Lucian Răus, Mariana Volf, Diana Elena Bolohan
ABSTRACT. In this study, four holding solutions very often used by florists and the final consumer were tested to analyse their impact on the development of the inflorescence, as well as the elongation, weight and degree of bending of the hyacinth flowers. The use of these solutions has a general character, and the flower species react differently due to both their genetics and the conditions in which they were cultivated, handled and stored. To verify the effectiveness of the preservative solutions on Hyacinthus orientalis vase life, four solutions were prepared with 2% sucrose and none or one of the follow-ing antimicrobial substances: sodium hypochlorite, acetic acid and ascorbic acid. During the study, measurements were made on the weight, elongation of the floral stems, chlorophyll content of the leaves and vase life days. The vase life of the hyacinths in this experiment was 5 days, except for the flowers from the 2% sucrose holding solution, which started to wilt on day 5. On day 6, the flowers showed signs of senescence. The increase in the length of the inflorescence stem showed significant differences for the sucrose + ascorbic acid holding solution, with a maximum of 0.8 cm on day 4. However, the interest was not to maximise the elongation of the floral stem but to slow down this process since elongation leads to the bending of the stem, causing it to require additional support.
Keywords: antimicrobial substances; holding solutions; inflorescence stem elongation.
Cite
ALSE and ACS Style
Răus, L.; Volf, M.; Bolohan, D.E. The influence of the usual holding solutions on Hyacinthus orientalis cut flower vase life. Journal of Applied Life Sciences and Environment 2023, 56 (2), 211-220.
https://doi.org/10.46909/alse-562096
AMA Style
Răus L, Volf M, Bolohan DE. The influence of the usual holding solutions on Hyacinthus orientalis cut flower vase life. Journal of Applied Life Sciences and Environment. 2023; 56 (2): 211-220.
https://doi.org/10.46909/alse-562096
Chicago/Turabian Style
Răus, Lucian, Mariana Volf, and Diana Elena Bolohan. 2023. “The influence of the usual holding solutions on Hyacinthus orientalis cut flower vase life” Journal of Applied Life Sciences and Environment 56, no. 2: 211-220.
https://doi.org/10.46909/alse-562096
View full article (HTML)
The Influence of The Usual Holding Solutions on Hyacinthus orientalis Cut Flower Vase Life
Lucian RĂUS, Mariana VOLF and Diana Elena BOLOHAN*
Department of Pedotechnics, Faculty of Agriculture, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 3, Mihail Sadoveanu Alley, 700490, Iasi, Romania; email: rauslucian@uaiasi.ro; mariana.volf@uaiasi.ro
*Correspondence: diana.bolohan@uaiasi.ro
Received: Jul. 03, 2023. Revised: Jul. 20, 2023. Accepted: Jul. 27, 2023. Published online: Aug. 23, 2023
ABSTRACT. In this study, four holding solutions very often used by florists and the final consumer were tested to analyse their impact on the development of the inflorescence, as well as the elongation, weight and degree of bending of the hyacinth flowers. The use of these solutions has a general character, and the flower species react differently due to both their genetics and the conditions in which they were cultivated, handled and stored. To verify the effectiveness of the preservative solutions on Hyacinthus orientalis vase life, four solutions were prepared with 2% sucrose and none or one of the follow-ing antimicrobial substances: sodium hypochlorite, acetic acid and ascorbic acid. During the study, measurements were made on the weight, elongation of the floral stems, chlorophyll content of the leaves and vase life days. The vase life of the hyacinths in this experiment was 5 days, except for the flowers from the 2% sucrose holding solution, which started to wilt on day 5. On day 6, the flowers showed signs of senescence. The increase in the length of the inflorescence stem showed significant differences for the sucrose + ascorbic acid holding solution, with a maximum of 0.8 cm on day 4. However, the interest was not to maximise the elongation of the floral stem but to slow down this process since elongation leads to the bending of the stem, causing it to require additional support.
Keywords: antimicrobial substances; holding solutions; inflorescence stem elongation.
INTRODUCTION
Among the first appearances in the spring is Hyacinthus orientalis, a flower highly appreciated both for its multitude of colours and for its strong perfume. It is used in flowerbeds in cities, decorative vases and bouquets (Drăghia, 2011). Although highly valued in bouquets, their short lifespan limits their use. Wilting is noticeable in 3–5 days. Rapid elongation of the stem leads to bending of the inflorescence, causing it to require additional support.
The vase life of hyacinths can be prolonged if the flowers are harvested with a small part of the bulb, approximately 1 cm. Afterwards, until they are delivered to the flower shops, they can be stored without water at 0–2°C for 7–14 days. Long periods of storage can cause the flowers to lose their fragrance and shorten their vase life to 3–5 days in the final consumer living space (Amăriuței, 2017). Improper storage leads to faster consumption of nutrients from the plant, and they lose their decorative value in a much shorter time. Preservative solutions are widely used to extend the flower life. The current research on the effect of different preservation solutions on the vase life of hyacinths is very limited, but there is some research on the general preservation of cut flowers.
The flowers should be hydrated by holding the stems for 12–24 hours in warm water (38–40°C), so that when they are removed from storage, the flower buds are stimulated with sucrose solutions (2–5%), and antimicrobial substances, organic acids, ethylene inhibitors, growth regulators or inhibitors are used (Cantor, 2015).
Carbohydrates represent the external food source of cut flowers. During vase life, they are used in the breathing process, the opening of flower buds and maintaining the quality of the flowers for as long as possible (Chuang and Chang, 2013). Among carbohydrates, sucrose is the most commonly used in the preparation of holding solutions. Its use in appropriate concentrations positively influences the absorption of the plant and implicitly the turgidity of the flowers. High concentrations of sugars can have a negative influence on petals, especially the leaves on flower stalks. However, the use of solutions with low concentrations of sugars will not have the desired effect (Yatendra et al., 2020).
Pathogenic microorganisms develop in the holding solutions, which may lead to a faster decline in the plants by blocking the conducting vessels (Van Doorn and De Witte, 1997). It is common practice to add antimicrobial substances, such as silver nitrate, silver thiosulphate and quinoline salts, to slow down/stop the development of fungi and bacteria, but these are more difficult for the final consumers to obtain. It is also recommended to use acids that lower the pH (up to 4) of the solution (de la Riva et al., 2009) or compounds with chlorine (Van Doorn and Perik, 1990). The use of chlorine compounds in the holding solution can also have unwanted effects, such as the yellowing of petals on flower stalks (Knee, 2000).
Tavakkoli et al. (2010) observed the negative influence of the increased concentration of sodium and chlorine ions in a soil solution in a study with Faba beans, which manifested as a lower chlorophyll content in leaves and chlorosis. Other negative influences have been observed by Ramoliya et al. (2004), such as changes in delayed flowering, accelerated ageing and an earlier loss of the plant’s ornamental value.
The purpose of this study was to investigate the response of Hyacinthus orientalis in vases to different holding solutions prepared with commercial products, widely available to customers, following the recipes found on the internet and wider media, to check the effectiveness of such solutions and to provide scientific backup to this anecdotal knowledge. This study followed the development of the Delft Blue hyacinth inflorescence, as well as the elongation, weight, freshness and degree of bending.
MATERIALS AND METHODS
The experiment was carried out in the agrochemistry laboratory of the Iasi University of Life Sciences on April 2022. Delft Blue hyacinth inflorescences were purchased from a wholesale flower store. The flowering stage of the shoots on the first day of the experiment was about 70% open buds. The inflorescence stems were harvested with a small part of the bulb (about 1.5 cm) that was attached to the inflorescence during the study. The cut was refreshed by removing 0.5 cm from the bulb portion with a cutter for better water absorption, and they were left in distilled water for 12 hours at 18°C. Afterwards, the flowers with the tip of the bulb and 4 leaves were weighed, measured and placed individually in 350 mL Erlenmeyer flasks, to which 200 mL of holding solution was added.
The holding solution was replaced every two days during the experiment. The room temperature where the experiment was conducted varied between 21 and 22°C, with an air humidity of 45–47%. In the experiment, four variants of holding solutions were studied, all of which were prepared with tap water: T1 – 2% sucrose – pH = 7, T2 – 2% sucrose + 0.025% sodium hypochlorite – pH = 7, T3 – 2% sucrose + 0.5% acetic acid (vinegar 9°) – pH = 4.5, and T4 – 2% sucrose + 0.01% ascorbic acid – pH = 4.3
The inflorescence shoots were measured using a measuring line glued to the worktable. The weight of the flowers selected for the experiment was recorded with an electronic balance. The chlorophyll content was also determined with the help of a chlorophyll metre (CCM-200 plus from Opti-Science), with the readings being made in the upper base of the leaf without a portion of 1 cm from the tip to avoid errors because the tip showed discoloured areas. The measurements were made during the same period of the day, starting at 11 o’clock.
The degree of stem bending was not measured directly but was assessed visually based on the comparison of the stems’ inclination to their vertical position. The degree of bending was categorised as follows: slight inclination: approximately 45 degrees; medium inclination: approximately 90 degrees; strong inclination: an angle greater than 90 degrees.
The experiment ended on day 5, when most of the flowers started to show signs of senescence, which manifested as translucent discolouration of the basal flower margins that had been observed visually. This phenomenon was referred to as wilting.
Each experimental variant was replicated 5 times with a single inflorescence shoot per replication. Data were analysed by one-way analysis of variance (ANOVA) using SPSS software. Means were grouped using the Duncan test at a significance level α = 0.05, comparing the average differences between treatments for each day.
RESULTS AND DISCUSSION
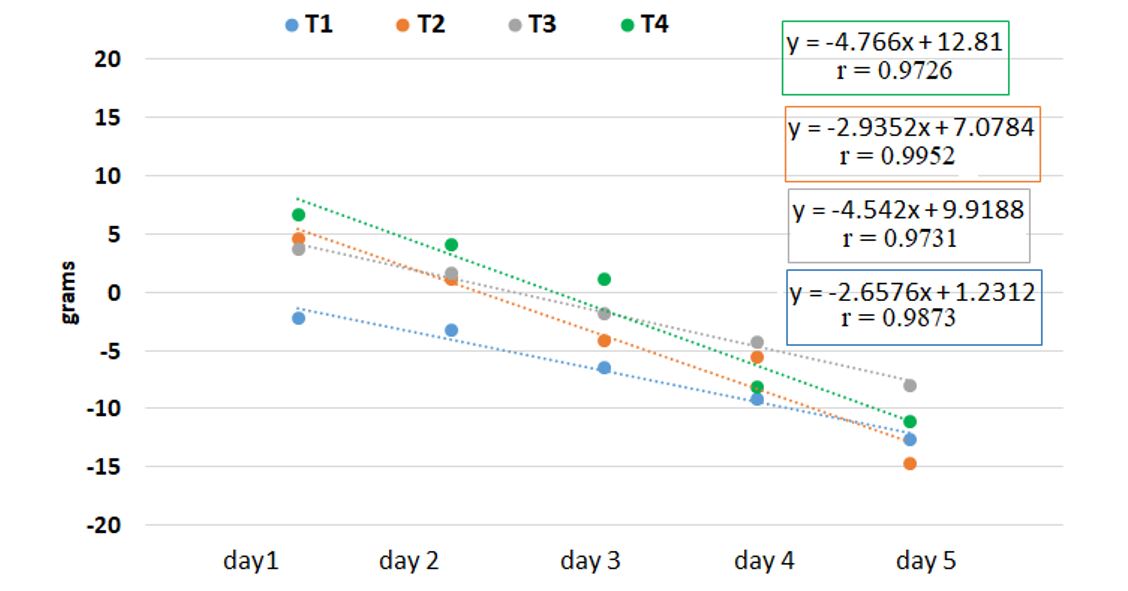
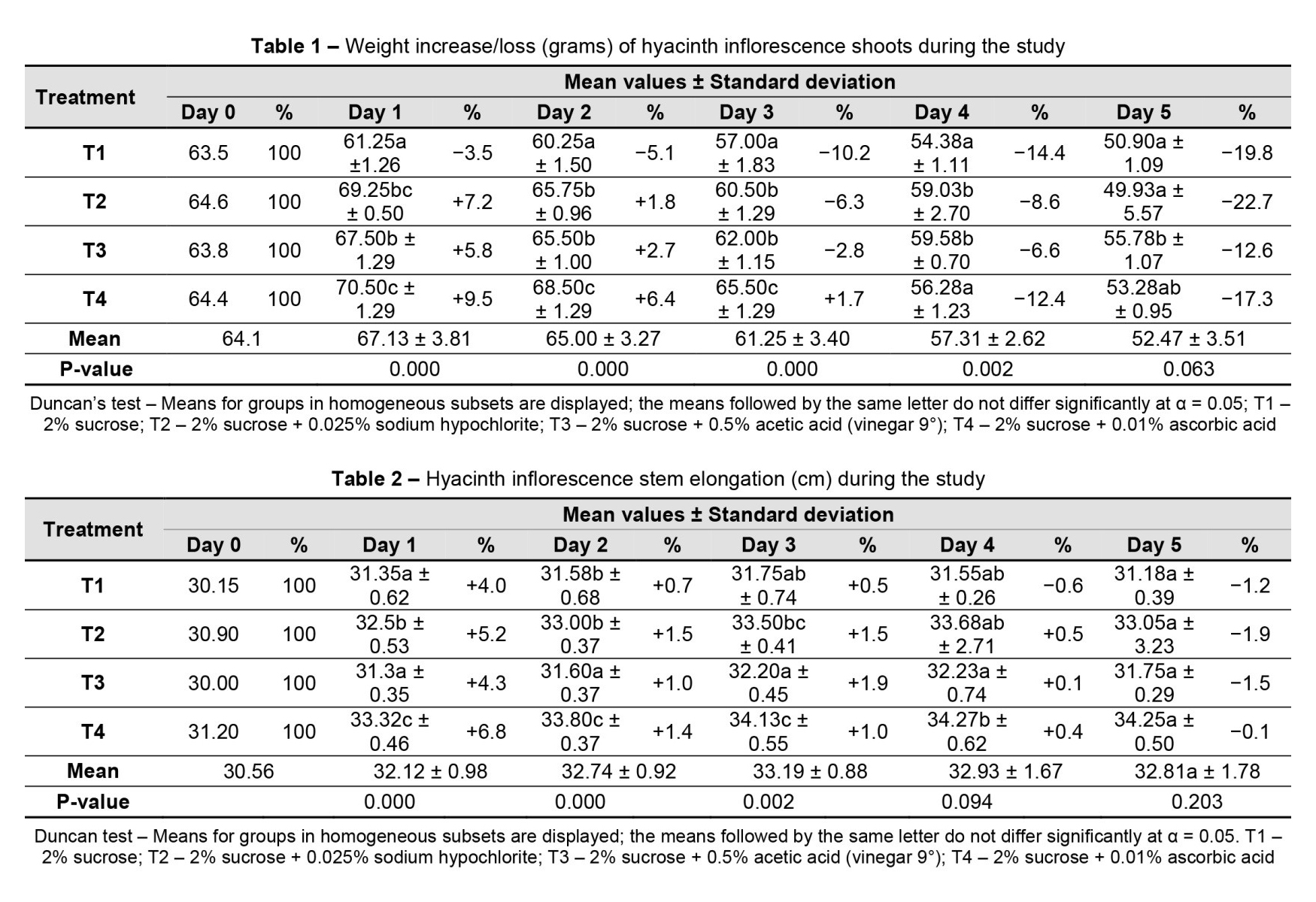
On the first day, all experimental variants recorded weight increases, except for T1, which slightly decreased, with a maximum of 70.5 g for T4. The increase in weight was correlated with the opening of the buds and the decorative quality of the flower. The inflorescence shoot weight decreased gradually from the second day until the end of the experiment. A slower decrease in weight was registered for the 2% sucrose + 0.5% acetic acid solution (T3) compared to the other holding solutions (Table 1).
Similar results were also recorded by Zeljković et al. (2021), who observed that the lowest average value of fresh weight in cut flowers was found with a 2% sucrose solution.
Duncan’s test determined which treatment means were significantly different from each other within the subsets created (T1 – subset a, T2 and T3 – subset b, T4 – subset c) for the first three days (Table 1). As shown in Figure 1, a negative correlation was observed between the holding solution and the weight loss of the flowers during the study (p < 0.05).
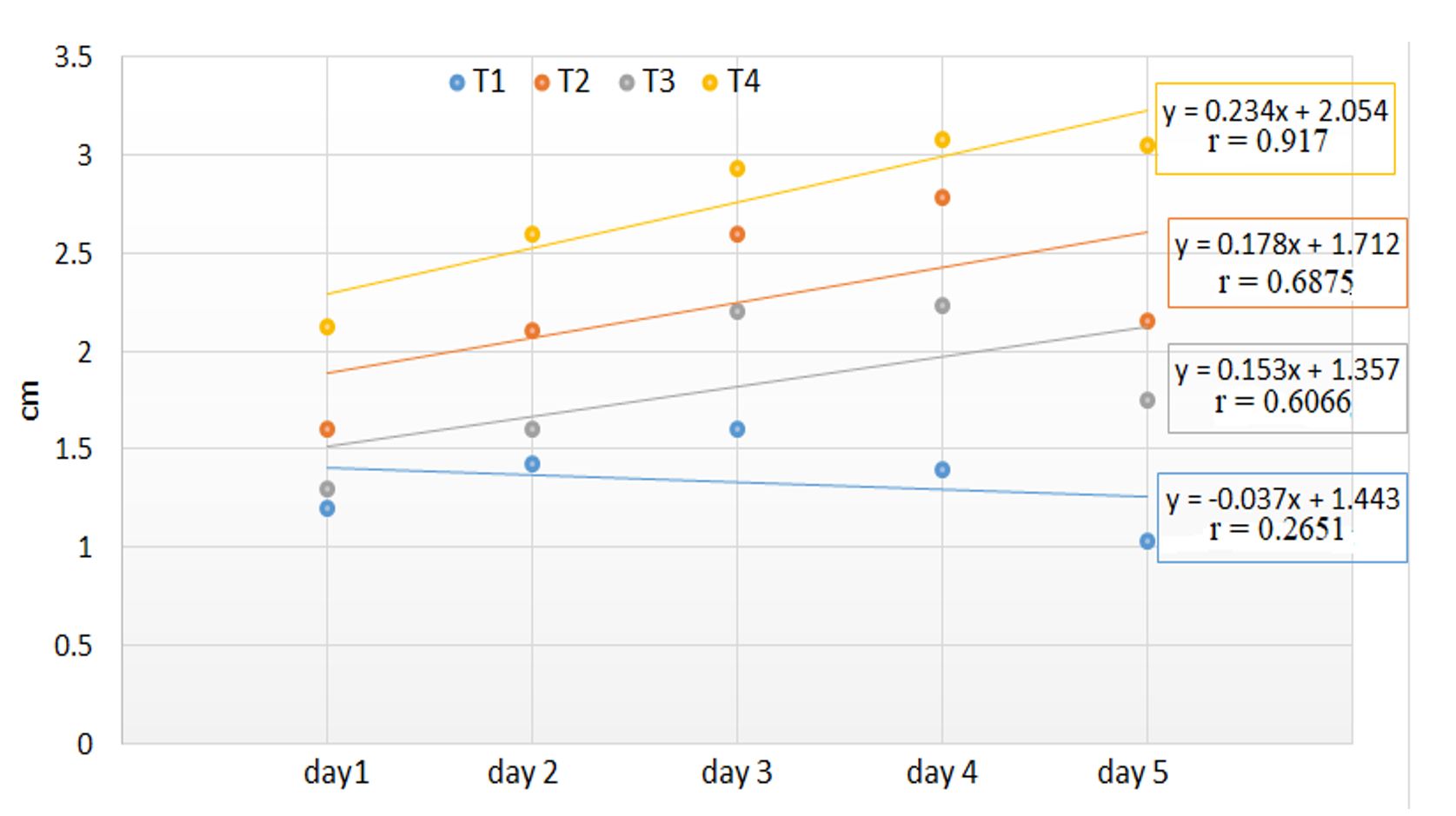
In the first three days, there was an increase in the length of the floral stem for all treatments. The increase in the length of the floral stem showed significant differences for the T4 holding solution, with a maximum of 0.8 cm. On day 4, only T1 with 2% sucrose registered a slight decrease (0.2 cm) due to the total opening of the buds and the twisting of the petals outward in the flowers at the top of the inflorescence. On the last day of the study, all treatments of holding solutions resulted in a decrease in inflorescence stem length, which was attributed to the loss of turgidity, as the plants exhibited signs of wilting (Table 2).
A positive correlation between the holding solution and stem elongation was statistically proven for T4 (2% sucrose + 0.01% ascorbic acid, p < 0.05). However, statistical significance could not be assured for all other holding solution treatments (Figure 2).
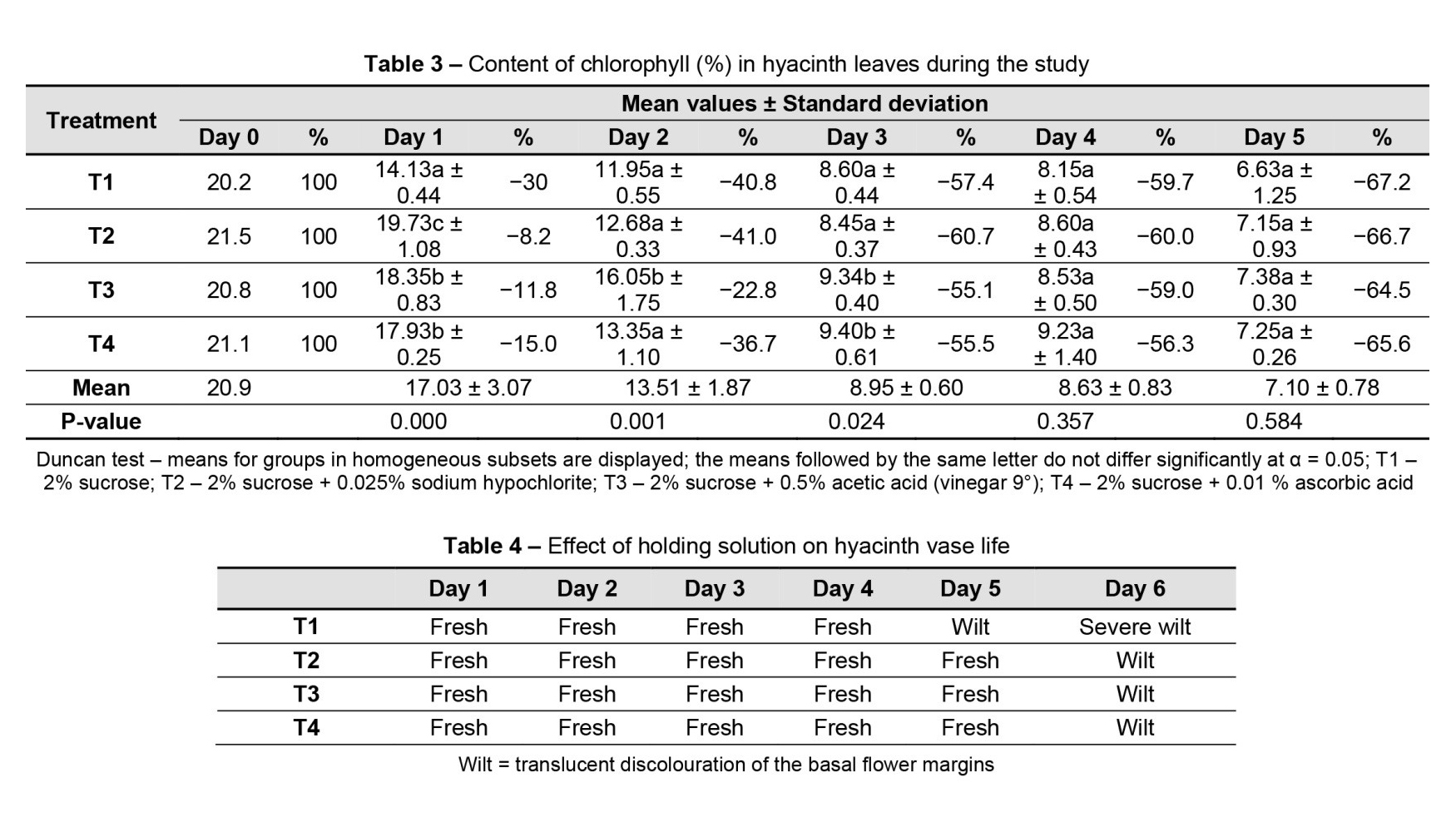
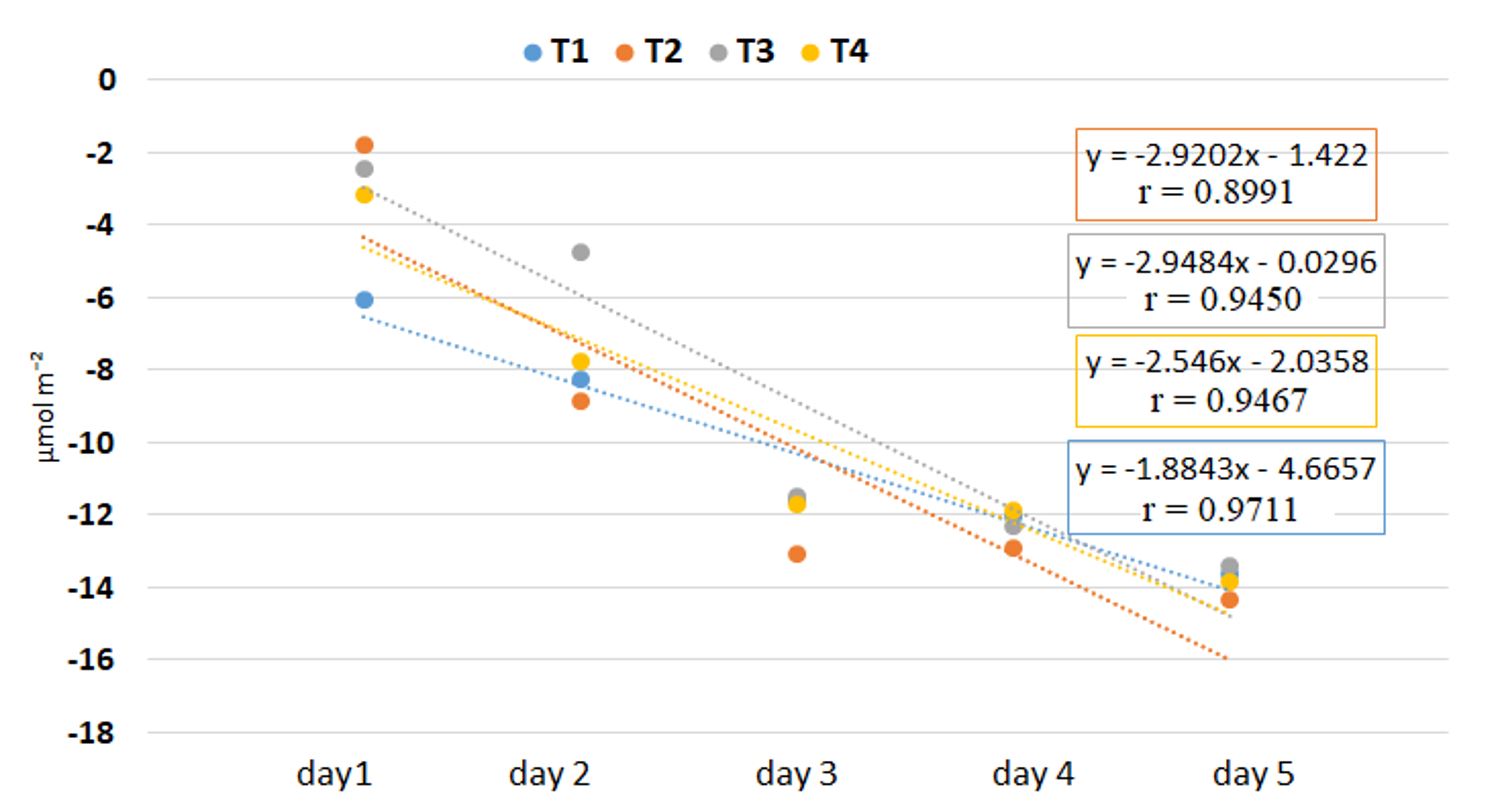
The chlorophyll content in the leaves decreased even from the first day until the third day, when the trend was constant for all four holding solutions. Significant differences appeared between the holding solutions with the highest chlorophyll concentrations for T2 and T3 on the first day of the experiment. On the second day, a sudden drop in chlorophyll concentration was recorded in the inflorescences from the T2 holding solution from 19.73 to 12.68 μmol m⁻². Its degradation was faster in solutions with sucrose (T1) and sugar with chlorine (T2) compared to the variants with sucrose and vinegar (T3) and sucrose and ascorbic acid (T4) (Table 3, Figure 3). There was a significant negative correlation between holding solutions and chlorophyll content decrease, for all holding solutions (p < 0.05, Figure 3).
The vase life of the flowers was between 5 and 6 days. The flowers in the solution with 2% sucrose (T1) showed signs of wilting after day 5, and on day 6, they showed an advanced degree of wilting. The flowers from the other holding solutions with antimicrobial substances showed signs of wilting on day 6 and severe wilting on day 7 (Table 4). Regarding the degree of bending, starting with day 3, T1 showed a slight inclination of the inflorescence, T2 and T4 showed strong bending, and T3 showed medium bending. These degrees of inclination can be correlated with the elongation of the inflorescence (Figure 4).
The addition of carbohydrates to the holding solution of cut flowers positively influenced the opening of the flower buds and extended their vase life. In addition to sucrose, substances with an antimicrobial effect, either by the nature of their composition or by reducing the pH of the holding solution, were added.
Adding chlorine to the solution is recommended for plants that require chlorine ions; however, chlorine vapour has negative effects on health, as well as phytotoxic effects on plants (Knee, 2000).
In addition, using products with chlorine had a limited effect on reducing microorganisms on the surface (Sapers, 2001) compared to other substances (Kitis, 2004). There is no information on the use of acetic acid in holding solutions for hyacinth. Its use in this study showed significant differences in chlorophyll content compared with other treatments, next to ascorbic acid with sucrose. The decrease in chlorophyll content occurred due to its degradation by mineral translocation but also due to the presence of chlorine ions, according to the studies carried out by Tavakkoli et al. (2010).
Chlorophyll degradation occurs more slowly because of decreased respiration and increased water absorption from cut flowers (Balouchi et al., 2012). This condition is critical due to the retention effect of the antioxidants in retinol solutions, which prevents water blockage (Banaee et al., 2013) and lengthens the blooming period of the flower. Even though sucrose is a carbohydrate source, Lee and Kim (2018) found that adding sucrose to the holding solution accelerated the onset of leaf chlorosis in cut roses and reduced the vase life of flowers in comparison to tap water or distilled water. The decrease in chlorophyll for T4 is not in line with the findings of Ghadimian and Danaee (2015), who studied roses and found that holding solutions with ascorbic acid increased the chlorophyll content of cut flowers during vase life. The presence of ascorbic acid in the holding solution at higher concentrations (200–300 ppm) had a positive effect on the chlorophyll concentration in the leaves, while the lower doses (100 ppm) better preserved the freshness of the petals (Budiarto, 2019). Stem length and stem thickness increases were observed and were directly proportional to the concentration of ascorbic acid applied (600–1200 ppm).
CONCLUSIONS
The solution containing 2% sucrose exhibited a slight decrease in weight and weak elongation, which positively affected the degree of bending. The vase life of the flowers in this solution was only 5 days, compared to the other solutions that lasted 6 days. Flowers from the other holding solutions displayed specific behaviours unique to each solution.
The addition of acids and chlorine extended the lives of the flowers compared to the use of sucrose solution. Among these, the solution containing sucrose and vinegar stood out, showing a slight inclination of inflorescence and a vase life of 6 days.
The slower decrease in chlorophyll content contributed to the prolonged freshness of the flowers, and the best results were observed for the flowers in the solution containing sucrose and ascorbic acid. The doses of sucrose and vitamin C could likely be corrected through further experiments, considering that each flowering species reacts specifically to the content of the holding solution. The elongation occurred due to both the blooming of the buds and the elongation of the stem, which will be investigated in another study, and the effect of the holding solution on the intensity of the colour will be examined.
Author Contributions: Conceptualization: LR and DEB; methodology: LR, MV and DEB; analysis DEB and MV; investigation: LR and DEB; writing: MV and LR; review: LR and DEB. All authors declare that they have read and approved the publication of the manuscript in this present form.
Funding: This research received no external funding.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
Amăriuței, A. Cut flowers – the secrets of their life (in Romanian). Elisavaros Publising House, Bucharest, 2017, pp. 50-100.
Balouchi, Z.; Peyvast, G.-A.; Ghasemnezhad, M.; Dadi, M. Effects of ascorbic acid in delaying florets senescence of broccoli during post-harvest storage. South Western Journal of Horticulture Biology and Environment. 2012, 3, 167-183.
Banaee, S.; Hadavi, E.; Moradi, P. Effect of ascorbic acid, 8-hydroxyquinoline sulfate and sucrose on the longevity and anthocyanin content of cut gerbera flowers. Current Agriculture Research Journal. 2013, 1, 29-33. http://dx.doi.org/10.12944/CARJ.1.1.03
Budiarto, K. Effects of Ascorbic Acids on Post-Harvest Longevity of Chrysantemum Cut Flowers. Planta Tropika: Journal of Agro Science. 2019, 7. https://doi.org/10.18196/pt.2019.091.33-40.
Cantor, M. General floriculture (in Romanian). Academic Pres Publising House, Cluj-Napoca, 2015, pp.205-212.
Chuang, Y.; Chang, A.Y. The Role of Soluble Sugars in Vase Solutions during the Vase Life of Eustoma grandiflorum. HortScience. 2013, 48, 222-226. http://dx.doi.org/10.21273/HORTSCI.48.2.222.
Drăghia, L. Floriculture (in Romanian).” Ion Ionescu de la Brad” Publising House, Iasi, 2011, pp. 260-269.
Ghadimian, S.; Danaee, E. Influences of ascorbic acid and salicyllic acid on vase life of cut flowers rose (Rosa hybrida cv. Black Magic). International Journal of Biology, Pharmacy and Allied Sciences. 2015, 5, 297-305.
Kitis, M. Disinfection of wastewater with peracetic acid: A review. Environment International. 2004, 30, 47-55. https://doi.org/10.1016/S0160-4120(03)00147-8.
Knee, M. Selection of biocides for use in floral preservatives. Postharvest Biology and Technology. 2000, 18, 227-234.
Lee, Y.B.; Kim, W.S. Improving Vase Life and Keeping Quality of Cut Rose Flowers Using a Chlorine Dioxide and Sucrose Holding Solution. Horticultural Science and Technology. 2018, 36, 380-387. https://doi.org/10.12972/kjhst.20180037
Ramoliya, P.J.; Patel, H.M.; Pandey, A.N. Effect of salinisation of soil on growth and macro- and micro- nutrient accumulation in seedlings of Acacia catechu (Mimosaceae). Annals of Applied Biology. 2004, 144, 321-332. https://doi.org/10.1111/j.1744-7348.2004.tb00347.x.
de la Riva, F.D.; Mazuela, P.C.; Álvaro, J.E.; Urrestarazu, M. Treatment with Peracetic Acid Extends the Vase Life of Lisianthus (Eustoma grandiflorum) Flowers. HortScience. 2009, 44, 418-420. http://dx.doi.org/10.21273/HORTSCI.44.2.418.
Sapers, G.M. Efficacy of washing and sanitizing methods for disinfection of fresh fruits and vegetable products. Food Technology and Biotechnology. 2001, 39, 305-311.
Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentrations of Na+ and Cl– ions in soil solution hale simultaneous detrimental effects on growth of Faba bean under salinity stress. Journal of Experimental Botany. 2010, 61, 4449-4459. https://doi.org/10.1093/jxb/erq251.
Van Doorn, W.G.; De Witte, Y. Source of the bacteria involved in vascular occulsion of cut rose flowers. Journal of the American Society for Horticultural Science. 1997, 122, 263-266.
Van Doorn, W.G.; Perik, R.R.J. Hydroxyquinoline citrate and low pH prevent vascular blockage in stems of cut rose flowers by reducing the number of bacteria. Journal of the American Society for Horticultural Science. 1990, 115, 979-981.
Yatendra, K.; Dwivedi, D.; Anurag, B. Enhancing the vase life of rose (Rosa hybrida L.) cv first red through different holding solutions. Environment & Ecology. 2020, 35, 2764-2768.
Zeljković, S.; Pašalić, M.; Pašalić, B.; Mladenovic, E. Vase life of cut flowers using different vase solution. Conference: XII International Scientific Agricultural Symposium “Agrosym 2021”, Jahorina, October 07-10, 2021, 51-56.
Academic Editor: Dr. Iuliana Motrescu
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.