Gabriela Ciobanu, Lidia Favier, Maria Harja
ABSTRACT. This work focused on the use of cellulose acetate polymer for the preparation of porous asymmetric membranes using a phase inversion process. These membranes were characterised by scanning electron microscopy, a bubble-point method and sorption measurements. The preparation method used induced membrane anisotropy. The capacity of the membranes in the removal of electrolyte (NaCl) from aqueous solution was investigated. A good retention of 58.6% and a high flux rate of 8.9 × 10–4 m/s using NaCl solution of 200 mg/L concentration were obtained by cellulose acetate membranes prepared with water as non-solvent. The results showed that the membrane performance was affected by the membrane structure, which was determined by the conditions of membrane preparation.
Keywords: cellulose acetate polymer; porous membrane; asymmetric structure; salt rejection.
Cite
ALSE and ACS Style
Ciobanu, G.; Favier, L.; Harja, M. Asymmetric cellulose acetate membranes used in separation applications. Journal of Applied Life Sciences and Environment 2021, 54(1), 70-76.
https://doi.org/10.46909/journalalse-2021-007
AMA Style
Ciobanu G, Favier L, Harja M. Asymmetric cellulose acetate membranes used in separation applications. Journal of Applied Life Sciences and Environment. 2021; 54(1): 70-76.
https://doi.org/10.46909/journalalse-2021-007
Chicago/Turabian Style
Ciobanu, Gabriela, Lidia Favier, and Maria Harja. 2021. “Asymmetric cellulose acetate membranes used in separation applications” Journal of Applied Life Sciences and Environment 54, no. 1: 70-76.
https://doi.org/10.46909/journalalse-2021-007
View full article (HTML)
Asymmetric Cellulose Acetate Membranes Used in Separation Applications
Gabriela Ciobanu1, Lidia Favier2, Maria Harja1
1Faculty of Chemical Engineering and Environmental Protection “Cristofor Simionescu”, „Gheorghe Asachi” Technical University of Iaşi, 73 D. Mangeron Bvd., Iaşi, 700050, Romania
2University of Rennes, Ecole Nationale Supérieure de Chimie de Rennes, CNRS, ISCR – UMR6226, F-35000 Rennes, France
*E-mail: gciobanu@tuiasi.ro
Received: Mar. 12, 2021. Revised: Mar. 22, 2021 Accepted: Mar. 26, 2021. Published online: May 14, 2021
ABSTRACT. This work focused on the use of cellulose acetate polymer for the preparation of porous asymmetric membranes using a phase inversion process. These membranes were characterised by scanning electron microscopy, a bubble-point method and sorption measurements. The preparation method used induced membrane anisotropy. The capacity of the membranes in the removal of electrolyte (NaCl) from aqueous solution was investigated. A good retention of 58.6% and a high flux rate of 8.9 × 10–4 m/s using NaCl solution of 200 mg/L concentration were obtained by cellulose acetate membranes prepared with water as non-solvent. The results showed that the membrane performance was affected by the membrane structure, which was determined by the conditions of membrane preparation.
Keywords: cellulose acetate polymer; porous membrane; asymmetric structure; salt rejection.
INTRODUCTION
Membrane processes are a good alternative to conventional water (Ladewig and Al-Shaeli, 2017; Peinemann and Nunes, 2010). Nanofiltration, reverse osmosis, microfiltration, ultrafiltration, etc. are membrane processes currently used to obtain drinking water and in the depollution of wastewater in order to protect the environment. One of the very important applications of membrane filtration is water desalination, i.e., the elimination of salts from seawater, but also the removal of toxic compounds existing in wastewater arising from various industries (Ciobanu et al., 2009; Ciobanu and Carja, 2010; Goh et al., 2016; Elimelech and Phillip, 2011).
Filter membranes, depending on their structure, can be symmetrical or asymmetrical, porous or non-porous. Also, depending on the materials from which they are made, the membranes can be polymeric, ceramic or polymer-polymer or polymer-ceramic composites.
A crucial moment in membrane technology was 1962, when Loeb and Sourirajan developed the phase inversion method for obtaining integrally skinned asymmetric membranes (Loeb and Sourirajan, 1962). This method involves the addition of a multicomponent solution (consisting of either a binary mixture of polymer and solvent or a combination of polymers, solvents, and non-solvents) in a coagulation bath (containing a non-solvent immiscible with the polymer). The counter diffusion between solvent and non-solvent leads to a phase instability in the solution, which results in the separation of the wet phase to form a membrane with an asymmetric structure (Ismail and Yean, 2003). The obtained membranes are formed by superimposed layers of different thicknesses and porosities: a top skin layer, a porous sub-layer (substructure) and a bottom skin (Ciobanu and Ciobanu, 2015; Kesting, 1985; Purkait et al., 2018).
In recent years, many studies have focused on the phase inversion process because it is one of the most versatile, economical and reproducible processes for forming polymeric asymmetric membranes (Baldino et al., 2017). In recent years, many categories of polymers with specific properties have been used to obtain asymmetric membranes, which have led to various applications in the separation and purification processes. A very important aspect in the research carried out by the specialists in the membrane science is to obtain membranes with a higher selectivity and high flow (Gao et al., 2011; Huang et al., 2019; Khan et al., 2020). Cellulose acetate is a modified natural polymer with a wide range of properties and applications (e.g., for films, membranes or fibres) (Fischer et al., 2008). Cellulose acetate is used in the preparation of ultra- and nanofiltration membranes for gas or liquid separation applications due to its good hydrophilicity, high toughness, and low price (Idress et al., 2021). The hydrophilic nature of cellulose acetate is desirable in a membrane process: wetting the membrane reduces the internal concentration polarisation (and this reduces membrane fouling) and increases water flux.
This study investigated whether cellulose acetate sheets could be used as semipermeable membranes in water desalination. The membranes were produced by a very easy method based on the phase inversion process. Membrane characterisations, such as morphology and sodium chloride rejection are reported.
EXPERIMENTAL
Materials
Cellulose acetate with 39.8% acetyl content and average molecular weight Mn of ~30,000, acetone, formamide and NaCl were purchased from Sigma-Aldrich (Germany). In the coagulation process, acetone acted as a solvent, while formamide or water acted as non-solvents. In order to evaluate the membrane performance in terms of percentage rejection and flux rate, NaCl solutions of different concentrations were prepared.
Membrane preparation
The membranes were prepared via the phase inversion method described elsewhere (Ciobanu and Bezdadea, 2004), starting from a 20 wt % cellulose acetate solution in acetone. Cellulose acetate and acetone were stirred in an Erlenmeyer flask until a homogeneous solution was formed (20-24 h). A fixed amount of non-solvent (deionised water or formamide) was added slowly to the polymer solution and stirred for another 2-3 h. The solution was then cast at ambient temperature on a glass dish with a special stainless-steel cast knife. The thickness of the fabricated membrane was ~300-350 µm. As soon as possible, the thin layer of cast solution together with the glass plate was immersed in the coagulation bath of pure water. The membranes formed were left in the coagulation bath for 30-60 min and then transferred to deionised water until characterisation.
Membrane characterisation
The sample morphology was investigated by scanning electron microscopy (SEM) with a TESLA-BS-300 instrument. The pore sizes were estimated by the bubble-point method with a home-made laboratory instrument.
The permeation experiments were carried out in a dead-end flow cell using circular membranes with a 12.5 cm2 effective area available for filtration. The flux rate and solute rejection were measured using NaCl solutions of different concentrations, at 25°C and at ~5 bar applied feed pressure. The conductivity of the permeate was measured using a conductivity meter, model CONSORT C831. Parameters used to quantify the membrane performance were flux rate of the solutions J (m/s), and salt rejection R(%):
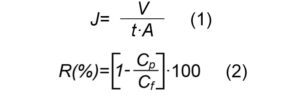
where, V/t is volume permeation rate (m3/s), A is membrane area (m2), and Cp and Cf are the concentrations of the permeate and feed solutions, respectively.
RESULTS AND DISCUSSION
Generally, the morphology of a membrane (i.e., thickness or porosity) is influenced by the process parameters, which ultimately lead to certain separation performances of the membrane (Idris et al., 2002; Wang et al., 2006). Phase inversion is a good method for obtaining integrally skinned asymmetric membranes (Loeb and Sourrirajan, 1962; Strathmann et al., 1975; Garcia et al., 2020).
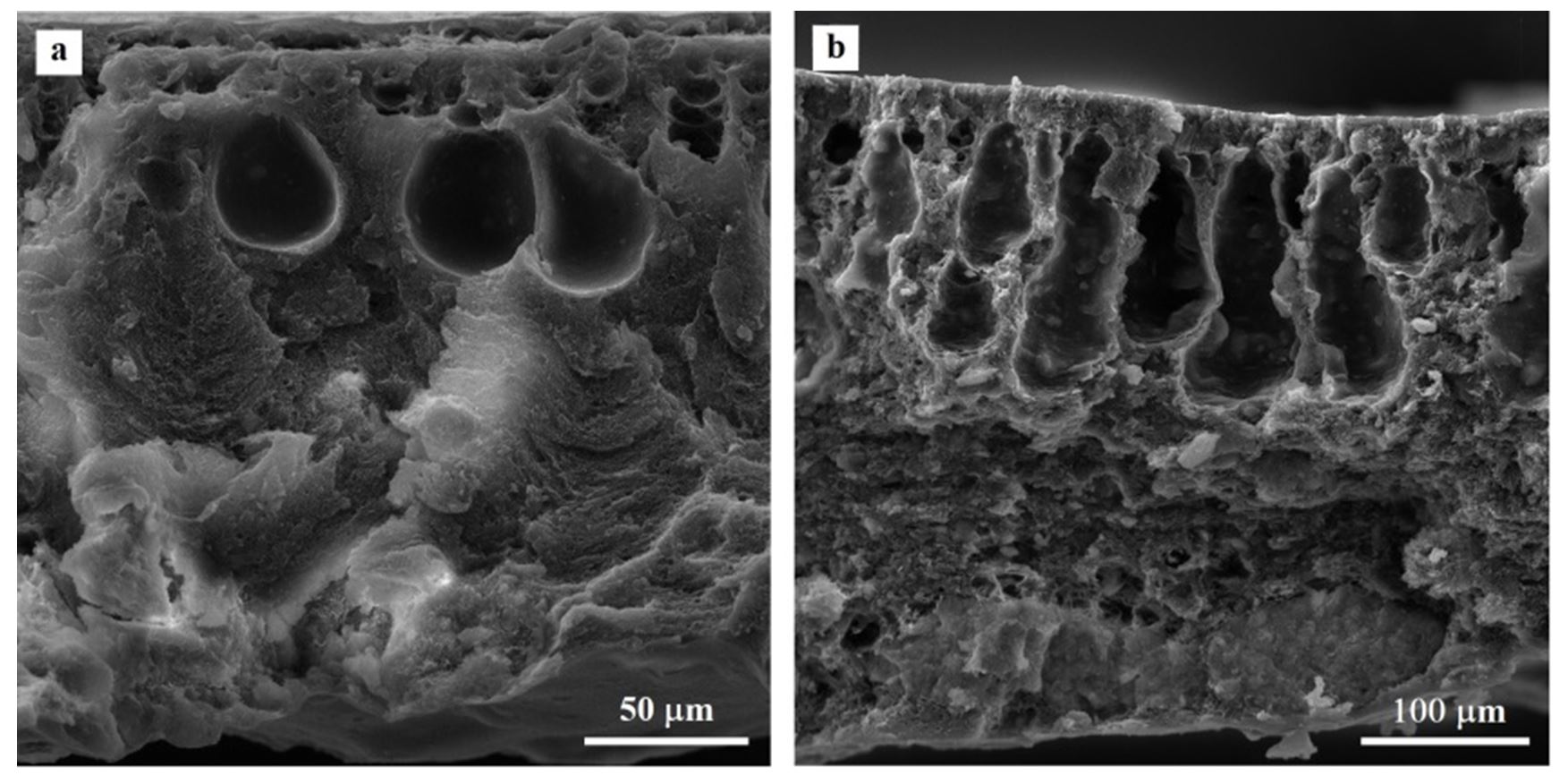
In our study, the obtained microporous membranes (i.e., CAM-F and CAM-W) have an open three-dimensional structure in which the pores are of molecular size consisted of a three-dimensional polymeric structure with pore sizes of molecular dimensions, which offer specific permeability to a component in a process feed to the membrane. These membranes have a quite complex, open, colloidal-type structure with a small fraction of the membrane volume occupied by the polymer substrate. The cellulose acetate membranes appeared to be a cohesive system consisting of open-cell foams (i.e., vacuoles with branched walls) composed of a cellular network bound together by chainlike ribs extending in three dimensions. The SEM images of the cellulose acetate membranes were evidence of the asymmetry/ heterogeneity of these systems (Fig. 1).
All the membranes have an asymmetric structure consisting of several layered areas, namely: the active layer (an ultra-thin skin layer), the transition layer (an open-cell and finger-like substructure) and the bottom layer. As reported by Kestingm (Kesting, 1985), generally large finger-like macrovoids and cavity-like structures are formed when the coagulation process is rapid, whereas a slow coagulation rate results in a porous sponge-like structure. The coagulation took place rapidly (in ~15 min) when the polymer solution was brought into contact with cold water; this explained the finger-like structure of our membranes. In the active layer, the estimated pore size is much smaller than that of the supported layer and, therefore, due to these very small pores results the molecular sieve property of cellulose acetate membranes.
Accordingly, the molecular sieve property of cellulose acetate membranes appears to be determined by the pore size in the top layer of the membranes.
Some physical characteristics of the prepared cellulose acetate membranes are presented in Table 1.
Table 1
Physical characteristics of the prepared cellulose acetate membranes
| Characteristic | CAM-F* | CAM-W* |
| Thickness (μm) | 240 | 375 |
| Pore diameter in top surface (μm) | 0.2-2.5 | 0.5-8.5 |
| Void diameter in substructure (μm) | 10-40 | 15-70 |
*The non-solvent was formamide (F) or water (W).
In the first stage of the study, the pure water flux of the membranes was determined. At 25°C and 5-bar applied pressure, the pure water fluxes values for CAM-F and CAM-W samples were about 4.5 × 10–4 m/s and 5.3 × 10–4 m/s, respectively, showing that the pure water flux was increased for cellulose acetate membranes prepared with water as non-solvent (CAM-W sample).
In the second step, the capacity of the membranes for electrolyte (NaCl) removal from aqueous solution was investigated. The results, in terms of flux rate (J) and percentage rejection (R%) are presented in Table 2, showing that the flux and percentage rejection were increased for cellulose acetate membranes obtained by using water as non-solvent (CAM-W sample).
Table 2
Performance of the prepared cellulose acetate membranes
| Characteristic | Membrane | |||
| CAM-F | CAM-W | |||
| Concentration of NaCl solution (mg/L) | ||||
| 10 | 200 | 10 | 200 | |
| J (m/s) × 10–4 | 7.2 | 7.4 | 8.3 | 8.9 |
| R% (%) | 49.3 | 51.2 | 55.1 | 58.6 |
According to the obtained results it can be observed that the performances of the analysed membranes are influenced by the membrane structure that depends on the conditions of membrane preparation, the composition of the casting solution being very important. During the phase inversion processes, due to some phenomena of microphase separation in the of evaporation and/or quenching of the polymeric solution, the formation of the asymmetric cellulose acetate membrane takes place.
Considering the experimental results, it can be concluded that these asymmetric cellulose acetate membranes can be used in various separation processes, especially for the removal of electrolytes from wastewater.
CONCLUSION
Asymmetric porous cellulose acetate membranes were prepared via a phase inversion method starting from a 20 wt % cellulose acetate solution in acetone to which a fixed amount of non-solvent (deionised water or formamide) was added. The cellulose acetate membranes consist of several layered areas, namely: the active layer (an ultra-thin skin layer), the transition layer (an open-cell and finger-like substructure) and the bottom layer The results showed that the membrane performance was affected by the membrane structure, which was determined by the conditions of membrane preparation, i.e., the nature of the non-solvent in the casting solution. In this study, cellulose acetate membranes prepared from a ternary system consisting of cellulose acetate, acetone and water using a phase inversion process were appropriate for a water desalination process. They showed a good pure water flux and a moderate percentage of rejection using a NaCl solution of 200 mg/L concentration.
REFERENCES
Baldino, L., Cardea, S. & Reverchon, E. (2017). Biodegradable membranes loaded with curcumin to be used as engineered independent devices in active packaging. J. Taiwan Inst.Chem.Eng., 71: 518-526, DOI: 10.1016/j.jtice.2016.12.020
Ciobanu, M.G. & Bezdadea, M. (2004). SAPO-5 zeolite-filled polyurethane membranes. 1. Preparation and morphological characterisation. Rev. Chim., 55(3): 140-143, https://revista dechimie.ro/Articles.asp?ID=301
Ciobanu, G., Ignat, D., Carja, G. & Luca C. (2009). Hydroxyapatite/polyurethane composite membranes for lead ions removal. Environ.Eng.Manag.J., 8(6): 1347-1350, https://eemj.eu/ index.php/EEMJ/article/view/2679
Ciobanu, G. & Carja, G. (2010). Electrolyte removal by mixed matrix membranes based on polyurethane. Desalination, 250 (1-3): 698-701, DOI: 10.1016/j.desal.2009.05.023
Ciobanu, G. & O Ciobanu, O. (2015). Mixed‐matrix membranes based on polyurethane containing nanohydroxyapatite and its potential applications. J.Appl.Polym.Sci., 132(17): 41813, DOI: 10.1002/app. 41813
Elimelech, M. & Phillip, W.A. (2011). The future of seawater desalination: energy, technology, and the environment. Science, 333: 712-717, DOI: 10.1126/science.1200488
Fischer, S., Thümmler, K., Volkert, B. & Hettrich, K. (2008). Properties and applications of cellulose acetate. Macromol.Symp., 262(1): 89-96, DOI: 10.1002/masy.200850210
Gao, W., Liang, H., Ma, J., Han, M., Chen, Z.I., Han, Z.S. & Li, G.B. (2011), Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination, 272: 1-8, DOI: 10.1016/j.desal.2011.01.051
Garcia, J.U., Iwama, T., Chan, E.Y., Tree, D.R., Delaney, K.T. & Fredrickson, G.H. (2020). Mechanisms of asymmetric membrane formation in nonsolvent-induced phase separation. ACS Macro. Lett., 9(11): 1617-1624, DOI: 10.1021/acsmacrolett.0c00609
Goh, P., Matsuura, T., Ismail, A. & Hilal, N. (2016). Recent trends in membranes and membrane processes for desalination. Desalination, 391: 43-60, DOI : 10.1016/j.desal.2015.12.016
Huang, T.F., Puspasari, T., Nunes, S.P. & Peinemann, K.V. (2019). Ultrathin 2D-layered cyclodextrin membranes for high-performance organic solvent nanofiltration. Adv.Funct.Mater., 30(4): 1906797, DOI: 10.1002/adfm.201906797
Idress, H., Zaidi, S.Z.J., Sabir, A., Shafiq, M., Khan, R.U., Harito, C., Hassan, S. & Walsh, F.C. (2021). Cellulose acetate based complexation-NF membranes for the removal of Pb(II) from waste water. Sci.Rep., 11: 1806, DOI: 10.1038/s41598-020-80384-0
Idris, A., Ismail, A.F., Noorhayati, M. & Shilton, S.J. (2002). Measurement of rheologically induced molecular orientation using attenuated total reflection infrared dichroism in reverse osmosis hollow fiber cellulose acetate membrane and influence on separation performance. J.Membr.Sci., 213: 45-54, DOI: 10.1016/S0376-7388(02) 00511-2
Ismail, A.F. & Yean, L.P. (2003). Review on the development of defect-free and ultrathin-skinned asymmetric membranes for gas separation through manipulation of phase inversion and rheological factors, J.Appl.Polym.Sci., 88: 442-451, 10.1002/app.11744
Kesting, R.E. (1985). Synthetic polymeric membranes: a structural perspective. 2nd Edition, Wiley‐Interscience, New York.
Khan, U., Biccai, S., Boland, C.S. & Coleman, J.N. (2020). Low cost, high performance ultrafiltration membranes from glass fiber-PTFE-graphene composites. Sci.Rep., 10: 21123, DOI: 10.1038/s41598-020-78091-x
Ladewig, B. & Al-Shaeli, M.N.Z. (2017). Fundamentals of membrane processes. In: Fundamentals of membrane bioreactors. Springer Transactions in Civil and Environmental Engineering, Springer, Singapore, DOI: 10.1007/978-981-10-2014-8_2
Loeb, S. & Sourirajan, S. (1962). Sea water demineralization by means of an osmotic membrane, Chapter 9. In: Saline Water Conversion II. Gould, R.F. (Ed.), ACS Symp.Ser. Am.Chem.Soc., 38: 117-132, DOI: 10.1021/ba-1963-0038.ch009
Peinemann, K.V. & Nunes, S.P. (2010). Membranes for water treatment, Volume 4. In: Membrane Technology, Wiley, Weinheim, DOI: 10.1002/9783527631407
Purkait, M.K., Sinha, M.K., Mondal, P. & Singh, R. (2018). Introduction to Membranes, Chapter 1. In: Interface Science and Technology, Vol. 25, Elsevier, London, pp. 1-37, DOI: 10.1016/B978-0-12-813961-5.00001-2
Strathmann, H., Kock, K., Amar, P. & Baker, R.W. (1975). The formation mechanism of asymmetric membranes. Desalination, 16(2): 179-203, DOI: 10.1016/S0011-9164 (00)82092-5
Wang, M., Wu, L. & Gao C. (2006). The influence of phase inversion process modified by chemical reaction on membrane properties and morphology. J.Membr.Sci., 270(1-2): 154-161, DOI: 10.1016/j.memsci.20 05.06.051