Enver Kendal
ABSTRACT. Triticale is an important source of protein in animal nutrition, both as grain and silage. Protein is a quality criterion that is strongly affected by the environment and genetic factors. The objectives of this study were to assess genotype–environment (G-E) interactions and determine and compare stable genotypes to recommend for further evaluation. The protein content of 12 genotypes obtained from 7 environments were evaluated using additive main effect and multiplicative interaction (AMMI) analysis in the 2014–2015 and 2015–2016 growing seasons in 4 locations in Turkey. The variance of AMMI analysis based on protein content showed a significant effect of environment, genotype and G–E interaction, with a 93.0, 2.3 and 4.5% total variation, respectively. Partitioning of the total sum of squares showed that the effect of environment was a predominant source of variation, followed by the G–E interaction and genotype effect. AMMI analysis showed that the first principal component was highly significant at P < 0.01. The mean grain protein content varied from 14.9 to 16.2% among the genotypes and from 10.9 to 18.8% among the environments. AMMI analysis indicated that G3, G12 and G1 were quite promising genotypes. G8, which was used as a variety candidate in this research, was quite stable but had a low protein value. In addition, G3, G11 and G12 had a higher protein content than the standard varieties evaluated in this research. G3 had both a high protein content and stable genotype; therefore, it can be recommended to release as a candidate. As AMMI analysis indicated, E1 and E7 environments were especially suitable for protein studies in triticale, while E4, E5 and E6 showed poor results and were not suitable environments.
Keywords: AMMI; location; protein content; triticale.
Cite
ALSE and ACS Style
Kendal, E. Protein content stability of triticale genotypes under multiple environments using AMMI analysis. Journal of Applied Life Sciences and Environment 2024, 57, 359-370.
https://doi.org/10.46909/alse-573141
AMA Style
Kendal E. Protein content stability of triticale genotypes under multiple environments using AMMI analysis. Journal of Applied Life Sciences and Environment. 2024; 57 (3): 359-370.
https://doi.org/10.46909/alse-573141
Chicago/Turabian Style
Kendal, Enver. 2024. “Protein content stability of triticale genotypes under multiple environments using AMMI analysis” Journal of Applied Life Sciences and Environment 57, no. 3: 359-370.
https://doi.org/10.46909/alse-573141
View full article (HTML)
Protein Content Stability of Triticale Genotypes Under Multiple Environments Using Ammi Analysis
Enver KENDAL1,2*
1Institute of Chemistry of Moldova State University, Chisinau, Republic of Moldova; e-mail: ionbulhac@yahoo.com; verzub@mail.ru
2Institute of Genetics, Physiology and Plant Protection of Moldova State University, Chisinau, Republic of Moldova; e-mail: anastasia.stefirta@gmail.com
3Institute for Research, Innovation and Technology Transfer of “Ion Creangă” State Pedagogical University, Chișinău, Republic of Moldova; e-mail: liliabrinza@mail.ru
*Correspondence: maria.cocu@sti.usm.md; mariacocu@gmail.com
Received: Oct. 04, 2023. Revised: Dec. 07, 2023. Accepted: Dec. 14, 2023. Published online: Feb. 09, 2024
ABSTRACT. Triticale is an important source of protein in animal nutrition, both as grain and silage. Protein is a quality criterion that is strongly affected by the environment and genetic factors. The objectives of this study were to assess genotype–environment (G-E) interactions and determine and compare stable genotypes to recommend for further evaluation. The protein content of 12 genotypes obtained from 7 environments were evaluated using additive main effect and multiplicative interaction (AMMI) analysis in the 2014–2015 and 2015–2016 growing seasons in 4 locations in Turkey. The variance of AMMI analysis based on protein content showed a significant effect of environment, genotype and G–E interaction, with a 93.0, 2.3 and 4.5% total variation, respectively. Partitioning of the total sum of squares showed that the effect of environment was a predominant source of variation, followed by the G–E interaction and genotype effect. AMMI analysis showed that the first principal component was highly significant at P < 0.01. The mean grain protein content varied from 14.9 to 16.2% among the genotypes and from 10.9 to 18.8% among the environments. AMMI analysis indicated that G3, G12 and G1 were quite promising genotypes. G8, which was used as a variety candidate in this research, was quite stable but had a low protein value. In addition, G3, G11 and G12 had a higher protein content than the standard varieties evaluated in this research. G3 had both a high protein content and stable genotype; therefore, it can be recommended to release as a candidate. As AMMI analysis indicated, E1 and E7 environments were especially suitable for protein studies in triticale, while E4, E5 and E6 showed poor results and were not suitable environments.
Keywords: AMMI; location; protein content; triticale.
INTRODUCTION
Triticale (x Triticosecale Wittmack) was developed by long-term breeding studies on wheat × rye hybrids utilizing marginal and poor agricultural areas to increase yield per hectare and to meet the food needs of the rapidly increasing world population. It is grown in many countries, including the USA, Poland, Canada and Mexico (CIMMYT’). It was initially considered animal feed, but in recent years, it has been used as human food directly or by mixing with wheat flour, with the development of varieties or lines with positive agricultural characteristics as a result of breeding studies (Peña, 2004). Triticale has an increasing cultivation area in Türkiye as well as throughout the world. Although the triticale cultivation area and production in the world and in Turkey vary depending on the year, the total production is 14.7 million tonnes from 3.8 million hectares of land in the world and 228,000 tonnes of production from 105,000 hectares of land in Turkey (FAOSTAT, 2022).
Triticale is more resistant to adverse environmental conditions than other grains and less input is used in its cultivation (Mamo et al., 2009). With this feature, its ability to protect the environment comes to the fore compared to other products. Triticale is also seen as an alternative grain to solve nutritional problems (Zhu, 2018). To adequately balance food for the increasing world population, it is of great importance to develop genotypes that give the highest yield and quality per unit area, and studies on this subject are developing rapidly (Kızılgeçi and Yıldırım, 2017). Therefore, limited advances have increased the demand for new varieties. For this purpose, variation is created in genotypes, and selections are made. In this way, new varieties that are high quality, productive, and disease and pest resistant can be obtained (Lalević et al., 2022).
According to studies conducted to improve yield and quality criteria in cereal breeding, there is a negative relationship between yield and quality (Neuweiler et al., 2020). Although plant breeders have worked hard to increase yield, the quality ratio in total yield has not increased to the desired level (Rapp et al., 2018). Therefore, when making a variety recommendation, quality parameters should be examined, in addition to yield. Quality parameters are not only gen-otypic features but are also highly affected by environmental conditions (Kilic, 2014). In this sense, evaluating them in terms of quality characteristics by growing them under different environmental conditions and identifying superior performers will contribute to the selection of breeding materials and the development of quality varieties in triticale breeding studies (Kendal, 2022; Rajičić et al., 2023).
The protein ratio stands out as a quality criterion in triticale. The protein content generally varies between 12 and 16% in triticale, depending on genotypes and environmental conditions. The protein ratio is also affected by envi-ronmental factors (Pattison and Trethowan, 2013), such as environmentally dependent growth conditions, climate factors, rain, temperature during triticale maturation, fertilization, rotation applied to the soil, disease, and irrigation time and amount. The genetic structure has a small but important effect on protein (Güngör et al., 2022). Extreme conditions may occur that affect the protein ratio in triticale, especially in cultivation regions where temperature changes are frequent and the distribution of precipitation is irregular (Tüik, 2021). Since the protein ratio of triticale varieties grown in these areas is also greatly affected by environmental factors, the effect of the environment on the protein ratio should be thoroughly investigated (Gebeyaw, 2019).
Today, the data obtained as a result of breeding studies have been analyzed and evaluated using many different methods. The main aim is to clearly reveal the sources of variation between genotypes, environments and their in-traction (Hassani et al., 2018).
Thus, many models are used. One of these models is the additive main effect and multiplicative interaction (AMMI) analysis model. The AMMI analysis model presents these effects in more detail with interaction principal component analysis (IPCA) (Shahriari et al., 2018).
In addition, the graphs created using the AMMI model provide researchers with the opportunity to visually comment on the stability of genotypes and the suitability of environments (Ghaed-Rahimi et al., 2014). Thus, the research results are presented with some very clear graphs instead of more complex numerical data tables. This analysis model is emphasized be-cause it is an issue that should be considered by both researchers and those who want to benefit from the re-search results.
The aim of this study was to (i) analyses the effect of GEI on the protein content of 12 triticale genotypes using the AMMI model, (ii) identify stable triticale genotype(s) across environments and (iii) detect appropriate genotype(s) for different environments based on the protein content.
MATERIALS AND METHODS
Experimental location
This study was conducted in seven different locations in the Southeastern Anatolia Region under at four locations of Turkey. Information about the locations where the study was conducted is given in Table 1.
Plant materials
Ten advanced spring triticale lines and two standards were evaluated in seven environments across two growing seasons. The genotypic code and pedigree of all genotypes are shown in Table 2.
Table 1
Years, codes and coordinate status of environments
|
Years |
Environments |
Altitude(m) |
Latitude |
Longitude |
Averag. of rainfall.(mm) |
|
2014–2015
|
E1(Diyarbakır) |
613 |
37° 55′ N |
40° 14′ E |
583 |
|
E2(Adıyaman) |
670 |
37° 76′ N |
38° 27′ E |
541 |
|
|
E3(Ceylanpınar) |
367 |
36° 84′ N |
40° 05′ E |
305 |
|
|
E7(Ceylanpınar) |
217 |
||||
|
2015–2016 |
E4(Diyarbakır) |
613 |
37° 55′ N |
40° 14′ E |
418 |
|
E5(Adıyaman) |
670 |
37° 76′ N |
38° 27′ E |
403 |
|
|
E6(Hazro) |
896 |
38° 15′ N |
40° 49′ E |
744 |
Table 2
Code, name and pedigree of triticale genotypes
|
Code |
Pedigree of lines and name checks |
|
Pedigree of lines and name checks |
|
G1 |
LIRON_2/5/……CTSS04Y……. |
G7 |
LIRON_2/5/DIS ….. CTSS02B….…. |
|
G2 |
PRESTO//2….CTSS03Y….…. |
G8 |
HX87-244/…CTSS03SH……. |
|
G3 |
LIRON_2/5/…… CTSS03Y…. |
G9 |
HX87-244/…/….. CTSS03S……… |
|
G4 |
TURACO/…..…CTSS02B……… |
G10 |
Presto(check) Transitional Zone Agr. Res. Inst. |
|
G5 |
Tacettinbey(check) —–Cukurova Univ. |
G11 |
LIRON_2/… ……CTSS03Y…. |
|
G6 |
DRIRA/2*CMH77A……CTSS02B…….. |
G12 |
LIRON_2/5/DIS …..CTSS03Y… |
Experimental design and treatment details
The experiments conducted in this research were set up with four replications in a random blocks trial design (RBTD). Each parcel was planted in 6 rows with a plot size of 5 × 1.2 m2 (5 m length, 1.2 m width) and spacing of 0.20 m between rows, 0.5 m between plots and 1 m between blocks.
Cultural practices
Seeds were sown with a planting machine at a rate of 20–22 kg.ha−1. The plant depth and soil compaction were kept at a minimum.
With seed planting, 60 kg.ha−1 P and N were applied, and during the till-ering period, only nitrogen fertiliser and 60 kg.ha−1 urea were applied. Other cultural practices were kept constant for all experimental units.
Data collection
The data on protein ratios obtained from the research were made according to the AMMI analysis model to determine the stability of genotypes according to their environments and to test the suitability of the en-vironments. In this model, principal component analysis (PCA) was used to decompose the multiplicative effects of genotype–environment interactions into a series of IPCAs (Purchase, 1997).
Statistical analysis
The Genstat statistical package programme (version 12) was used to create this model. AMMI analysis was performed according to the method presented by Shahriari et al. (2018).
RESULTS
This study aimed to evaluate the grain protein content across different years.
However, due to changes in environments and locations each year, a combined analysis over the years was not feasible.
Instead, the study focused on analyzing data based on specific environments using AMMI analysis.
The regression coefficient (bi), standard deviation (Sd), and deviations from regression (S2di) analyses were not provided using the AMMI technique, and the inclusion of such data would overly lengthen the article and introduce semantic complexity.
AMMI analysis interprets IPCA scores based on the percentage effect of variation sources. This information, along with the stability results, is clearly presented through variance analysis, IPCA scores, and visual representation in Figure 1.
Table 3
The analysis of variance for grain yield using AMMI model
|
Source |
Degrees of freedom |
Sum of squares |
Mean square |
F |
% of total MS |
% of total interactions MS |
|
Total |
167 |
1394.2 |
8.35 |
* |
– |
|
|
Treatments |
83 |
1357 |
16.35 |
44.72 |
– |
|
|
Genotypes |
11 |
31.4 |
2.85** |
7.81 |
2.30 |
|
|
Environments |
6 |
1264.7 |
210.78** |
162.41 |
92.6 |
|
|
Block |
7 |
9.1 |
1.3 |
3.55 |
0.66 |
|
|
Interactions |
66 |
60.9 |
0.92** |
2.53 |
4.46 |
61.90 |
|
IPCA 1 |
16 |
37.7 |
2.36** |
6.45 |
|
14.61 |
|
IPCA 2 |
14 |
8.9 |
0.64ns |
1.74 |
|
|
|
Residuals |
36 |
14.3 |
0.4 |
1.09 |
– |
|
|
Error |
77 |
28.2 |
0.37 |
* |
– |
|
**: significant at 1% probability level, MS: mean of squares
According to the results of the combined analysis of variance, all sources of variation and IPCA 1 were significant (P < 0.001), while IPCA 2 was insignificant (Table 3).
The presence of variability among tested genotypes showed the possibility of obtaining desirable protein content. The significant variation in GEI indicated the possibility of performing stability analysis to understand the nature of GEI and the performance of the genotypes over tested environments.
The impact of environments was calculated for 92.6% of all variation, compared with 2.3% for genotype and 4.46% for their interaction (Table 3).
According to the AMMI analysis, variance analysis showed that the protein ratio was affected most by location, followed by the genotype environment interaction, and least by the genotype.
The multiplicative variance of the variance sum of squares due to the interaction was divided into two basic interaction components, but only one of them, the effect of PCAI, was significant. PCI and PCII accounted for 61.90 and 14.61% of the variation in triticale protein content values, respectively (Table 3).
In AMMI analysis, the figure is interpreted from two perspectives: the x-axis expresses the effect of genotypes and environment, and the y-axis expresses the effect of their interaction (Figure 1).
The environments in which the research was conducted and the genotypes used in the research showed great variability in terms of both the main effect and interaction. The environments located in the positive region of the y-axis are desirable and, at the same time, highly productive, while the environments located in the negative region of the y-axis have low production and are undesirable (Kendal and Tekdal, 2016). According to these definitions, the AMMI (Figure 1) showed that the protein content was between 15.0 and 16.5% among genotypes and between 10.9 and 18.8% among environments (Figure 1 and Table 4).
Based on the evaluations the protein content average among environments, E1, E2, E3 and E7 had values above the mean (y-axis) in the first growing season (2014–2015), and E4, E5 and E6 had values below the mean (y-axis) in the first and second growing seasons (2015–2016).
The protein content in the 2014–2015 growing season was higher than that in the 2015–2016 growing season in all locations. Considering the environments as independent of each other, the highest protein ratio average was found in E3, and the lowest protein ratio was found in E6 (Figure 1 and Table 5).
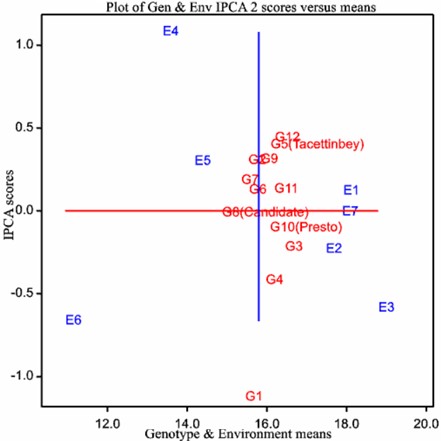
Figure 1 – The AMMI model shows the stability and adaptability genotypes based on environments
Table 4
Protein mean values and IPCA scores of genotypes according to six environments
|
Genotypes |
Gen mean |
IPCAg [1] |
IPCAg [2] |
|
G1 |
15.5 |
0.3773 |
-1.1262 |
|
G2 |
15.5 |
0.68429 |
0.30281 |
|
G3 |
16.5 |
-0.79356 |
-0.22052 |
|
G4 |
16.0 |
-0.9084 |
-0.42144 |
|
G5(Tacettinbey) |
16.1 |
-0.59684 |
0.39734 |
|
G6 |
15.6 |
0.43004 |
0.12386 |
|
G7 |
15.4 |
0.35112 |
0.18264 |
|
G8(Candidate) |
14.9 |
1.05342 |
-0.01363 |
|
G9 |
15.8 |
0.24208 |
0.30923 |
|
G10(Presto) |
16.1 |
0.09493 |
-0.10352 |
|
G11 |
16.2 |
-0.45094 |
0.12999 |
|
G12 |
16.2 |
-0.48342 |
0.43943 |
The AMMI graph (Figure 1) is described by two curves (vertical and horizontal curve indicated by arrow). The vertical curve shows the average protein ratio, and the horizontal curve, indicated by the arrow, shows which genotypes are more stable in terms of protein average based on two-year values.
Genotypes located close to the horizontal line showing the x-axis and to the right of the vertical line showing the y-axis indicate that they are stable and have high protein values, while genotypes located away from the horizontal line showing the x-axis and to the left of the vertical line showing the y-axis have low stability and low protein content (Yan and Tinker, 2002).
G8 was near the x-axis and on the right of the y-axis, showing that this genotype had a stable and low protein content. However, G11, G10 and G3 are located to the right of the y-axis and near the x-axis; therefore, they had favorable genotypes across environments. Moreover, G1 and G12 were far from the x-axis; thus, they were unstable based on protein con-tent in different environments. AMMI (Figure 1) showed that G3 and G11 were favorable, but G6 and G7 were the poorest genotypes based on protein content across the tested environments (Table 5).
As a result of this analysis, the genotypes that were preferred and should be selected across environments showed the following order: G3 > G12 > G11 > G5 and G10 (Table 6).
As shown in Table 6, genotypes that had a high protein ratio in the first or second order and were stable for each environment or more than one environment could be selected. AMMI analysis is an analysis model that can convey extremely important results on the graph supporting it, with different PCA solutions used to evaluate the data obtained from studies conducted in different environments, determine the most stable and preferred variety candidates and the most suitable environments, and support the accuracy of the results.
Table 5
The Protein content of genotypes in 7 environment (%)
|
Genotypes |
E1 |
E2 |
E3 |
E4 |
E5 |
E6 |
E7 |
Mean |
|
G1 |
17.8 |
16.9 |
18.8 |
11.8 |
13.6 |
11.9 |
17.5 |
15.5 |
|
G2 |
17.5 |
16.9 |
17.7 |
13.8 |
14.2 |
11.7 |
17.2 |
15.5 |
|
G3 |
18.0 |
18.4 |
20.5 |
13.4 |
15.5 |
10.5 |
19.1 |
16.5 |
|
G4 |
17.5 |
18.6 |
20.2 |
13.4 |
13.4 |
10.2 |
18.6 |
16.0 |
|
G5 (Tacettinbey) |
18.3 |
17.8 |
19.3 |
14.0 |
14.1 |
10.1 |
19.1 |
14.8 |
|
G6 |
17.4 |
17.3 |
17.6 |
13.1 |
14.4 |
11.4 |
18.1 |
15.6 |
|
G7 |
18.2 |
16.8 |
17.7 |
12.9 |
13.9 |
10.7 |
17.2 |
15.3 |
|
G8(Candidate) |
17.7 |
17.2 |
16.7 |
12.8 |
13.0 |
11.6 |
15.9 |
15.0 |
|
G9 |
18.3 |
16.6 |
18.7 |
13.6 |
14.9 |
11.1 |
17.9 |
15.8 |
|
G10(Presto) |
18.1 |
17.1 |
19.2 |
13.6 |
14.8 |
11.4 |
17.8 |
16.1 |
|
G11 |
18.3 |
17.6 |
19.9 |
14.1 |
14.3 |
10.6 |
18.2 |
16.2 |
|
G12 |
18.1 |
17.9 |
19.7 |
14.4 |
14.5 |
10.4 |
18.4 |
16.2 |
|
Mean |
17.9 |
17.4 |
18.8 |
13.4 |
14.2 |
10.9 |
17.9 |
15.7 |
Table 6
The first four AMMI selections for per environments, variance and IPCA scores
|
Environments |
Score |
1 |
2 |
3 |
4 |
IPCAe [1] |
IPCAe [2] |
Variance |
|
E1 |
0.4456 |
G10(S) |
G3 |
G12 |
G11 |
0.11836 |
0.313 |
17.9 |
|
E2 |
-0.4308 |
G3 |
G4 |
G11 |
G12 |
-0.23271 |
0.933 |
17.4 |
|
E3 |
-11.422 |
G3 |
G4 |
G11 |
G5(S) |
-0.58810 |
1.536 |
18.8 |
|
E4 |
0.0807 |
G12 |
G5(S) |
G11 |
G9 |
107.850 |
0.642 |
13.4 |
|
E5 |
0.2205 |
G12 |
G3 |
G11 |
G5(S) |
0.29791 |
0.737 |
14.2 |
|
E6 |
14.759 |
G1 |
G8(Can) |
G2 |
G10(S) |
-0.66586 |
0.538 |
10.9 |
|
E7 |
-0.6497 |
G3 |
G4 |
G12 |
G11 |
-0.00809 |
0.935 |
17.9 |
Lower IPCA1 score indicates higher environmental stability
DISCUSSION
Studies conducted in multiple environments enable the determination of the most superior genotypes for each environment or more than one environment. Due to the risk of traits in changing environments, different statistical methods (GGE, AMMI) are used to reveal the effects of genotype, environment and genotype–environment interactions in a broad way and remain usable in many plants (Goyal et al., 2011; Shahriari et al., 2018). Moreover, AMMI and GGE models have been used to determine the effect of genotype, environment and genotype–environment interactions on the protein ratio (Akcura et al., 2016; Has-sani et al., 2018; Oral et al., 2018; Yan and Tinker, 2006). AMMI analysis is used to identify superior genotypes and their stability under multiple environmental conditions and to identify the most ideal and favorable sites (Tekdal and Kendal, 2018). In this study, AMMI analysis indicated that G3 was stable, while G3 and G11 were the best based on protein content in the tested environments. However, the protein ratio fluctuated according to year and environ-mental factors. Even in different years, some environments (E3 and E7) had high protein values, while some environments (E6) had low protein values. Some research results evaluated by AMMI analysis have found that this model can be used to show the genotype–environment effect in a two-way graph. The two-way graph provides information about the stability of genotypes and the suitability of environments (Derejko et al., 2020; Gauch et al., 2008; Ghaed-Rahimi et al., 2014; Goyal et al., 2011). It is used to determine the most suitable varieties in terms of one or more traits in research carried out in more than one year and in different environments, to determine candidate varieties, and to identify and recommend the most suitable varieties (Bocianowski et al., 2021). Similar to studies on different traits (Đekić et al., 2014), in this study, the impact of environment on the protein content in triticale was higher than the impact of genotype and the genotype–environment interaction. Descriptive and representative environments in terms of protein content in triticale are good test sites for the identification of specific cultivars (Bilgin et al., 2018). In addition, the GGE biplot analysis have shown that genotypes provide insight into both their specific and general adaptability and that genotypes can be preferred accordingly. This study showed that in the four mega-environment sectors, which consisted of different test sites, G4 and G9 were poor genotypes based on protein content, and G3 and G11 were favorable among crop seasons and environments. G8 (candidate) was stable among changing environment conditions. Some research results have reported that an ideal genotype should have both high mean values and stability in test environments (Lule et al., 2014; Yan and Tinker, 2006). This research consisted of more than one year and different sites. If there were no significant differences between two or more sites, these sites were included in the same group, and this situation was called the mega-environment. In similar studies on the same subject, it is recommended to conduct the study in only one of the environments in the same group, as it reduces the cost. The effect of genotype–environment interaction in a study can generally be determined to a high extent because of studies conducted in more than two mega-environments (Gauch, 2013; Stoyanov, 2020). The AMMI models showed that some genotypes had general adaptation to environmental condi-tions, while others had specific adaptation. Results similar to other studies were obtained (Branković-Radojčic et al., 2018; Ferreira et al., 2015). AMMI indicated that the variability in MET data due to genotype–environment in-teractions was significant. This is a good model to evaluate genotypes in terms of both stability and protein ratio. However, the GGE biplot results visually give a more detailed representation by evaluating the genotypes from dif-ferent angles with more graphics, but the AMMI model, along with numerical data, lists both the stability values and the genotypes to be recommended for each environment more easily (Bocianowski et al., 2021).
CONCLUSIONS
AMMI indicated that the protein content of triticale genotypes was highly affected by the environment, followed by genotype–environment interaction and genotypic effects. This model was effective in identifying the stability states of triticale genotypes in terms of grain protein content and in determining ideal environments. G4 and G9 showed specific adaptability, while G8 (candidate) showed general adaptability across test sites. Furthermore, G3 should be used in E2, E3 and E7, while G9 should be used in E1 and E5 and G8 in E6. Moreover, G3 and G11 can be registered as varieties for studying the protein content in test environments. These two genotypes were quite favorable and ideal among genotypes at the test sites. This study demonstrated that the AMMI biplot model is very useful for determining the most suitable environments and stable genotypes for traits in studies conducted in different envi-ronments and years. This model can be used by researchers when evaluating different plants and their properties.
Author Contributions: I have read and approved the publication of the manuscript in the present form.
Funding: There was no external funding for this study.
Acknowledgments: The author expresses their gratitude to the GAP International Agricultural Research and Training Center.
Conflicts of Interest: Author declare no conflict of interest.
REFERENCES
Akcura, M.; Kokten, K.; Akcacik, A.G.; Aydogan, S. Pattern analysis of Turkish bread wheat landraces and cultivars for grain and flour quality. Turkish Journal of Field Crops. 2016, 21, 120-130. https://doi.org/10.17557/tjfc.72407
Bilgin, O.; Balkan, A.; Korkut, Z.K.; Başer, İ. Multi-environmental evaluation of triticale, wheat and barley genotypes by GGE biplot analysis. Journal of Life Sciences. 2018, 12, 13-23. https://doi.org/10.17265/1934-7391/2018.01.002
Bocianowski, J.; Tratwal, A.; Nowosad, K. Genotype by environment interaction for main winter triticale varieties characteristics at two levels of technology using additive main effects and multiplicative interaction model. Euphytica. 2021, 217, 26. https://doi.org/10.1007/s10681-020-02756-x
Branković-Radojčić, D.; Babić, V.; Girek, Z.; Živanović, T.; Radojčic, A.; Filipović, M.; Srdić, J. Evaluation of maize grain yield and yield stability by AMMI analysis. Genetika. 2018, 50, 1067-1080. https://doi.org/10.2298/GENSR1803067B
Đekić, V.; Milivojević, J.; Branković, S. The interaction of genotype and environment on yield and quality components in triticale. Biologica Nyssana. 2018, 9. https://doi.org/10.5281/zenodo.1470850
Derejko, A.; Studnicki, M.; Wójcik-Gront, E.; Gacek, E. Adaptive grain yield patterns of Triticale (× Triticosecale Wittmack) cultivars in six regions of Poland. Agronomy. 2020, 10, 415. https://doi.org/10.3390/agronomy10030415
FAOSTAT. Statistics division of food and agriculture organization of the united nations. Online at: http://faostat3.fao.org/browse/Q/QC/E, 2022.
Ferreira, V.; Grassi, E.; Ferreira, A.; Santo, H.D.; Castillo, E.; Paccapelo, H. Genotype-environment interaction and stability of grain yield in triticales and tricepiros. Chilean Journal of Agricultural and Animal Sciences. 2015, 31, 93-104.
Gauch Jr, H.G. A simple protocol for AMMI analysis of yield trials. Crop science. 2013, 53, 1860-1869. https://doi.org/10.2135/cropsci2013.04.0241
Gauch Jr, H.G.; Piepho, H.P.; Annicchiarico, P. Statistical analysis of yield trials by AMMI and GGE: Further considerations. Crop science. 2008, 48, 866-889. https://doi.org/10.2135/cropsci2007.09.0513
Gebeyaw, A. Genotype by environment interaction and stability on yield, yield components and protein content of Faba bean (Vicia faba L.) across Faba bean growing area of Ethiopia (Doctoral dissertation). Bahir Dar University College Of Agriculture And Environmental Science, Graduate Program, 2019.
Ghaed-Rahimi, L.; Heidari, B.; Dadkhodaie, A. Genotype × environment interactions for wheat grain yield and antioxidant changes in association with drought stress. Archives of Agronomy and Soil Science. 2014, 61, 153. https://doi.org/10.1080/03650340.2014.926004
Goyal, A.; Beres, B.L.; Randhawa, H.S.; Navabi, A.; Salmon, D.F.; Eudes, F. Yield stability analysis of broadly adaptive triticale germplasm in southern and central Alberta, Canada, for industrial end-use suitability. Canadian Journal of Plant Science. 2011, 91, 125-135. https://doi.org/10.4141/cjps10063
Güngör, H.; Cakir, M.F.; Dumlupinar, Z. Evaluatıon of Trıtıcale: Genotype by Envıronment Interactıon and GGE Bıplot Analysıs. Journal of Animal and Plant Sciences. 2022, 32. https://doi.org/10.36899/JAPS.2022.6.0573
Hassani, M.; Heidari, B.; Dadkhodaie, A.; Stevanato, P. Genotype by environment interaction components underlying variations in root, sugar and white sugar yield in sugar beet (Beta vulgaris L.). Euphytica. 2018, 214. https://doi.org/10.1007/s10681-018-2160-0
Kendal, E. Using biplot analysis technique to selection in Tritikale breeding studies. Yuzuncu Yıl University Journal of Agricultural Sciences. 2022, 32, 186-198. https://doi.org/10.29133/yyutbd.1035375
Kendal, E.; Tekdal, S. Application of AMMI model for evaluation spring barley genotypes in multi-environment trials. Bangladesh Journal of Botany. 2016, 45, 613-620.
Kiliç, H. Additive main effect and multiplicative interactions (AMMI) Analysis of grain yield in barley genotypes across environments. Journal of Agricultural Sciences. 2014, 20, 337-344. https://doi.org/10.15832/tbd.44431
Kizilgeçi, F.; Yildirim, M. Bazı tritikale (X Triticosecale Wittmack) genotiplerinin verim ve kalite özelliklerinin belirlenmesi. Türkiye Tarımsal Araştırmalar Dergisi. 2017, 4, 43-49. https://doi.org/10.19159/tutad.300616
Lule, D.; Tesfaye, K.; Mengistu, G. Genotype by environment interaction and grain yield stability analysis for advance triticale (X. Triticosecale Wittmack) genotypes in western Oromia, Ethiopia. SINET: Ethiopian Journal of Science. 2014, 37, 63-68. https://www.ajol.info/index.php/sinet/article/view/111062
Lalević, D.; Miladinović, B.; Biberdžić, M.; Vuković, A.; Milenković, L. Differences in grain yield and grain quality traits of winter triticale depending on the variety, fertilizer and weather conditions. Applied Ecology & Environmental Research. 2022, 20. http://dx.doi.org/10.15666/aeer/2005_37793792
Mamo, B.; Gelalcha, S.; Yaie, B.; Debelo, D.; Girma, B. Stability and performance evaluation of triticale (X Triticosecale wittm Ex A.) genotypes in different environments of Ethiopia. Sebil, 91, 2009.
Neuweiler, J.E.; Maurer, H.P.; Würschum, T. Genetic architecture of phenotypic indices for simultaneous improvement of protein content and grain yield in triticale (× triticosecale). Plant Breeding. 2021, 140, 232-245. https://doi.org/10.1111/pbr.12894
Oral, E. Effect of nitrogen fertilization levels on grain yield and yield components in triticale based on AMMI and GGE biplot analysis. Applied Ecology and Environmental Research. 2018, 16, 4865-4878. http://dx.doi.org/10.15666/aeer/1604_48654878
Pattison, A.L.; Trethowan, R.M. Characteristics of modern triticale quality: Commercially significant flour traits and cookie quality. Crop and Pasture Science. 2013, 64, 874-880. https://doi.org/10.1071/CP13056
Peña, R.J. Food uses of triticale. In Triticale improvement and production, FAO, 2004, 37-48.
Purchase, J.L. Parametric analysis to describe genotype x environment interaction and yield stability in winter wheat. PhD Thesis, University of the Free State, Bloemfontein, South Africa, 1997.
Rapp, M.; Lein, V.; Lacoudre, F.; Lafferty, J.; Müller, E.; Vida, G.; Bozhanova, V.; Ibraliu, A.; Thorwarth, P.; Piepho, H.P.; Leiser, W.L.; Würschum, T.; Longin, C.F.H. Simultaneous improvement of grain yield and protein content in durum wheat by different phenotypic indices and genomic selection. Theoretical and Applied Genetics. 2018, 131, 1315-1329. https://doi.org/10.1007/s00122-018-3080-z
Rajičić, V.; Popović, V.; Đurić, N.; Biberdžić, M.; Babić, V.; Stojiljković, J.; et al. Impact of agro-ecological conditions and fertilization on yield and quality of triticale on pseudogley soil. Notulae Botanicae Horti Agro-botanici Cluj-Napoca. 2023, 51. https://doi.org/10.15835/nbha51413387
Shahriari, Z.; Heidari B.; Dadkhodaie, A. Dissection of genotype × environment interactions for mucilage and seed yield in Plantago species: Application of AMMI and GGE biplot analyses. Plos One. 2018, 13. https://doi.org/10.1371/journal.pone.0196095
Stoyanov, H. Analysis on test weight of Bulgarian triticale cultivars. Bulgarian Journal of Crop Science. 2020, 57. https://cropscience-bg.org/page/en/details.php?article_id=879
Tekdal, S.; Kendal, E. AMMI model to assess durum wheat genotypes in multi-environment trials. Journal of Agricultural Science and Technology. 2018, 20, 153-166.
Yasar, M. Yield and fiber quality traits of cotton (Gossypium hirsutum L.) cultivars analyzed by biplot method. Journal of King Saud University-Science. 2023, 35. https://doi.org/10.1016/j.jksus.2023.102632
Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Canadian Journal of Plant Science. 2006, 86, 623-645. https://doi.org/10.4141/P05-169
Zhu, F. Triticale: Nutritional composition and food uses. Food Chemistry. 2018, 241, 468-479. https://doi.org/10.1016/j.foodchem.2017.09.009
Academic Editor: Dr. Iuliana Motrescu
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Kendal Enver



