are considered to have been first domesticated in the Middle East, or, as the symbolic name suggests, the “Fertile Crescent”. The genome of common wheat consists of three different genitors (2n=6x=42; AA, BB and DD) derived from three diploid ancestors. Triticum urartu and Aegilops tauschii are considered for genomes A and D, while Aegilops speltoides is considered the donner of the B genome. Common wheat originated from the cross between a tetraploid wheat, Triticum turgidum, and a diploid ancestor, Aegilops tauschii, which generated the hexaploid AABBDD genome (Matsuoka, 2011).
The analysis of phenotypic diversity is useful for determining the genetic diversity of plant genetic resources, an important condition for making successful crosses between different cultivated varieties of wheat, and the results can be used to design effective breeding programmes (Voss-Fels et al., 2018). An effective plant breeding strategy uses the genetic diversity of germplasm resources as sources of new alleles to improve a crop’s traits. This is crucial in the context of climate change and in increasing productive potential and crop quality (Žilić et al., 2011). Phenotypic variability and correlations between different characteristics are widely used parameters in the selection of valuable parental forms and in the design of successful breeding programmes (Dagnaw et al., 2022).
Phenome analysis of diverse wheat varieties in a certain well-defined geographical area is crucial for plant breeders and contributes extensively to genotype by environmental estimates that predict the agronomic performance of offspring and facilitate the increased rate of genetic gain. The study of statistical parameters, between different agronomic descriptors is useful both to assess the environmental plasticity and genetic stability of crosses and to determine the best selection for a particular characteristic (Azimi et al., 2017).
Wheat productivity is described in the literature as a complex quantitative trait that is influenced by many genetic loci and a large number of characteristics that contribute to wheat yield (Xie, 2015). The best plants are then selected from mixed populations and stabilised for several years. These newly developed cultivars with superior traits are tested for suitability in different climates and propagated and distributed to farmers, a process that can take 12–15 years. An example of the results of plant breeding is the wheat variety Veery, which was developed through 3170 crosses from 51 parent plants from 21 countries (Rajaram et al., 1996). Obtaining a newly improved cultivar with a high production capacity and good yield under different agroclimatic conditions depends on the degree of variability of the initial breeding material (Ahmad et al., 2018; Baye et al., 2020).
The aim of this study was to estimate the genetic diversity and relationships between 200 Triticum cultivars using morphophysiological markers to: i) determine the genetic variability of studied Triticum cultivars and ii) compare results based on morphophysiological markers.
MATERIALS AND METHODS
The biological material used to evaluate the phenotypic variability of the Triticum germplasm consisted of the following:
- 177 cultivars of aestivum ssp. aestivum;
- 3 cultivars of aestivum ssp. spelta;
- 3 cultivars of turgidum;
- 17 cultivars of monococcum.
The four species of the genus Triticum mentioned above had a biological status as local populations, breeding lines or old varieties, as follows:
- Triticum aestivum aestivum;
- local population – 63;
- breeding lines – 93;
- old varieties – 44.
- Triticum aestivum spelta;
- local population – 1;
- breeding lines – 2.
- Triticum turgidum;
- local population – 1;
- breeding lines – 2.
- Triticum monococcum;
- local population – 17.
The choice of the 200 genotypes from the strategic national collection of the Suceava Gene Bank, Romania, was made based on the differentiation between species and biological status. Wheat germplasm stored in the Suceava Gene Bank was received from breeding institutions or collected by research teams from multiple locations, as presented in Table 1.
Local population play an important role in breeding efforts and are among the most valuable genetic resources in a gene bank. Four Triticum species, which are described as local populations, were collected in rural localities from Transylvania and Maramureș (Figure 1).
Breeding lines and varieties of Triticum were received from different agriculture research units in Romania or other countries, such as Germany and the Czech Republic (Table 2).
Table 1
Geographical distribution of local populations of Triticum analysed in 2021
|
County |
Sites of collection (no) |
Number of assessed populations |
|
Triticum aestivum ssp. aestivum – 63 local populations |
||
|
Alba |
4 |
8 |
|
Bihor |
2 |
2 |
|
Bistrița Năsăud |
10 |
14 |
|
Cluj |
2 |
3 |
|
Covasna |
1 |
1 |
|
Hunedoara |
2 |
2 |
|
Maramureș |
17 |
23 |
|
Sălaj |
5 |
7 |
|
Satu Mare |
1 |
2 |
|
Suceava |
1 |
1 |
|
Triticum aestivum ssp. spelta – 1 local population |
||
|
Cluj |
1 |
1 |
|
Triticum monococcum – 1 local population |
||
|
Alba |
2 |
13 |
|
Hunedoara |
2 |
4 |
|
Triticum turgidum – 1 local population |
||
|
Cluj |
1 |
1 |
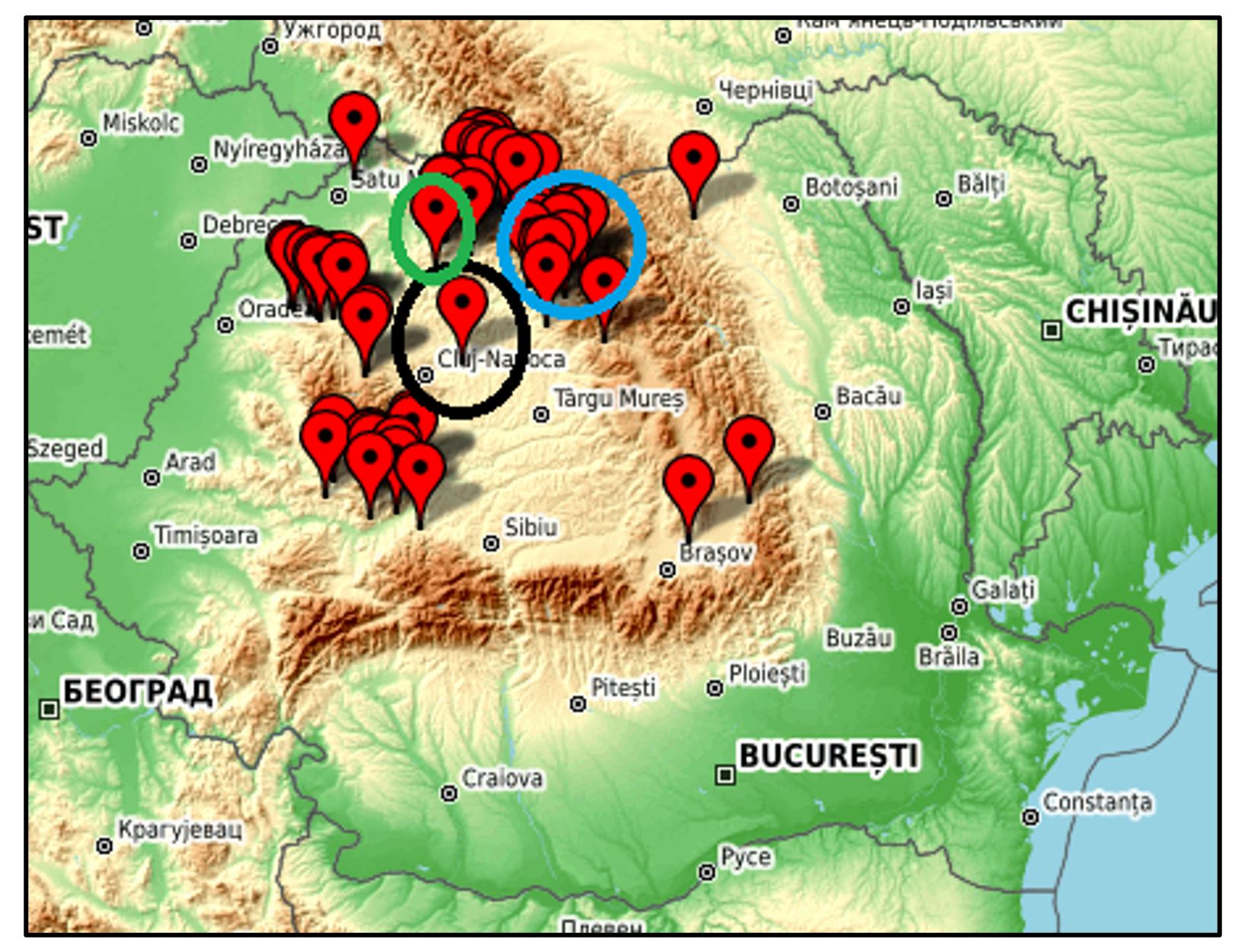
Figure 1 – Origin of local populations of Triticum. Colour codes: green circle for Triticum turgidum; blue for Triticum monococcum; black circle for Triticum aestivum ssp. spelta.
Table 2
Institutes that donated breeding lines and varieties
|
Institute |
Number of breeding lines |
Number of varieties |
|
Triticum aestivum ssp. aestivum – 137 samples |
||
|
SCDA Suceava |
93 |
44 |
|
Triticum aestivum ssp. spelta – 2 samples |
||
|
SCDA Turda |
1 |
1 |
|
Triticum turgidum – 2 samples |
||
|
Germany |
1 |
|
|
Czech Republic |
1 |
|
Notes: SCDA – Agricultural Development Research Institute
The 200 wheat varieties were sown in small blocks in the experimental field of the Suceava Gene Bank. Each sample was sown in two rows at a distance between rows of 25 cm and a row length of 3 m, with three replications. From each replication and genotype, 10 plants were extracted for morphological descriptors. During the growing season, determinations were made regarding the resistance of plants to foliar diseases. The morphophysiological descriptors used to evaluate the phenotypic diversity of Triticum germplasm are presented in Table 3.
Table 3
International Plant Genetic Resources Institute (IPGRI) phenotypes data standards used for the morphophysiological description of wheat cultivars
|
Descriptor |
Significance |
|
Growth type (FAO notes) |
1. autumn; 2. optional; 3. spring. |
|
Plant height (cm) |
Measured at maturity of plant height (ground level to tip of the spike), for 10 plants per genotype |
|
Spike density (FAO notes) |
Visual scoring using a scale from 1 to 9, ranging from very lax (1) to intermediary (5) to very dense (9) |
|
Awn (FAO notes) |
0 – without awn; 1 – short awn; 7 – high awn. |
|
Colour of the glumes (FAO notes) |
1 – white; 2 – red to brown; 3 – purple to black. |
|
Presence of hair/glumes (FAO notes) |
0 – absent; 3 – low; 7 – significantly. |
|
Number of spikelets/spike |
Average number of spikelets per spike in 5 spikes selected from a genotype |
|
Number of seeds/spikelets |
Average number of seeds in a spikelet – sampled from the central area of the spike. 5 spikes from each genotype |
|
Seeds color (FAO notes) |
0 – white; 2 – red; 3 – purple (it is tested with NaOH 5%) Seeds were placed in Petri dishes and covered with 5% NaOH solution. Performed with 60–90 seeds. Red seeds – dark brown–orange; white seeds – pale yellow |
|
Resistance to foliar diseases (%) |
FAO notes (1-9): 1. 0–0.75, Immune; 2. >0.75–2, Extremely resistant (ER); 3. >2–4, Resistant (R); 4. >4–7, Moderately resistant (MR); 5. >7–13, Transition from MR–MS; 6. >13–21 Moderately susceptible (MS); 7. >21–36, Susceptible (S); 8. >36–60, Transition from S–HS; 9. >60–100, Highly susceptible (HS) |
RESULTS AND DISCUSSION
For measured descriptors, we calculated the plant height at maturity (in cm), average number of spikelets/spike and number of seeds/spikelet for one genotype (x), variance of each trait (s2), standard deviation () and coefficients of variation.
Results of these measurements referring to plant, spikelet and seed architecture are shown in Table 4. The results were interpreted by determining the coefficient of variation, which showed the diversity of the wheat genotypes studied. The coefficient of variation was high, at over 20% for the investigated descriptors.
All selected descriptors were based on FAO scoring notes and showed a wide range of differences among the selected population. The growth type was similar for all Triticum genotypes investigated, namely winter types. The spike density of genotypes presented a high amplitude of variation, ranging between 3 and 9.
Moreover, one cultivar had a very loose spike, 4 genotypes had a loose spike, 90 had an intermediary spike, 93 had a dense spike and 12 had a very dense spike.
Table 4
Values of calculated coefficients for measurements made on Triticum cultivars
|
Coefficients |
Plant height (cm) |
Number of spikelets/spike |
Number of seeds/spikelets |
|
Average |
69.9 |
17.33 |
2.92 |
|
Max. value |
118.7 |
36.8 |
5 |
|
Cultivar name |
Trticum monococcum, 1 local population |
Trticum aestivum 1 variety |
Trticum aestivum 1 variety |
|
Min. value |
47.2 |
11.2 |
1 |
|
Cultivar name |
Trticum aestivum 1 breeding line |
Trticum aestivum 1 breeding line |
Trticum monococcum 3 local populations Trticum aestivum 1 breeding line |
|
Standard deviation |
15.54 |
3.778 |
0.685 |
|
Variance |
241.504 |
14.275 |
0.4696 |
|
Coefficients of variation (%) |
22.23 |
21.80 |
23.45 |
Regarding awns, 5 cultivars were awnless, 71 presented short awns, and the remaining 124 had obvious awns. The colour of the glumes was similar for all genotypes, with white glumes.
Additionally, the presence of hair/glumes varied, 127 samples had no hair/glumes, 71 samples had little hair, and 2 samples had many hair/glumes. The seed colour of all cultivars was white.
Regarding the resistance to foliar diseases of the Triticum genotypes, following the observations made in 2022, only the pathogen Septoria tritici was identified.
This pathogen was the only one that appeared during the growing season from March to June.
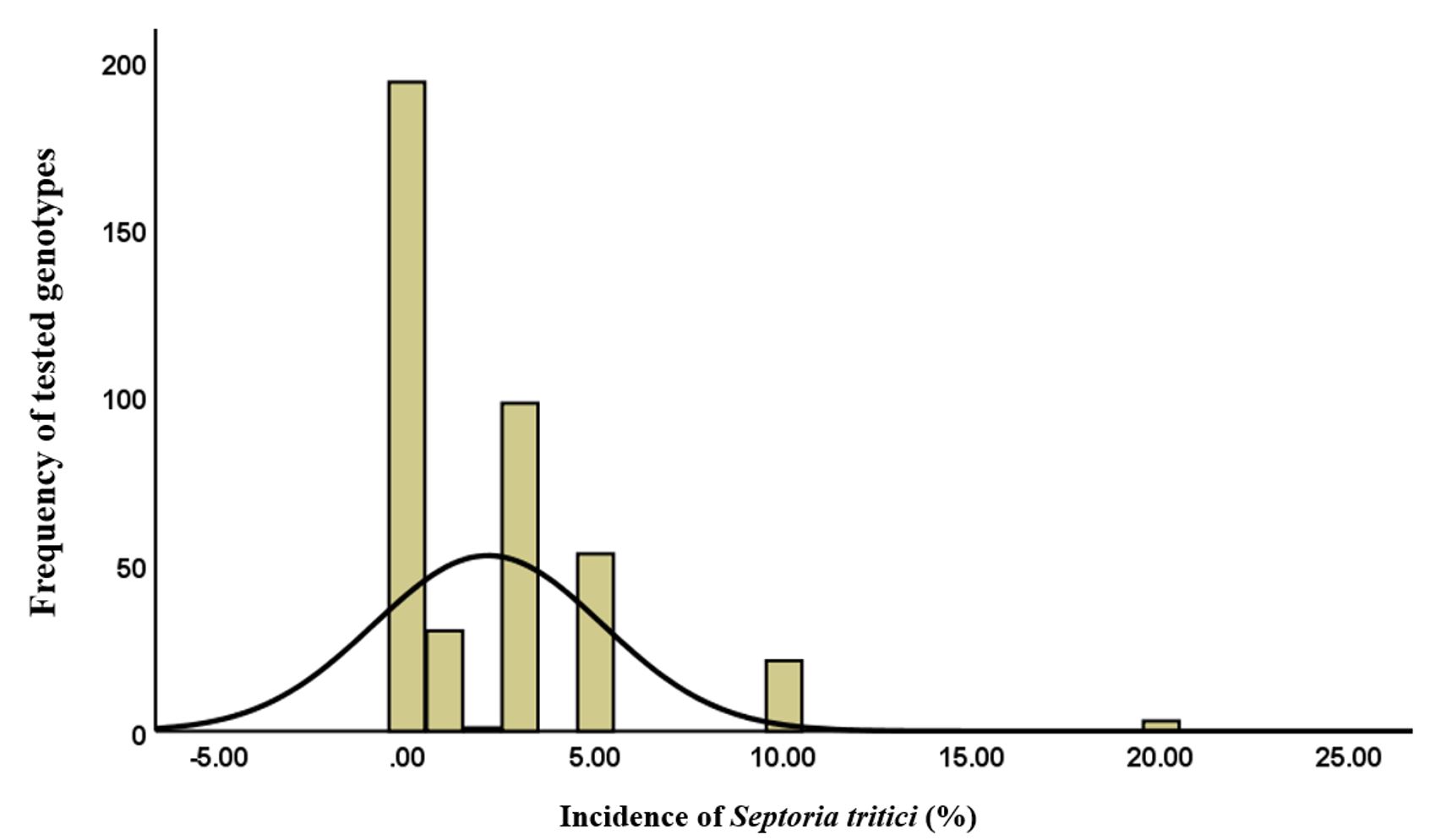
The intensity of the micromycete Septoria tritici showed high variability, with an amplitude of variation (Xmin–Xmax) between 0 and 20 (Table 5). The wheat genotypes analysed were ranked in the resistance range of immune to moderately susceptible.
The differentiated values of the variance and the coefficient of variation (Table 6) showed a wide dispersion of the degree of attack, indicating a large heterogeneity of Triticum cultivars regarding resistance to Septoria tritici (S% – 140.3). A total of 83 genotypes were immune, 62 were resistant, and 50 were medium or moderately resistant. The 17 samples of Triticum monococcum were immune to Septoria tritici.
Statistical investigation of the micromycete Septoria tritici are presented in Figure 2, as a histogram showing a leptokurtic distribution (Kurtosis > 3/ 8.64) with a homogeneous distribution of values around the average.
The variation curve was inclined to the left with a series of extreme values to the right (Skewness > 0/ 2.37), which represents the transition from the resistance reaction in most genotypes to a moderate susceptibility for some Triticum cultivars.
Table 5
Resistance reaction of investigated Triticum genotypes to Septoria disease
|
Pathogen |
Number of genotypes |
Number of infected genotypes |
Percentage of infection |
Note |
Resistance type of wheat genotypes |
|
Septoria tritici |
200 |
83 |
0–0.75 |
1 |
Immune |
|
16 |
0.75–2 |
2 |
Extremely resistant |
||
|
46 |
2–4 |
3 |
Resistant (R) |
||
|
38 |
4–7 |
4 |
Moderately resistant (MR) |
||
|
15 |
7–13 |
5 |
Transition from MR–MS |
||
|
2 |
13–21 |
6 |
Moderately susceptible (MS) |
Table 6
Statistical indicators regarding the incidence of Septoria tritici
|
Indicators / Pathogen |
Average (X) |
Min. (Xmin) |
Max. (Xmax) |
Standard deviation |
Variance (S²) |
Coefficients of variation (S %) |
|
Septoria tritici |
2.18 |
0.0 |
20 |
3.06 |
9.41 |
140.3 |
Using the linkage method between groups of characteristics (SPSS statistical calculation programme), the links between different characteristics of wheat cultivars were determined. In this way, useful information was obtained for the choice of the initial material in wheat breeding programmes (Table 7).
Similar results were observed by Gabur et al. (2022) in a study that investigated 135 cultivars for the Vegetal Genetic Resources Bank “Mihai Cristea” Suceava (VGRB) germplasm collection. Agronomical traits showed a high degree of variance for flowering time, biotic stress resistance and yield components.
Gabur et al. (2022) confirmed that plant genetic resources with high variance are key for future breeding programmes.
As shown in Table 7, there were two statistically significant correlation coefficients, namely plant height with the number of seeds per spikelet and the number of seeds per spikelet with spike density.
The other characteristics correlated with each other but were not statistically significant.
The classification of the characteristics recorded in the 200 genotypes of Triticum was achieved by drawing a dendrogram (Figure 3), and they were grouped into two clusters.
Cluster 1 included the following descriptors: plant height, number of seeds/spikelets, spike density and awn.
Cluster 2 included the following descriptors: presence of hair/glumes, number of spikelets/spike and resistance to Septoria tritici.
In addition, according to Figure 3, there was a close relationship between the presence of hair/glumes, resistance to Septoria tritici and the number of spikelets/spike.
The dendrogram based on the morphophysiological characteristics (Figure 4) was divided into 2 main clusters (I and II), each with 2 subclusters: subclusters Ia and Ib and subclusters IIa and IIb.
Table 7
Classification of morphophysiological traits into clusters using the linkage method between characteristic groups and Pearson correlations
|
The descriptor |
Descriptor number in the dendrogram |
Combining clusters by the linkage method between groups of characteristics |
Pearson correlation coefficient |
|
|
Cluster 1 |
Cluster 2 |
|||
|
Plant height |
1 |
1 |
6 |
***0.540 |
|
Spike density |
2 |
2 |
1 |
0.021 |
|
Awn |
3 |
2 |
3 |
0.077 |
|
Presence of hair/glumes |
4 |
4 |
1 |
0.063 |
|
Number of spikelets per spike |
5 |
5 |
4 |
0.126 |
|
Number of seeds per spikelets |
6 |
6 |
2 |
***0.420 |
|
Resistance to foliar diseases |
7 |
4 |
7 |
0.034 |
Subcluster Ia was subdivided into two other divisions (Ia1 and Ia2) and subcluster Ib was split into two divisions (Ib1 and Ib2). For cluster II, there were only subdivisions IIa and IIb.
Among the 200 Triticum genotypes, 160 were included in cluster I and were quite similar to each other.
In cluster II, 40 genotypes were grouped, and 10 of them presented the highest dissimilarity coefficients compared to the other genotypes analysed.
This result signified the high variability in morphophysiological characteristics.
The 10 genotypes, marked with red in Figure 4, presented the following characteristics regarding taxon, origin and biological status:
- Triticum aestivum ssp. vulgare – 8 cultivars (4 breeding lines and 4 local populations); the local populations originated from Bihor county (Roșia, Renghet and Budureasa) and Suceava county (Măgura Ilvei), and three breeding lines were from the Fundulea Institute, and one was from Suceava Agricultural Development Research Institute;
- Triticum monococcum – 1 genotype (local population originating from Blăjeni, Hunedoara county);
- Triticum aestivum spelta – 1 genotype (local population received from the University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca).
Further studies should be conducted to determine the genetic architecture of Septoria tritici resistance genes. Investigations will be conducted to develop new breeding material with a high tolerance to unfavourable growing conditions.
Moreover, molecular screening for the identification of wheat germplasm with adaptation to climatic conditions, such as severe drought, is an essential aspect for future plant growth.
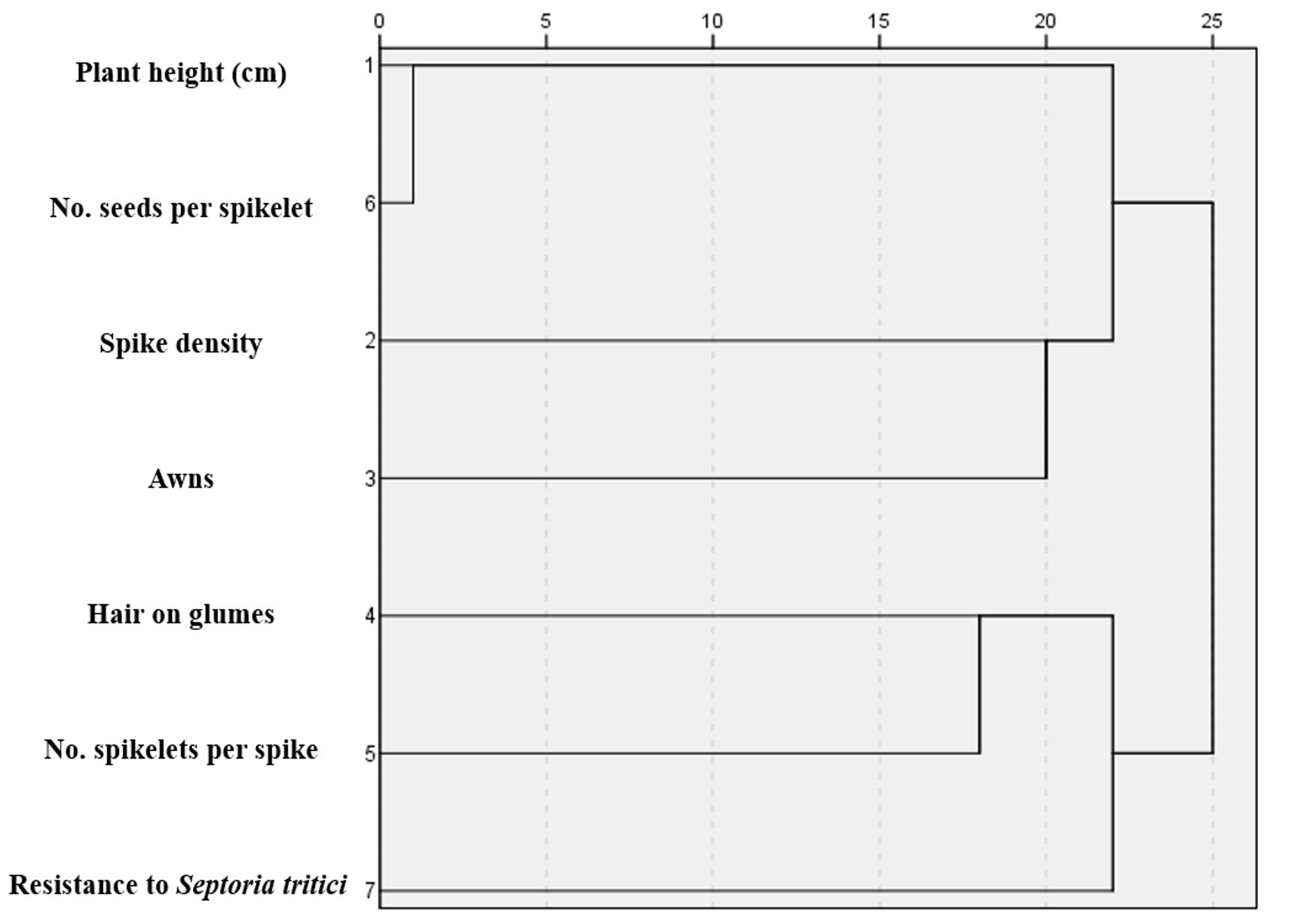
Figure 3 – Dendrogram analysis using average linkage of 7 morphophysiological descriptors of 200 wheat cultivars, based on Pearson correlations
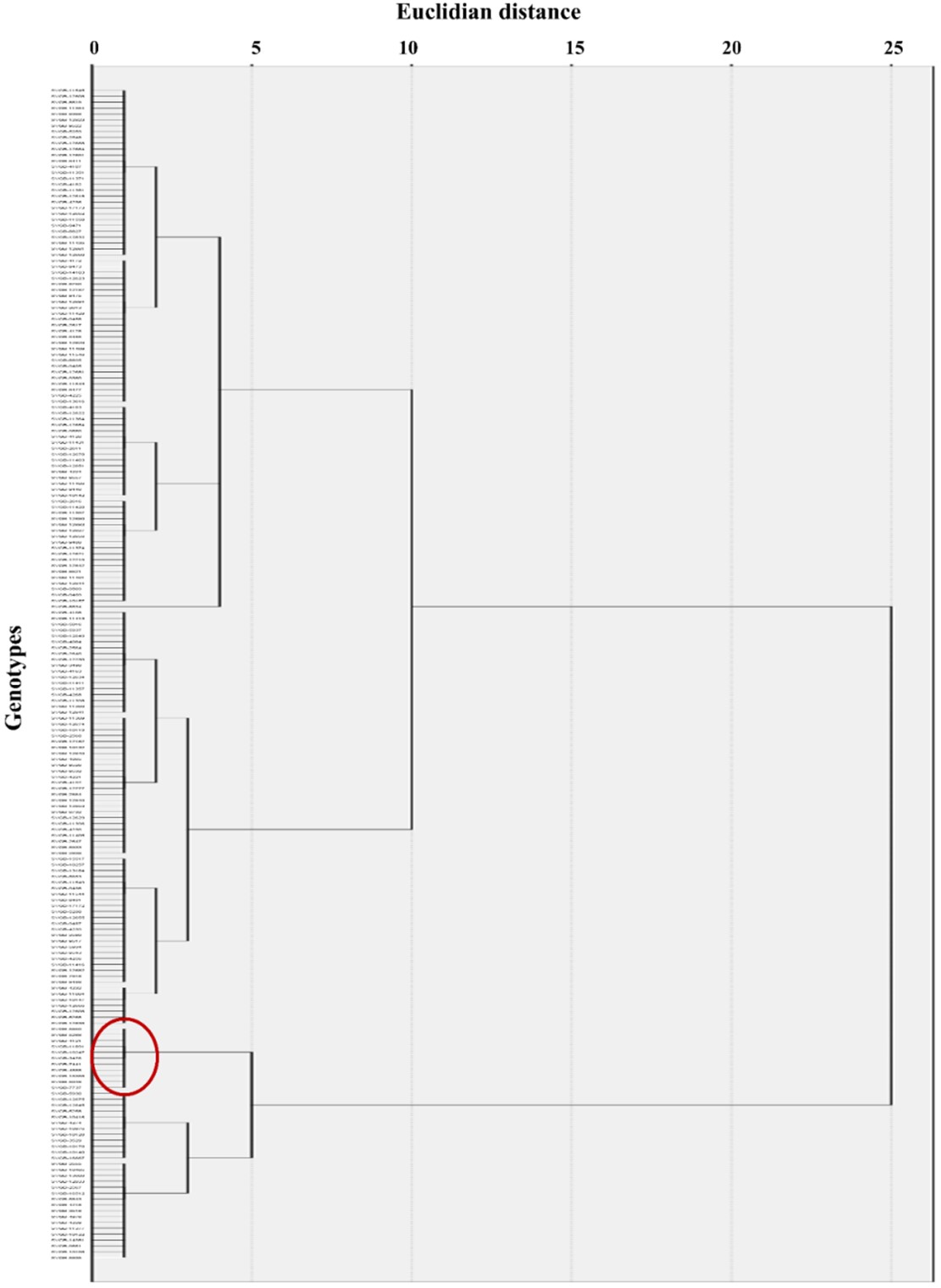
Figure 4 – Dendrogram of Triticum genotypes built based on the Euclidean distance, according to the analysed phenome data. Red marking for 10 genotypes with the highest dissimilarity coefficients
Unfortunately, genetics controlling seedling drought stress seem to be quantitative, as suggested by Schierenbeck et al. (2023). They used 17,093 molecular markers for a comprehensive genome-wide association study and identified 8 of 70 stable QTN spread across the entire wheat genome and a series of candidate genes.
Similar investigations will potentially reveal allelic variation in the Suceava Gene Bank material, which plays a role in multi-trait genetic variation.
New insights into allelic composition and genetic mechanisms linked to important agronomic traits are needed for further wheat breeding and adaptation to biotic and abiotic stress.
CONCLUSIONS
The results revealed substantial genetic variability within the examined Triticum germplasm. All assessed morphological traits exhibited coefficients of variability surpassing 20%, and the cultivars displaying the highest values hold promise as foundational materials for future wheat breeding initiatives.
Of the 200 investigated Triticum genotypes, 83 displayed immunity against attacks from the Septoria tritici pathogen. Correlation analysis established a positive association among specific phenotypic traits. Notably, a positive correlation arose between resistance to Septoria tritici and the presence of glume hair. This particular trait could serve as an indicator for identifying genotypes resistant to this pathogen’s onslaught.
By applying the Euclidean distance method, this study identified 10 distinct genotypes exhibiting considerable phenotypic diversity. These genotypes are prime candidates for inclusion in forthcoming pre-breeding programs aimed at enhancing winter wheat varieties.
Author Contributions: Conceptualisation, MD, IG and VS; Methodology, TS, IG, DDP and DM; Writing-original draft preparation, TS; Writing-review and editing, IG, VS, DPS; DDP, DM. VS; Supervision, IG and DM, Funding acquisition, IG. All authors have read and agreed to the published version of the manuscript.
Funding: This study was supported by a grant from the Romanian Ministry of Education and Research, CNCS-UEFISCDI, project number PN-III-P2-2.1-PED-2019-0175, within PNCDI III and project “PROINVENT”, Contract no. 62487/03.06.2022 – POCU/993/6/13 – Code 153299, financed by The Human Capital Operational Programme 2014–2020 (POCU), Romania.
Acknowledgments: The work of Iulian GABUR was supported by the project “PROINVENT”, Contract no. 62487/03.06.2022 – POCU/993/6/13 – Code 153299, financed by The Human Capital Operational Programme 2014–2020 (POCU), Romania.
Conflicts of Interest: All authors declared no conflict of interest.
REFERENCES
Ahmad, T.; Kumar, A.; Pandey, D.; Prasad, B. Correlation and path coefficient analysis for yield and its attributing traits in bread wheat (Triticum aestivum L. em Thell). Journal of Applied and Natural Science. 2018, 10, 1078-1084. https://doi.org/10.31018/jans.v10i4.1867.
Azimi, A.M.; Marker, S.; Bhattacharjee, I. Genotypic and phenotypic variability and correlation analysis for yield and its components in late sown wheat (Triticum aestivum L.). Journal of Pharmacognosy and Phytochemistry. 2017, 6, 167-173.
Baye, A.; Berihun, B.; Bantayehu, M.; Derebe, B. Genotypic and phenotypic correlation and path coefficient analysis for yield and yield-related traits in advanced bread wheat (Triticum aestivum L.) lines. Cogent Food & Agriculture. 2020, 6, 1752603. https://doi.org/10.1080/23311932.2020.1752603.
Crespo-Herrera, L.A.; Gargava-Gustavsson, L.; Ahman, I. A systematic review of rye (Secale cereale L.) as a source of resistance to pathogens and pests in wheat (Triticum aestivum L.). Hereditas. 2017, 154, 1-9. https://doi.org/10.1186/s41065-017-0033-5.
Dagnaw, T.; Mulugeta, B.; Haileselassie, T.; Geleta, M.; Tesfaye, K. Phenotypic variability, heritability and associations of agronomic and quality traits in cultivated Ethiopian durum wheat (Triticum turgidum L. ssp. Durum, Desf.). Agronomy. 2022, 12, 1714. https://doi.org/10.3390/agronomy12071714.
Gabur, I.; Sârbu, T.; Gabur, G.D.; Simioniuc, V.; Murariu D.; Simioniuc, D.P. Phenotypic Analysis of Vegetal Genetic Resources Bank “Mihai Cristea” Suceava Germplasm in North-East Romanian Field Conditions, E-Health and Bioengineering Conference (EHB), Iasi, Romania, 2022, 01-04. https://doi.org/10.1109/EHB55594.2022.9991613.
Geisslitz, S.; Scherf, K.A. Rediscovering ancient wheats. Cereal Foods World 2020; 65(2). https://doi.org/10.1094/CFW-65-2-0013.
Matsuoka, Y. Evolution of polyploid Triticum wheats under cultivation: the role of domestication, natural hybridization and allopolyploid speciation in their diversification. Plant and cell physiology. 2011, 52, 750-764. https://doi.org/10.1093/pcp/pcr018.
Rosset, P.; Collins, J.; Lapp, F.M. Lessons from the Green Revolution: do we need new technology to end hunger? Tikkum Mag. 2000, 15, 52-56.
Rajaram, S.; Braun, H.J.; van Ginkel, M. CIMMYT’s approach to breed for drought tolerance. Euphytica. 1996, 92, 147-153. https://doi.org/10.1007/BF00022840.
Schierenbeck, M.; Alqudah, A.M.; Thabet, S.G.; Lohwasser, U.; Simón, M.R.; Börner, A. Association mapping unravels the genetics controlling seedling drought stress tolerance in winter wheat. Frontiers in Plant Science. 2023 14:1061845. https://doi.org/10.3389/fpls.2023.1061845.
Voss-Fels, K.P.; Stahl, A.; Wittkop, B.; Lichthardt, C.; Nagler, S.; et al. Breeding improves wheat productivity under contrasting agrochemical input levels. Nature Plants. 2019, 5, 706-714. https://doi.org/10.1038/s41477-019-0445-5.
Voss-Fels, K.P; Qian, L.; Gabur, I.; Obermeier, C.; Hickeyet, L.T.; et al. Genetic insights into underground responses to Fusarium graminearum infection in wheat. Scientific Reports. 2018, 8, 13153. https://doi.org/10.1038/s41598-018-31544-w.
Xie, Q. Physiological and genetic determination of yield and yield components in a bread wheat× spelt map-ping population. PhD Thesis. University of Nottingham. May/2015.
Žilić, S.; Barać, M.; Pešić, M.; Dodig, D.; Ignjatović-Micić, D. Characterization of proteins from grain of different bread and durum wheat genotypes. International Journal of Molecular Sciences. 2011, 12, 5878-5894. https://doi.org/10.3390/ijms12095878.
Academic Editor: Dr. Mihaela Roșca
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Gabur Iulian, Murariu Danela, Plăcintă Daniela Domnica, Sârbu Tiberiu Emilian, Simioniuc Dănuț Petru, Simioniuc Violeta