Alexandra-Andreea Cherșunaru, Mihaela Claudia Spataru, Constantin Spataru
ABSTRACT. The anatomical peculiarities of the tiger’s skull reflect the ecological and behavioural needs of this predator. The study aims to observe the morphological adaptations that grant it exceptional hunting and survival abilities. The anatomical study was conducted on the skulls of two adult male tigers. The tiger’s skull is wide and rounded, providing a robust base for the attachment of masticatory muscles, which is essential for powerful bites. The sagittal crest, a bony prominence located on the upper part of the skull, serves as an attachment point for the temporal muscles. A distinct process is present on the maxillary tubercle, from which a strong tendon of the masseter muscle originates. The nasal bones are elongated, extending beyond the frontal processes of the maxillae, while the frontal area is elevated in the region of the postorbital processes. The anterior nasal opening is narrow, and the lower margin of the mandible is typically concave, with a prominent mental process. The front part of the mandibular symphysis is concave. Compared to other large felines, tigers have a more rounded skull and a downward-sloping dorsal surface. The tiger’s skull exhibits remarkable morpho-functional adaptations, such as a robust bony head, a prominent sagittal crest, a strong mandible, and well-developed insertions for masticatory muscles. These features are essential for their predatory lifestyle, allowing them to capture and control large prey efficiently.
Keywords: adaptation; mandible; skull; tiger.
Cite
ALSE and ACS Style
Cherșunaru, A.-A.; Spataru, M.C.; Spataru, C. Morpho-functional adaptations of the tiger skull (Panthera tigris) in relation to forceful biting.
Journal of Applied Life Sciences and Environment 2025, 58 (1), 1-12.
https://doi.org/10.46909/alse-581161
AMA Style
Cherșunaru A-A, Spataru MC, Spataru C. Morpho-functional adaptations of the tiger skull (Panthera tigris) in relation to forceful biting. Journal of Applied Life Sciences and Environment. 2025; 58 (1): 1-12.
https://doi.org/10.46909/alse-581161
Chicago/Turabian Style
Cherșunaru, Alexandra-Andreea, Mihaela Claudia Spataru, and Constantin Spataru. 2025. “Morpho-functional adaptations of the tiger skull (Panthera tigris) in relation to forceful biting.” Journal of Applied Life Sciences and Environment 58, no. 1: 1-12.
https://doi.org/10.46909/alse-581161
View full article (HTML)
Morpho-functional adaptations of the tiger skull (Panthera tigris) in relation to forceful biting
Alexandra-Andreea CHERȘUNARU1*, Mihaela Claudia SPATARU2 and Constantin SPATARU1
1Department of Preclinics, Faculty of Veterinary Medicine, “Ion Ionescu de la Brad” Iasi University of Life Sciences,
8, Mihail Sadoveanu Alley, 700490, Iasi, Romania; email: constantin.spataru@iuls.ro
2Department of Public Health, Faculty of Veterinary Medicine, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 8, Mihail Sadoveanu Alley, 700490, Iasi, Romania; email: mihaela.spataru@iuls.ro
*Correspondence: alexandra.sikra@iuls.ro
Received: Nov. 30, 2024. Revised: Jan. 09, 2025. Accepted: Jan. 22, 2025. Published online: Feb. 12, 2025
ABSTRACT. The anatomical peculiarities of the tiger’s skull reflect the ecological and behavioural needs of this predator. The study aims to observe the morphological adaptations that grant it exceptional hunting and survival abilities. The anatomical study was conducted on the skulls of two adult male tigers. The tiger’s skull is wide and rounded, providing a robust base for the attachment of masticatory muscles, which is essential for powerful bites. The sagittal crest, a bony prominence located on the upper part of the skull, serves as an attachment point for the temporal muscles. A distinct process is present on the maxillary tubercle, from which a strong tendon of the masseter muscle originates. The nasal bones are elongated, extending beyond the frontal processes of the maxillae, while the frontal area is elevated in the region of the postorbital processes. The anterior nasal opening is narrow, and the lower margin of the mandible is typically concave, with a prominent mental process. The front part of the mandibular symphysis is concave. Compared to other large felines, tigers have a more rounded skull and a downward-sloping dorsal surface. The tiger’s skull exhibits remarkable morpho-functional adaptations, such as a robust bony head, a prominent sagittal crest, a strong mandible, and well-developed insertions for masticatory muscles. These features are essential for their predatory lifestyle, allowing them to capture and control large prey efficiently.
Keywords: adaptation; mandible; skull; tiger.
INTRODUCTION
The Order Carnivora exhibits greater ecological diversity than any other order of mammals and includes approximately 270 existing species. It comprises many of the world’s largest terrestrial predators, such as the cheetah, lion, leopard, tiger, hyena, and wolf, as well as popular pets like dogs and cats, and iconic wild animals such as the panda, polar bear, and brown bear (Agnarsson et al., 2010; Christiansen and Wroe, 2007; Gündemir et al., 2023; Mazák, 1981; Thorley, 2017). These species vary significantly in traits such as body size, dietary ecology, social behaviour, locomotion, activity patterns, and more (Christiansen and Wroe, 2007; Finarelli and Flynn, 2006; Larivière and Kruuk, 2004; Wroe and Milne, 2007;). Felids are distinguished by anatomical adaptations that allow them to effectively hunt, using a strong and precise bite to kill (Christiansen and Wroe, 2007; Gürbüz et al., 2022). They have short, robust skulls with a reduced and wide rostrum, a convex frontal region, and broad zygomatic arches to generate high biting forces (Holliday and Steppan, 2004; Mazák, 2010).
Studies illustrate that the skull morphology varies between large and small felid species; however, this variation is closely related to the absolute size of the skull, indicating that large felids are not anatomically different from small species but simply larger (Gürbüz et al., 2022; Sicuro and Oliveira, 2011). The generation of uniform and powerful biting forces has led to the elongation and elevation of the aboral region of the skull, as well as a more robust zygomatic arch to support a more developed musculature (Christiansen, 2008; Mazák, 2010).
The tiger, the largest member of the Felidae family, is a carnivorous predator that dominates many ecosystems in Asia. The skulls of large males are massive, with a total length of over 255 mm, typically ranging between 285 and 360 mm (Mazák, 1981). The skull is a set of interconnected mechanical parts designed to work together to perform and support powerful bites (Christiansen, 2008; Christiansen and Adolfssen, 2005; Christiansen and Wroe, 2007). The functionality of the bite is an essential part of predatory behaviour. Therefore, it is important to assess it alongside the proportions of the skull and the bending strength of the teeth used for killing prey, especially the canines (Gündemir et al., 2023; Gürbüz et al., 2022; Mazák, 2010). The morphological adaptations of the carnivorous skull that underscore the ability to exert high biting forces are represented by a long and prominent external sagittal crest, robust and well-developed zygomatic arches, wide and convex temporal fossae, extremely long and sharp canines, and a strong mandible (Christiansen, 2008; Christiansen and Adolfssen, 2005; Christiansen and Wroe, 2007; Figueirido et al., 2010; Gündemir et al., 2023; Gürbüz et al., 2022; Meiri, 2005; Palmqvist et al., 2007).
This paper focuses on the morphological characteristics of the bony head in tigers, which influences mechanical performance during the killing bite.
MATERIALS AND METHODS
This study was conducted within the Department of Preclinical Sciences in the Anatomy discipline at the Faculty of Veterinary Medicine in Iași. The anatomical study was performed on the axial skeleton of two adult tigers received from the Bârlad Zoo after their natural death and from the Anatomy Museum of the Faculty of Veterinary Medicine.
Classical anatomical methods were employed to describe the morphological features of two male tigers’ skull skeletons. These included boiling, dissection, and cleaning of adjacent tissues, with the objective of observing the shape, length, and development of bony and articular processes compared to other members of the Felidae family. The description, identification, and homologation were conducted in accordance with the Nomina Anatomica Veterinaria (NAV) 2017.
RESULTS AND DISCUSSION
The robust structure of the skull
The tiger’s skull has a trapezoidal shape, conferred by the length of the longitudinal axis, which is approximately 35 cm, measured from the plane of the incisors to the level of the occipital protuberance. It consists of four surfaces: two symmetrical lateral surfaces, a dorsal surface, and a ventral surface.
The lateral surface of the skull, viewed as a whole, appears rather wide in the middle third of the skull. The facial skeleton represents a relatively small extension of about 10 cm, while the neurocranium, oriented aborally towards the articular condyles, measures about 23 cm in length. Thus, the viscerocranium constitutes one-third of the total length of the skull.
The dorsal surface of the skull is the largest surface. At the aboral extremity, it detaches from the external occipital protuberance (Protuberantia occipitalis externa), from which the external sagittal crest (Crista sagittalis externa) extends rostrally (Christiansen and Adolfssen, 2005; Gheție, 1971; Mazák, 2010; Spataru, 2003). The sagittal crest is a bony prominence, with a height of 0.5–2 cm, located on the upper part of the skull. It offers a large insertion to temporal muscles, essential in generating the bite force (Christiansen and Wroe, 2007; Ellis et al., 2009) (Figure 1).
The robust and well-developed zygomatic arches (Arcus zygomaticus) feature a frontal process that provides insertion for the powerful masseter muscles, significantly contributing to the pressure exerted during biting (Christiansen, 2008; Ellis et al., 2009; Mazák, 2010). The zygomatic processes of the frontal bone (Processus zygomaticus ossis frontalis) are approximately triangular and are about 2.8 cm in length. The orbit is large and incomplete on its aboral side because the zygomatic process of the frontal bone does not connect with the frontal process of the zygomatic bone (Processus frontalis ossis zygomaticum). Thus, the orbit generously communicates with the temporal fossa (Fossa temporalis). The anterior margin of the orbit is interrupted by the infratrochlear tubercle (Processus infratrochlearis). The infratrochlear foramen and the lacrimal foramen (Foramen lacrimale) are located at the base of the infratrochlear tubercle medially. At the base of the temporal process of the zygomatic bone, there is an infraorbital foramen (Foramen infraorbitale) that is oval and measures 1 cm by 1.3 cm (Figure 2A).
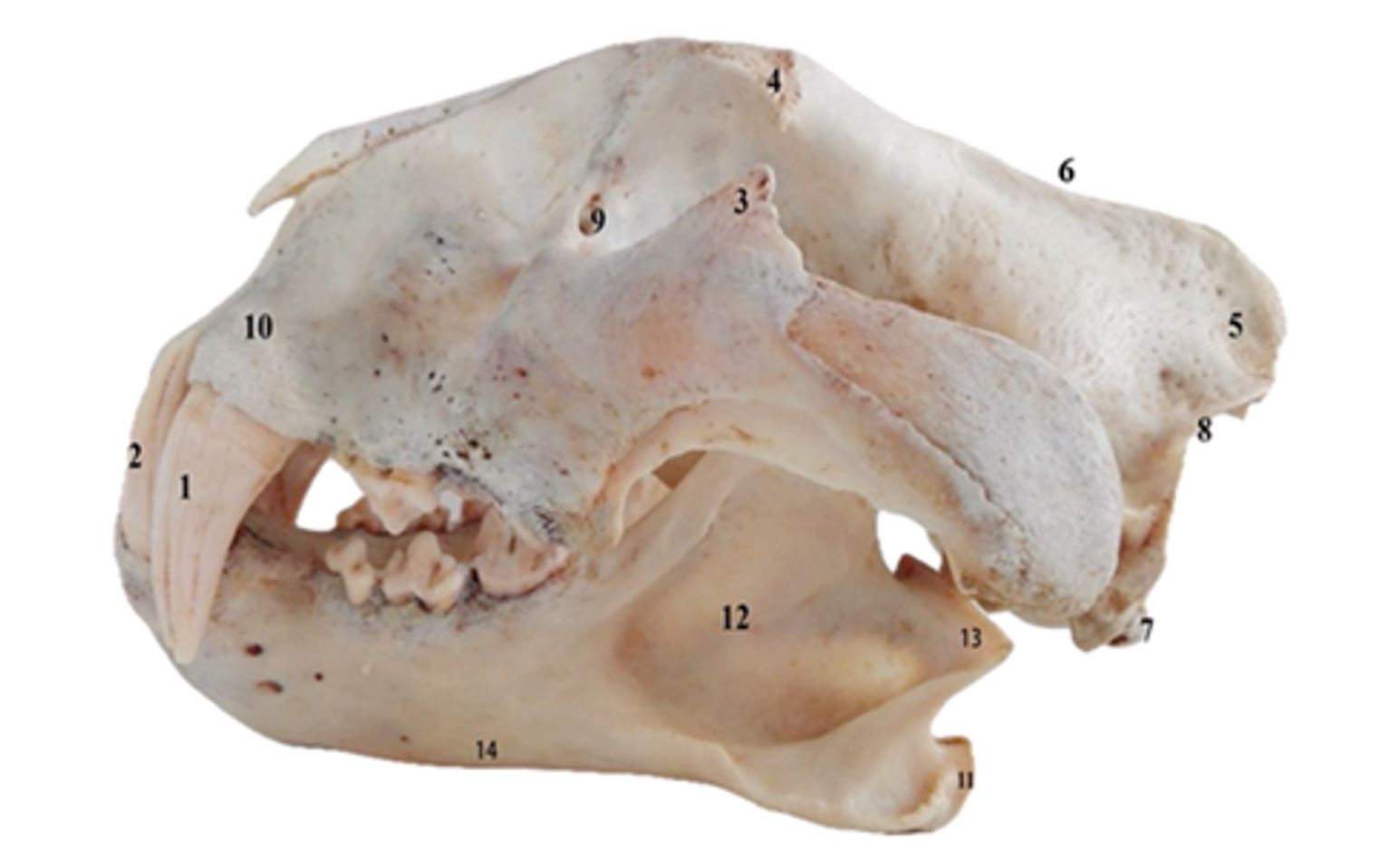
Figure 1 – Skull of Panthera tigris in lateral view
1–2. Dentes canini; 3. Processus frontalis ossis zygomaticum; 4. Processus zygomaticus ossis frontalis; 5. Protuberantia occipitalis externa; 6. Crista sagittalis externa; 7. Condylus occipitalis; 8. Crista nuchae; 9. Foramen lacrimale; 10. Crista alveolaris; 11. Processus angularis; 12. Fossa masseterica; 13. Processus condylaris; 14. Corpus mandibulae
The bite force must be considered in combination with other morphological indicators, especially the dental morphology (Christiansen and Wroe, 2007; Ellis et al., 2008; Ellis et al., 2009; Slater et al., 2009). The carnivorous diet, based almost exclusively on meat, is reflected in the dental pattern, which consists of highly developed, sharp, and robust canines and a pair of carnassial teeth, the first lower molar (M1) and last upper premolar (P4), that exert a shearing effect (Meiri, 2005; Różycka et al., 2024).
The hard palate is laterally bordered by four molars (the first and last being greatly reduced), one canine, and anteriorly by six incisors. The canine alveoli enclose conical and slightly curved canines (Dentes canini), which are concave posteriorly and convex on the anterior edge. In adult tigers, they measure about 6 cm long, with a transverse diameter of 1.8 cm and a craniocaudal diameter of 3 cm. The incisors (Dentes incisivi) are arranged approximately in a straight line, with the lateral ones being much more developed; they have sharp edges being well-suited for gripping and stripping flesh from prey (Christiansen and Wroe, 2007; Cooper et al., 2022; Gürbüz et al., 2022; Gündemir et al., 2023) (Figure 2).
The roots of the canines are strongly anchored in the alveoli, providing stability and reducing the risk of fracture during powerful bites (Christiansen and Adolfssen, 2005; Long, 1965; Różycka et al., 2024; Van Valkenburgh, 2007; Van Valkenburgh and Ruff, 1987).
In comparison to other felines, the tiger exhibits a large nasal opening characterised by oblique nasal bones that protrude rostrally and describe a wide oval-shaped nasoincisive notch (Incisura nasoincisiva; Figure 2B) (Cooper et al., 2022; Díaz Martínez et al., 2024a, b; Slater and Van Valkenburgh, 2009). The transverse diameter at the level of the zygomatic processes of the frontal bones is about 27 cm wide.
Thus, in proportion to body weight, the skull has an increased width, allowing for the development of a larger nasal cavity (Díaz Martínez et al., 2024a, b; Mazák, 2010; Torregrosa et al., 2010).
Considering the influence of environmental conditions in skull morphometry, research made by Cooper et al. (2022) showed a larger anterior facial length in captive individuals, while the skull length, nasal length, and posterior facial length are reduced.
The ventral surface of the tiger’s skull has an approximately trapezoidal shape, with a longitudinal axis of 35 cm, measured from the incisor plane to the plane of the occipital protuberance. The distance from the incisor plane to the plane of the occipital condyles (Condylus occipitalis) is about 30 cm (Ellis et al., 2009; Mazák, 2010; Slater and Van Valkenburgh, 2009). The region of the base of the skull extends from the occipital foramen to the transverse plane that crosses the bases of the pterygoid processes. In this area, the occipital foramen (Foramen magnum) is found; it is flanked by the two condyles, which are elongated (Ellis et al., 2009; Long, 1965; Slater and Van Valkenburgh, 2009). The tuberculum articulare of the pars squamosa of the temporal bone has a hemicylindrical shape, with a longitudinal axis of 5.5 cm and a transverse axis of 2 cm (Figure 3).
In the tiger, we also notice a bony process (Tuber maxillae) located in the caudo-lateral region of the alveolar margin (the rostro-ventral part of the zygomatic arch), which represents the point for insertion of the superficial portion of the masseter muscle (Figure 3). The presence of this process suggests that the tiger performs strong rostro-ventral and dorso-ventral bites, resulting in the suffocation of prey and the maximum shearing effect at the level of the first lower molar and the last upper premolar (Christiansen and Wroe, 2007; Ellis et al., 2008; Slater and Van Valkenburgh, 2009).
Some studies regarding the skull in other Felidae species, such as puma, leopard, and jaguar, have shown that this tubercle present at the maxilla suggests a strong tendon of the masseter muscle that acts with considerable force (Christiansen and Adolfssen, 2005; Sasaki, 2000). The absence of this bony prominence in small felines indicates that the tendon of the superficial portion of the masseter muscle is less developed (Mazák, 2010; Sasaki, 2000).
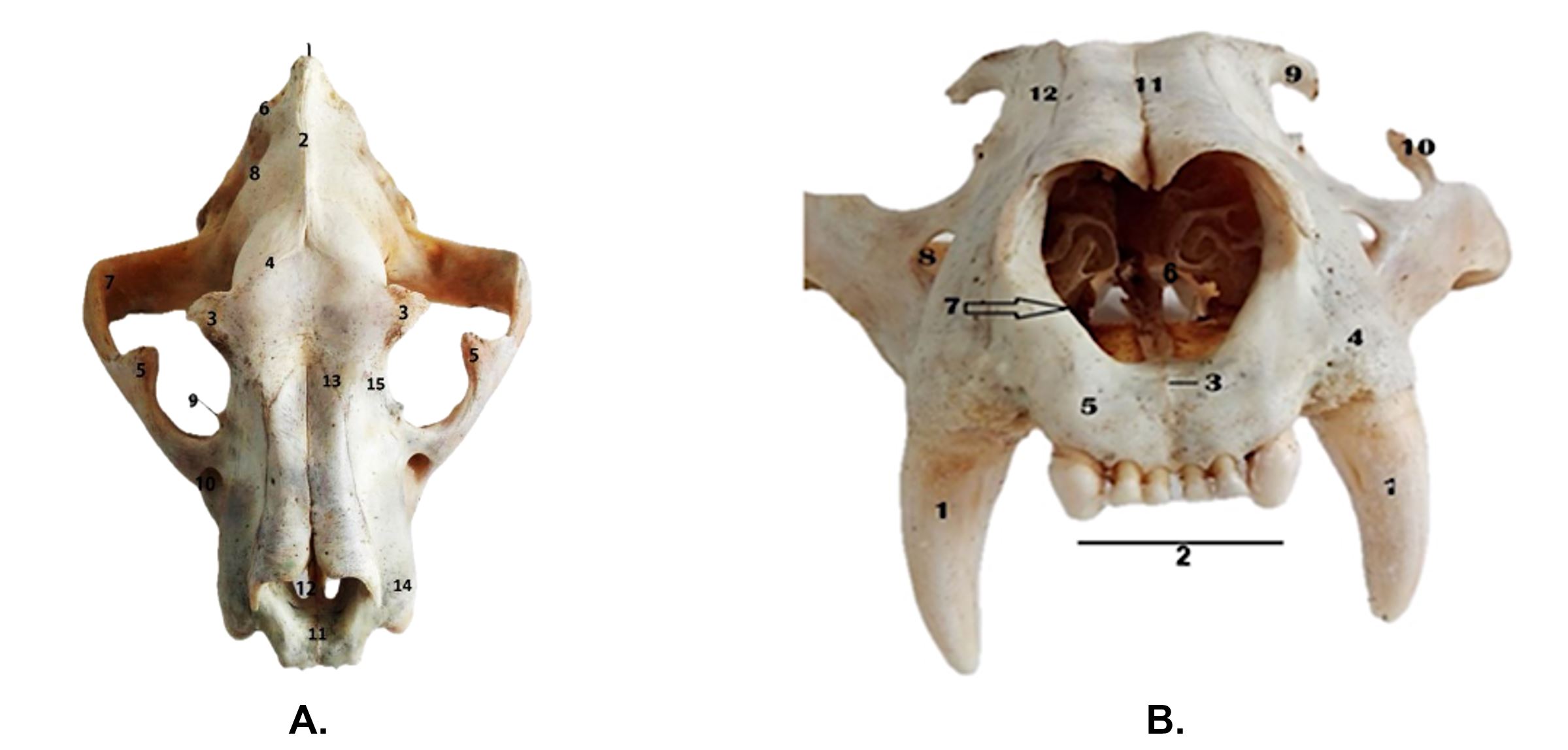
Figure 2 – Skull of Panthera tigris in dorsal (A) and ventral (B) view A.
Dorsal view:1. Protuberantia occipitalis externa; 2. Crista sagittalis externa; 3. Processus zygomaticus ossis frontalis; 4. Linea temporalis; 5. Processus frontalis ossis zygomatici; 6. Crista nuchae; 7. Arcus zygomaticus; 8. Fossa temporalis; 9. Processus infratrochlearis; 10. Foramen infraorbitale; 11. Fissura interincisiva; 12. Fissura palatina; 13. Sutura nasolacrimalis; 14. Cristae alveolares; 15. Sutura frontomaxillaris.
B. Rostral view:1. Dentes canini; 2. Dentes incisivi; 3. Fisura interincisiva; 4. Crista alveolares; 5. Facies labialis corporis ossis incisivi; 6. Conchae nasales 7. Incisura nasoincisiva; 8. Foramen infraorbitale; 9. Processus zygomaticus ossis frontalis; 10. Processus frontalis ossis zygomaticus; 11. Sutura internasalis; 12. Sutura nasomaxillaris
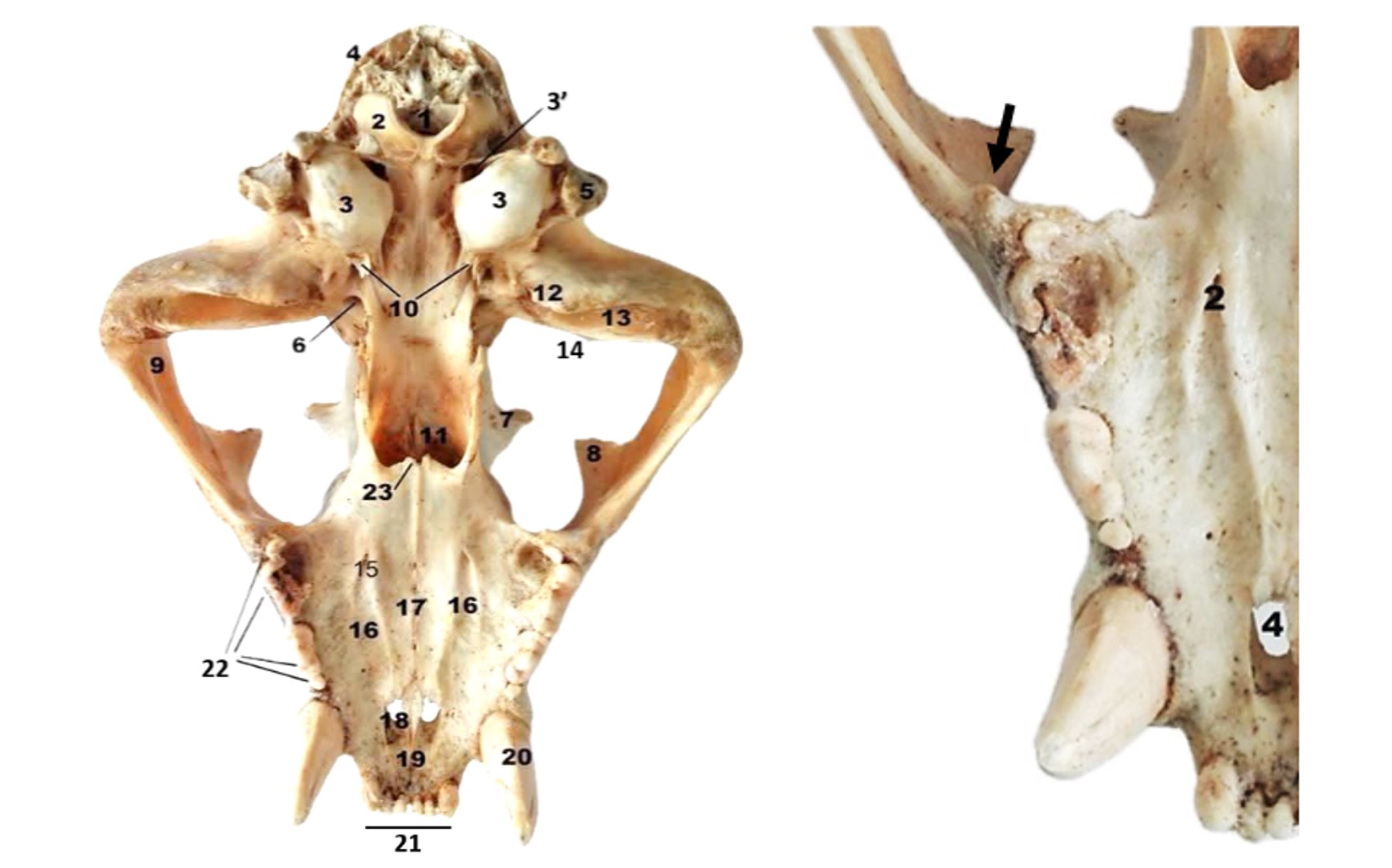
Figure 3 – Tiger skull–ventral view
1. Foramen magnum; 2. Condylus occipitalis; 3. Bulla tympanica; 3’. Foramen jugulare; 4. Crista nucha; 5. Processus paracondylaris; 6. Foramen ovale; 7. Processus zygomaticus ossis frontalis; 8. Processus frontalis ossis zygomaticum; 9. Arcus zygomaticus; 10. Processus muscularis; 11. Choanae; 12. Processus retroarticularis; 13. Fossa mandibularis; 14. Tuberculum articulare; 15. Foramen palatinum; 16. Sulcus palatinus; 17. Sutura palatina mediana; 18. Fissura palatina; 19. Fissura interincisiva; 20. Dentes canini; 21. Dentes incisivi; 22. Dentes molares; 23. Spina nasalis; Tuber maxillae (Black arrow)
Strong jaw and long, conical canines
The tiger’s jaw is massive and powerful, and it can withstand the large forces generated during bites (Long, 1965; Slater and Van Valkenburgh, 2009; Tiwari, 2011). The body of the mandible (Corpus mandibulae) is short, measuring approximately 7 cm in length. Together with the horizontal branch of the mandible, it forms an angle of 105–110°. The lower edge of the mandible, which is slightly concave or, very rarely, straight in the middle, is characteristic of the type of food and feeding habits (Mazák, 1981, 2010; Thorley, 2017).
In the front of the mandible, there are dental alveoli (Alveoli dentales) for the six incisors. The first and last dental alveoli are more developed. This portion appears triangular and is thicker than the rest of the mandible. On the anterior plane of the mandible, the very developed canines (Dentes canini), also known as fangs, are found. They are slightly curved, about 6 cm long, and 3 cm in diameter. Rostrally, three mental foramina (Foramen mentale) are visible.
The molar section is elongated, exhibiting two surfaces and two margins. The lateral (buccal) surface (Facies buccalis) is smooth, tapering towards the rostral end and expanding aborally. This section contains only three molar alveoli (Figure 4A).
The mandibles articulate to each other through a fibrocartilaginous joint, which does not ossify in adults, permitting them slight mobility (Tiwari et al., 2011). The articular extremity consists of a developed angular process (Processus angularis), a triangular-shaped condylar process, and a highly developed coronoid process (Processus coronoideus) that curves caudally without surpassing the plane of the articular condyle (Figure 4A). The coronoid process, when articulated, projects into the temporal fossa (Fossa temporalis) and serves as the insertion point for the temporalis muscle (Cooper et al., 2022; Long, 1965; Tiwari et al., 2011). The articular condyle (Processus condylaris) is located below the level of the dental table, arranged transversely, and measuring approximately 7 cm in length. It is well-adapted to allow precise and powerful jaw-closing movements (Murphy et al., 2013; Nanci, 2003). The angular process of the mandible appears rounded and is oriented caudally. On the lateral surface of the mandibular branch, there is a deep masseteric fossa (Fossa masseterica), bordered by bony ridges. This includes a dorsal masseteric ridge (Crista masseterica dorsalis), which ascends towards the dorsal edge of the coronoid process; an intermediate sharp and prominent masseteric ridge (Crista masseterica intermedia), which extends towards the articular condyle; and a ventral masseteric ridge (Crista masseterica ventralis), which stretches toward the mandibular angle (Figure 4B).
The temporomandibular joint (Articulatio temporomandibularis) is adapted to allow for extensive movements of lowering and raising the mandibles. The articular surfaces of the temporomandibular joint are almost congruent. The condylar process of the ramus mandibulae fits into a groove (Fossa mandibularis) on the underside of the zygomatic process of the temporal bone (Figure 1). This groove is bordered caudally by a prominent retroarticular process (Processus retroarticularis), which securely fixes the articular condyle and prevents its displacement in the caudal direction.

Figure 4 – Mandible in Tiger
A. Dorsal view. 1. Articulatio intermandibularis; 2. Dentes incisivi; 3. Dentes canini; 4. Margo interalveolaris; 5. Dentes molares; 6. Facies lingualis pars molaris; 7. Ramus mandibulae; 8. Processus coronoideus; 9. Crista masseterica intermedia; 10. Processus angularis; 11. Caput mandibulae. B. Lateral view. 1. Processus angularis; 2. Processus condylaris; 3. Processus coronoideus; 4. Fossa masseterica; 5. Corpus mandibulae; 6. Foramen mentale; 7. Dentes canini; 8. Margo alveolaris; 9. Dentes molares; 10. Incisura mandibularis; Insertio of the masseter muscle; 11. Crista masseterica intermedia; 12. Crista masseterica ventralis; 13. Crista masseterica dorsalis
Due to the congruency of the joint, the articular disc (Discus articularis) is thin. The articular capsule is reinforced by a lateral ligament (Murphy et al., 2013; Nanci, 2003).
All these particularities highlight the ability to exert strong bite forces. The robust mandible, well-formed lower molars, and a prominent angular process are indications of well-developed force arms for the temporal and masseter muscles, which act to raise the mandible (Christiansen and Adolfssen, 2005; Figueirido et al., 2010; Van Valkenburgh, 2007).
Christiansen (2007) hypothesised that the neck grip, so often applied in prey-killing by tigers, was a special functional adaptation to handle large ungulates. Felines also have stronger, more flexible forelimbs, which are critically useful while the prey is held down by the neck, and they can recruit the ventral cervical muscles to assist in closing the jaws (Anton et al., 2004; Van Valkenburgh, 2007).
In carnivores, bite force plays a crucial role due to the challenges of a carnivorous diet, which requires capturing and killing prey. There is a close relationship between the morphology and diet of canids (Christiansen and Wroe, 2007; Mazák, 2010; Sicuro and Oliveira, 2011; Thorley, 2017; Van Valkenburg and Koepfli, 1993;). Hypercarnivorous species have relatively deep jaws to withstand the forces involved in killing and consuming large prey, larger canines and incisors, and molar adaptations for shearing at the expense of grinding. In the evolution of the skull in carnivores, significant changes have been observed, such as the development of temporal ridges and the sagittal crest, which are associated with the masticatory apparatus. According to García-Perea (1994), although these structures present various patterns of evolutionary changes, they have similar functional effects. The increase in size and extension of the origin areas of the temporal muscles with age allows for muscle mass development by increasing the number of muscle fibres. This likely leads to a stronger bite force.
CONCLUSIONS
The skull of the tiger presents the morpho-functional characteristics specific to felines adapted to habitat conditions. The very high external sagittal crest and the detached zygomatic arches outline a wide temporal fossa occupied by temporalis muscles, which play an important role in elevating the mandible. The mandibular articular condyle descends below the level of the dental table, which increases the force arm. The coronoid process of the mandible is extremely wide and high, serving as the insertion point for a well-developed temporalis muscle.
The distanced alveolar processes of the canines represent a typical feature of predatory carnivores. This spacing allows for efficient gripping and piercing of prey, being a specific adaptation for killing. The presence of a bony eminence in the posterior part of the maxilla suggests the insertion of a strong tendon that acts with considerable force to execute an efficient bite. In conclusion, there is a close relationship between the morphological characteristics of the bony head and the killing behaviour in felines.
Author contributions: Conceptualization of the manuscript and development of the methodology: AAC and MCS; Data collection and curation: AAC and CS; Data analysis and interpretation: AAC, MCS and CS; Writing of the orginal manuscript: AAC; Writing, review and editing: MCS and CS. All authors declare that they have read and approved the publication of the manuscript in this present form.
Funding: There was no external funding for this study.
Conflicts of interest: The authors declare no conflict of interest.
REFERENCES
Anton, M.; Salesa, M.J.; Pastor, J.F.; Sanchez, I.M.; Fraile, S.; Morales, J. Implications of the mastoid anatomy of larger extant felids for the evolution and predatory behaviour of sabretoothed cats (Mammalia, Carnivora, Felidae). Zoological Journal of the Linnean Society 2004, 140 (2), 207-221. https://doi.org/10.1111/j.1096-3642.2003.00093.x
Agnarsson, I.; Kuntner, M.; May-Collado, L.J. Dogs, cats, and kin: A molecular species-level phylogeny of Carnivora. Molecular Phylogenetics and Evolution 2010, 54 (3), 726-745. https://doi.org/10.1016/j.ympev.2009.10.033
Christiansen, P.; Adolfssen, J.S. Bite forces, canine strength and skull allometry in carnivores (Mammalia, Carnivora). Journal of Zoology 2005, 266 (2), 133-151. https://doi.org/10.1017/S0952836905006643
Christiansen, P.; Wroe, S. Bite Forces and Evolutionary Adaptations to Feeding Ecology in Carnivores. Ecology 2007, 88 (2), 347-358. https://doi.org/10.1890/0012-9658(2007)88[347:BFAEAT]2.0.CO;2
Christiansen, P. Canine morphology in the larger Felidae: implications for feeding ecology. Biological Journal of the Linnean Society 2007, 91 (4), 573-592. https://doi.org/10.1111/j.1095-8312.2007.00819.x
Christiansen, P. Evolution of Skull and Mandible Shape in Cats (Carnivora: Felidae). PLoS One 2008, 3 (7), e2807. https://doi.org/10.1371/journal.pone.0002807
Cooper, D.M.; Yamaguchi, N.; Macdonald, D.W.; Nanova, O.G.; Yudin, V.G.; Dugmore, A.J.; Kitchener, A.C. Phenotypic plasticity determines differences between the skulls of tigers from Mainland Asia. Royal Society Open Science 2022, 9 (11), 220697. https://doi.org/10.1098/rsos.220697
Díaz Martínez, E.; Arencibia Espinosa, A.; Soler Laguía, M.; Ayala Florenciano, M.D.; Kilroy, D.; García García, M.I.; Ramírez Zarzosa, G. The Bony Nasal Cavity and Paranasal Sinuses of Big Felids and Domestic Cat: A Study Using Anatomical Techniques, Computed Tomographic Images, and 3D Printing Models. Animals 2024a, 14 (17), 2609. https://doi.org/10.3390/ani14172609
Díaz Martínez, E.; Arencibia Espinosa, A.; Soler Laguía, M.; Kilroy, D.; Martínez Gomariz, F.; Casas García, D.L.; Ramírez Zarzosa, G. An Anatomical Study Using Computed Tomography, Magnetic Resonance Imaging, and Rhinoscopy of the Nasal Cavity of Domestic Cat (Felis silvestris catus L.) and Big Cats: Lion (Panthera leo leo L.), Leopard (Panthera pardus kotiya L.), and Cheetah (Acinonyx jubatus jubatus S.). Animals 2024b, 14 (8), 1172. https://doi.org/10.3390/ani14081172
Ellis, J.L.; Thomason, J.J.; Kebreab, E.; France, J. Calibration of estimated biting forces in domestic canids: comparison of post‐mortem and in vivo measurements. Journal of Anatomy 2008, 212 (6), 769-780. https://doi.org/10.1111/j.1469-7580.2008.00911.x
Ellis, J.L.; Thomason, J.; Kebreab, E.; Zubair, K.; France, J. Cranial dimensions and forces of biting in the domestic dog. Journal of Anatomy 2009, 214 (3), 362-373. https://doi.org/10.1111/j.1469-7580.2008.01042.x
Figueirido, B.; Serrano‐Alarcón, F.J.; Slater, G.J.; Palmqvist, P. Shape at the cross‐roads: homoplasy and history in the evolution of the carnivoran skull towards herbivory. Journal of Evolutionary Biology 2010, 23 (12), 2579-2594. https://doi.org/10.1111/j.1420-9101.2010.02117.x
Finarelli, J.A.; Flynn, J.J. Ancestral State Reconstruction of Body Size in the Caniformia (Carnivora, Mammalia): The Effects of Incorporating Data from the Fossil Record. Systematic Biology 2006, 55 (2), 301-313. https://doi.org/10.1080/10635150500541698
García-Perea, R. The Pampas cat group (Genus Lynchailurus Severtzov, 1858) (Carnivora, Felidae): a systematic and biogeographic review, American Museum novitates. American Museum of Natural History, New York, U.S.A., 1994, 3096, 1-36.
Gheție, V.; Hillebrand, A. Anatomy of Domestic Animals: Vol. 1: The Locomotor System (in Romanian); Publishing House of the Academy of the Socialist Republic of Romania. Bucharest, Romania, 1971.
Gündemir, O.; Koungoulos, L.; Szara, T.; Duro, S.; Spataru, M.C.; Michaud, M.; Onar, V. Cranial morphology of Balkan and West Asian livestock guardian dogs. Journal of Anatomy 2023, 243, 951-959. https://doi.org/10.1111/joa.13929
Gürbüz, I.; Demiraslan, Y.; Rajapakse, C.; Weerakoon, D.K.; Fernando, S.; Spataru, M.C.; Gündemir, O. Skull of the Asian (Paradoxurus Hermaphroditus) and the Golden (Paradoxurus Zeylonensis) Palm Civet: Geometric Morphometric Analysis Using Palate, Tooth, and Frontal Landmarks. Anatomia, Histologia, Embryologia 2022, 51 (6), 718-727. https://doi.org/10.1111/ahe.12847
Holliday, J.A.; Steppan, S.J. evolution of hypercarnivory: The effect of specialization on morphological and taxonomic diversity. Paleobiology 2004, 30 (1), 108-128. http://dx.doi.org/10.1666/0094-8373(2004)030%3C0108:EOHTEO%3E2.0.CO;2
Larivière, S.; Kruuk, H. Hunter and Hunted: Relationships Between Carnivores and People. Journal of Mammalogy 2004, 85 (1), 167-168. https://doi.org/10.1644/1545-1542(2004)085%3C0168:BR%3E2.0.CO;2
Long, C.A. Functional Aspects of the Jaw-Articulation in the North American Badger, with Comments on Adaptiveness of Tooth-Wear. Transactions of the Kansas Academy of Science 1965, 68 (1), 156-162. https://doi.org/10.2307/3626358
Mazák, V. Panthera tigris. Mammalian Species 1981, 152, 1-8. https://doi.org/10.2307/3504004
Mazák, Ji.H. Craniometric Variation in the tiger (Panthera tigris): Implications for patterns of diversity, taxonomy and conservation. Mammalian Biology 2010, 75, 45-68. https://doi.org/10.1016/j.mambio.2008.06.003
Meiri, S.; Dayan, T.; Simberloff, D. Variability and correlations in carnivore crania and dentition. Functional Ecology 2005, 19 (2), 337-343. https://doi.org/10.1111/j.1365-2435.2005.00964.x
Murphy, M.K.; Arzi, B.; Vapniarsky-Arzi, N.; Athanasiou, K.A. Characterization of degenerative changes in the temporomandibular joint of the bengal tiger (Panthera tigris tigris) and siberian tiger (Panthera tigris altaica). Journal of Comparative Pathology 2013, 149 (4), 495-502. https://doi.org/10.1016/j.jcpa.2013.05.003
Nanci, A. Temporomandibular Joint. In Ten Cate’s Oral Histology, Development, Structure, and Function, 7th Mosby, St. Louis, 2003, pp 358-378.
NAV. Nomina Anatomica Veterinaria. The International Committee on Veterinary Gross Anatomical Nomenclature; Editorial Committee: Hannover, Germany, Columbia, MO, U.S.A., Ghent, Belgium, Sapporo, Japan, 6th ed. (Revised version), 2017. http://www.wava-amav.org.
Palmqvist, P.; Torregrosa, V.; Pérez-Claros, J.A.; Martínez Navarro, B.; Turner, A. A re-evaluation of the diversity of Megantereon (Mammalia, Carnivora, Machairodontinae) and the problem of species identification in extinct carnivores. Journal of Vertebrate Paleontology 2007, 27 (1), 160-175. http://dx.doi.org/10.1671/0272-4634(2007)27[160:AROTDO]2.0.CO;2
Różycka, K.; Skibniewska, E.; Rajkowski, Ł.; Skibniewski, M. Craniometric Characteristics of Selected Carnivora Species Kept in Captivity in Relation to Bite Force and Bending Strength of the Upper Canines. Animals 2024, 14 (9), 1367. https://doi.org/10.3390/ani14091367
Sasaki, M.; Endo, H.; Yamamoto, M.; Arishima, K.; Hayashi, Y. The superficial layer of the Musculus masseter and the well-developed process of the maxilla in the tiger Panthera tigris. Mammal Study 2000, 25 (1), 27-34. https://doi.org/10.3106/mammalstudy.25.27
Slater, G.J.; Van Valkenburgh, B. Allometry and performance: the evolution of skull form and function in felids. Journal of Evolutionary Biology 2009, 22 (11), 2278-2287. https://doi.org/10.1111/j.1420-9101.2009.01845.x
Sicuro, F.L.; Oliveira, L.F.B. Skull morphology and functionality of extant Felidae (Mammalia: Carnivora): a phylogenetic and evolutionary perspective. Zoological Journal of the Linnean Society 2011, 161 (2), 414-462. https://doi.org/10.1111/j.1096-3642.2010.00636.x
Spataru, C.; Spataru, M. Practical Manual of Veterinary Anatomy – The Locomotor System (In Romanian); Publishing House Tehnopress, Iaşi, Romania, 2003.
Tiwari, Y.; Taluja, J.S.; Vaish, R. Biometry of Mandible in Tiger (Panthera Tigris). Annual Research & Review in Biology 2011, 1 (1), 14-21.
Thorley, D. Naming the Tiger in the Early Modern World. Renaissance Quarterly 2017, 70 (3), 977-1006. https://doi.org/10.1086/693884
Torregrosa, V.; Petrucci, M.; Pérez-Claros, J.A.; Palmqvist, P. Nasal Aperture Area and Body Mass in Felids: Ecophysiological Implications and Paleobiological Inferences. Geobios 2010, 43 (6), 653-661. https://doi.org/10.1016/j.geobios.2010.05.001
Van Valkenburgh, B.; Ruff, C.B. Canine tooth strength and killing behaviour in large carnivores. Journal of Zoology 1987, 212 (3), 379-397. https://doi.org/10.1111/j.1469-7998.1987.tb02910.x
Van Valkenburgh, B.; Koepfli, K.P. Cranial and dental adaptations to predation in canids. Symposia of the Zoological Society of London 1993, 65, 15-37.
Van Valkenburgh, B. Déjà Vu: the evolution of feeding morphologies in the Carnivora. Integrative and Comparative Biology 2007, 47 (1), 147-163. https://doi.org/10.1093/icb/icm016
Wroe, S.; Milne, N. Convergence and Remarkably Consistent Constraint in the Evolution of Carnivore Skull Shape. Evolution 2007, 61 (5), 1251-1260. https://doi.org/10.1111/j.1558-5646.2007.00101.x.
Academic Editor: Prof. Dr. Gheorghe SOLCAN
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Cherșunaru Alexandra-Andreea, Spataru Constantin, Spataru Mihaela-Claudia



