Tarikul Islam, Simul Bhuyan, Mala Khan, Mrityunjoy Kunda, Sumi Akter, Nayan Kumer Kundu
ABSTRACT. Jellyfish (JF) are essential to marine ecosystems. However, JF that increases rapidly can have negative effects. On 3-4 August 2022, a significant JF (Lobonemoides robustus Stiasny, 1920) bloom was observed along Cox’s Bazar coast (from Najdirartek to Sabrang) in Bangladesh. The goal of the current investigation was to identify the fatty acids (FAs) and amino acids (AAs) of L. robustus. The AAs were determined using liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis, while the FAs were determined using a gas chromatographic system with a flame ionisation detector. The most prevalent AA was glycine. The most common FA was linoleic acid (C18:3) (0.43%), followed by myristic acid (0.12%), cis-9-oleic acid (0.18%), gamma-linolenic acid (0.24%), and heptadecanoic acid (0.29%). Based on its AA and FA contents, L. robustus can be a great candidate for the potentially sustainable manufacture of nutraceutical, cosmeceutical, and biomedical natural products to improve health and well-being. In addition, the edible L. robustus could be exported to other countries, thus way it can play a major role in achieving a blue economy.
Keywords: amino acids; bloom; blue economy; fatty acids; jellyfish.
Cite
ALSE and ACS Style
Islam, Md.T.; Bhuyan, Md.S.; Khan, M.; Kunda, M.; Akter, S.; Kundu, N.K. First report of the amino acid and fatty acid composition of jellyfish (Lobonemoides robustus Stiasny, 1920) collected during jellyfish bloom along the Cox’s Bazar coast, Bangladesh. Journal of Applied Life Sciences and Environment 2024, 57 (1), 107-122.
https://doi.org/10.46909/alse-571126
AMA Style
Islam MdT, Bhuyan MdS, Khan M, Kunda M, Akter S, Kundu NK. First report of the amino acid and fatty acid composition of jellyfish (Lobonemoides robustus Stiasny, 1920) collected during jellyfish bloom along the Cox’s Bazar coast, Bangladesh. Journal of Applied Life Sciences and Environment. 2024; 57 (1): 107-122.
https://doi.org/10.46909/alse-571126
Chicago/Turabian Style
Islam, Md. Tarikul, Md. Simul Bhuyan, Mala Khan, Mrityunjoy Kunda, Sumi Akter, and Nayan Kumer Kund. 2024. “First report of the amino acid and fatty acid composition of jellyfish (Lobonemoides robustus Stiasny, 1920) collected during jellyfish bloom along the Cox’s Bazar coast, Bangladesh” Journal of Applied Life Sciences and Environment 57, no. 1: 107-122.
https://doi.org/10.46909/alse-571126
View full article (HTML)
First Report of The Amino Acid And Fatty Acid Composition of Jellyfish (Lobonemoides Robustus Stiasny, 1920) Collected During Jellyfish Bloom Along the Cox’s Bazar Coast, Bangladesh
Md. Tarikul ISLAM1, Md. Simul BHUYAN1,2*, Mala KHAN3, Mrityunjoy KUNDA2, Sumi AKTER4,5 and Nayan Kumer KUNDU3
1Bangladesh Oceanographic Research Institute, Cox’s Bazar-4730, Bangladesh; email: taruimscu@gmail.com
2Sylhet Agricultural University, Sylhet, Bangladesh; email: kunda.arm@sau.ac.bd
3Bangladesh Reference Institute for Chemical Measurements (BRiCM), Dhaka, Bangladesh;
email: dg@bricm.gov.bd; nayan@bricm.gov.bd
4Department of Marine Bioresource Science, Chattogram Veterinary and Animal Sciences University, Chattogram, Bangladesh; email: sumi@cvasu.ac.bd
5School of Ocean Science and Engineering, Division of Coastal Sciences, The University of Southern Mississippi, USA
*Correspondence: simulbhuyan@gmail.com
Received: Dec. 18, 2024. Revised: Feb. 08, 2024. Accepted: Feb. 13, 2024. Published online: Mar. 18, 2024
ABSTRACT. Jellyfish (JF) are essential to marine ecosystems. However, JF that increases rapidly can have negative effects. On 3-4 August 2022, a significant JF (Lobonemoides robustus Stiasny, 1920) bloom was observed along Cox’s Bazar coast (from Najdirartek to Sabrang) in Bangladesh. The goal of the current investigation was to identify the fatty acids (FAs) and amino acids (AAs) of L. robustus. The AAs were determined using liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis, while the FAs were determined using a gas chromatographic system with a flame ionisation detector. The most prevalent AA was glycine. The most common FA was linoleic acid (C18:3) (0.43%), followed by myristic acid (0.12%), cis-9-oleic acid (0.18%), gamma-linolenic acid (0.24%), and heptadecanoic acid (0.29%). Based on its AA and FA contents, L. robustus can be a great candidate for the potentially sustainable manufacture of nutraceutical, cosmeceutical, and biomedical natural products to improve health and well-being. In addition, the edible L. robustus could be exported to other countries, thus way it can play a major role in achieving a blue economy.
Keywords: amino acids; bloom; blue economy; fatty acids; jellyfish.
INTRODUCTION
The oceans are a nearly untapped reservoir of biochemicals that cover 70% of Earth’s surface. They are home to over 194,000 known species of microorganisms, flora, and fauna (Primavera et al., 2019), but between 2011 and 2017, only a tiny number of these marine creatures were utilised, yielding roughly 9,000 unique natural compounds (Romano et al., 2022). Among these marine organisms are JF, a generic term that refers to medusae of the phylum Cnidaria, specifically the class Scyphozoa. Many people value JF for their elegant appearance, but they are also feared for their severe stings. Compared with other taxa, cnidarians have been subjected to relatively little natural product exploitation (Das et al., 2023; Haider et al., 2022).
Globally, JF populations seem to have risen in the last few decades. The overall increase and its causes are unclear because JF abundance is not routinely monitored (Brotz et al., 2012). The natural rhythms of JF blooms may be disrupted by several human-driven activities, including overfishing, pollution, and high temperatures (Haider et al., 2022). This could result in a substantial rise in JF populations in specific coastal areas and major marine ecosystems. Only a small number of bioactive substances have been recovered from oceanic cnidarians; the majority of natural goods are derived from benthal cnidarians. However, there are many significant potential human uses for the natural compounds that pelagic cnidarians synthesise (Fonseca et al., 2023). Substantial scientific data supports the idea that JF are valuable bioresources for a variety of high-end applications such as human food; feed for cultivated species; and the discovery of untapped bioactive compounds for use in pharmaceutical, cosmetic, nutraceutical, and other biotechnological applications (Das and Patel, 2020; Duarte et al., 2022; Romano et al., 2022).
FAs are the building blocks of lipids. They are divided into saturated fatty acids (SFAs), which lack double bonds between carbons, and unsaturated fatty acids (Ulrich et al., 2011), including monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), classified based on the number and location of double bonds (Monroig et al., 2022). FAs are essential parts of cells and are involved in digestion, signaling pathways, somatic development, and breeding (Yao et al., 2020). Arachidonic acid (ARA) (20:4(ω-6)), eicosapentaenoic acid (EPA) (20:5(ω-3)), and docosahexaenoic acid (DHA) (20:6(ω-3)), also referred to as ω6 and ω3 FAs, are three very important PUFAs (Crawford et al., 2023). Despite considerable interspecific variability, PUFAs are often more prevalent than SFAs and MUFAs in the FAs composition of scyphomedusae (Duarte et al., 2022).
AAs have numerous functions, including an important contribution to the creation of hydrogen bonds and the stability of the collagen triple helix structure and thermal behaviour (Xu et al., 2019). It is normal for marine creatures to have low levels of AAs, which causes collagen to denature at lower thermal denaturation temperatures (Barzideh et al., 2014). The essential amino acids (EAAs) are histidine (His), isoleucine (Ile), leucine (Leu), lysine (Lys), methionine (Met), phenylalanine (Phe), threonine (Thr), valine (Val), and tryptophan (Trp); the conditionally EAAs comprise arginine (Arg), cystine (Cys), tyrosine (Tyr), glycine (Gly), proline (Pro), and serine (Ser); and the non-essential acids (NEAAs) include aspartic acid (Asp), glutamic acid (Glu), and alanine (Ala) (dos Santos, 2013).
During 3–4 August 2022, numerous dead JF of the species L. robustus were found along the shore at Cox’s Bazar, Bangladesh. They were carried onto the beach at high tide and they stuck in the sand deposit during low tide. According to Kitamura and Omori (2010), L. robustus are marketed as ‘white-type’ JF and are typically seen in huge quantities during certain seasons. They live along the Bay of Bengal (BoB) coast and may be harvested for export or human use. No scientists in Bangladesh have yet researched the biochemical composition of L. robustus. Hence, the purpose of the current investigation was to ascertain L. robustus’s AA and FA content. This information could increase the export of L. robustus and contribute to the blue economy of Bangladesh.
MATERIALS AND METHODS
Study area
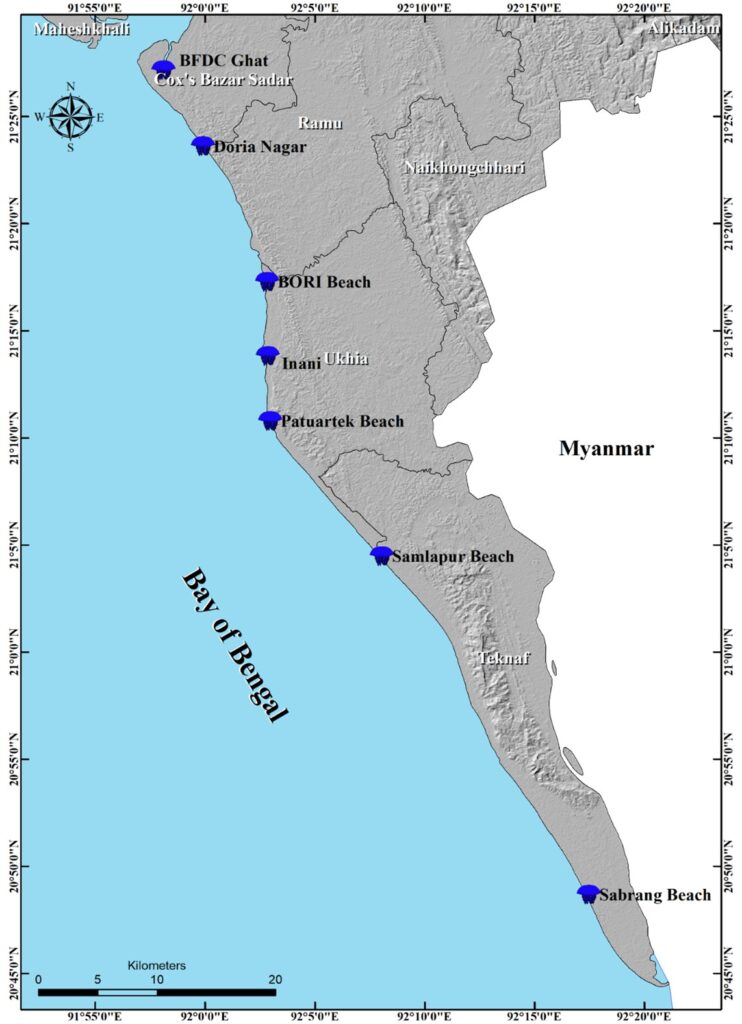
The current study was conducted in the following areas: the Sabrang coast, the Patuartek coast, the Shamlapur coast, the Bangladesh Oceanographic Research Institute (BORI) beach, Inani Beach, the Daria Nagar coast, and Bangladesh Fisheries Development Corporation (BFDC) Ghat. Each site is located along the Cox’s Bazar shore, which is part of the BoB coast (Figure 1). Samples were collected on 3–4 August 2022 during a massive L. robustus bloom.
Sample collection and preservation
Using hand gloves, a total of 14 L. robustus samples (average weight 30 kg) were collected from each sampling site during the peak JF occurrence. The samples were collected in plastic buckets (due to their large size, only one specimen per bucket) and cleaned onsite with seawater. The samples were transported to BORI’s Biological Oceanography Laboratory after being preserved in 10% formalin (Haider et al., 2022). The specimens had minimal damage and were in generally good condition. Along with live specimens, photographs and videos were captured in the field for species identification. As soon as possible after capture, specimens were photographed to capture their natural hue (Haider et al., 2022).
Determination of amino acids (AAs)
Preparation of stock solution and intermediate stock solution
A stock solution of 2500 µM of AAs was prepared in methanol and water (50:50, v/v), sonicated for 1 min, and stored at -4°C. The stock solution was diluted in methanol and water (50:50, v/v) to produce solutions containing 2.0–100 µM of AAs. These solutions were filtered with a 0.232-µm syringe filter (PTFE).
Sample preparation
A 10–100 mg sample was weighed in a 15 ml tube. Then, 2 mL of 6 N HCl was added, and the mixture was incubated at 120°C for hours. Following digestion, the solvent was removed and the sample was resuspended in methanol and water (50:50, v/v; 2 mL).
Analytical conditions
The liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis used an ultra-fast liquid chromatography system (Shimadzu Corporation, Kyoto, Japan) with binary pumps, an autosampler, an on-line degassing unit, and a column oven connected to a Shimadzu LCMS-8050 triple quadrupole system mass spectrometer, which has an electrospray ionisation (ESI) source. Twenty genetically encoded AAs were subjected to an improved gradient elution method using a novel combined mode.
LC and MS conditions
The AA analysis required an Intrada 100 × 3 mm, 3 μm column that was kept at 35°C. The mobile phase comprised solution A (acetonitrile [can], tetrahydrofuran [THF], 25 mM NH₄HCO₂, and HCO2H, 9:75:16:0.3, v/v) and solution B (can and 100 mM NH₄HCO₂, 20:80, v/v). The elution programme was 0% B (0–3.0 min), 0%–17% B (3.0–9.0 min), 17%–100% B (9.0–16.0 min), 100% B (16.0–22.0 min), and 0% B (22.0 min) at a flow rate of 0.6 mL/min. The chromatographic injection volume was 10 µL, and the AAs were retained for approximately 22 min.
Table 1 presents the MS acquisition conditions, and Table 2 presents the multiple reaction monitoring (MRM) transition events of the AAs.
Determination of fatty acids (FAs)
A Shimadzu GC 2010 Plus gas chromatographic apparatus with a flame ionisation detector was utilised to identify FAs. One hundred milligrams of C6H6O3 and 2 mL of ethanol were added to a flask containing 100–200 mg of material and thoroughly mixed. Then, 10 mL of 8.3 M HCl was added and the contents were stirred. The flask was incubated in a water bath heated to 70–80°C for 40 min, with gentle shaking every 10 min. Then, the flask was allowed to cool to ambient temperature (20–25°C). While stirring carefully, enough ethanol was added to fill the flask’s bottom reservoir.
After adding 20 mL of diethyl ether and 20 mL of petroleum ether, the flask was centrifuged at 600 rpm for 5 min (if a centrifuge is not available, then the contents should be allowed to settle for at least 1 h until the upper layer is transparent). In a steam bath, the top layer was removed and the ether was evaporated.
After dissolving the residue in 2–3 mL of CHCl3 and 2–3 mL of (C2H5)2O, the mixture was shifted to a 3-dram glass vial and dried in a water bath at 40°C. Then, 1 mL of toluene and 2 mL of 7% BF3 methanol were added. The vial was closed with a screwcap top with a teflon/silicone septum. The vial was heated in an oven to 100˚C for 45 min, with gentle shaking every 10 min. The vial was cooled to room temperature (20–25°C). After adding 1 mL hexane, 5 mL water, and 1 g Na2SO4, the vial was shaken. Then, the upper layer was transferred to a new vial containing 1 g of Na2SO4 for gas chromatography.
RESULTS AND DISCUSSION
JF represents a vital part of marine food webs. Although their function as consumers has long been recognised, they are also consumed by a diverse range of species (Schaub et al., 2023).
Amino acids (AAs) in L. robustus
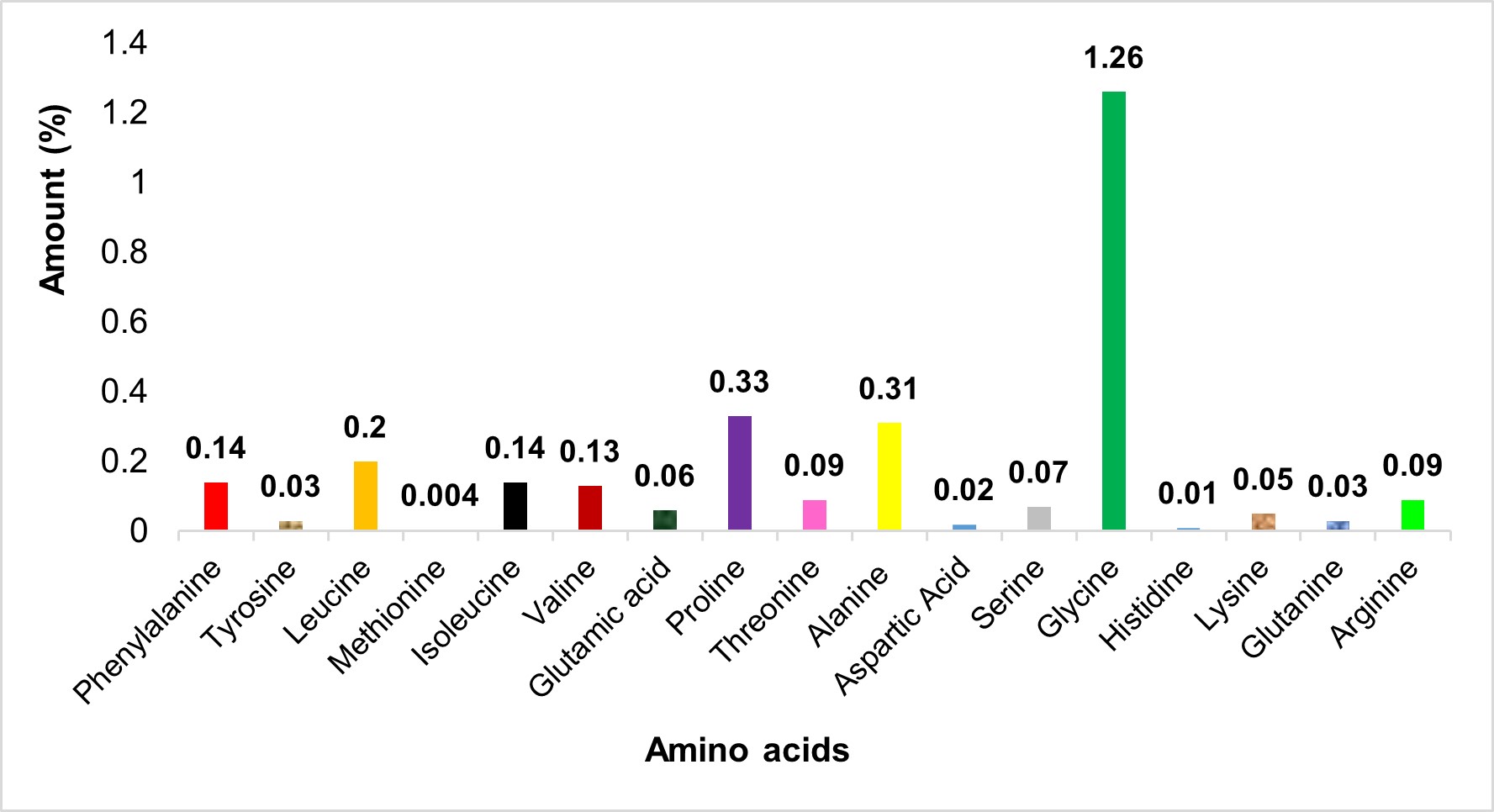
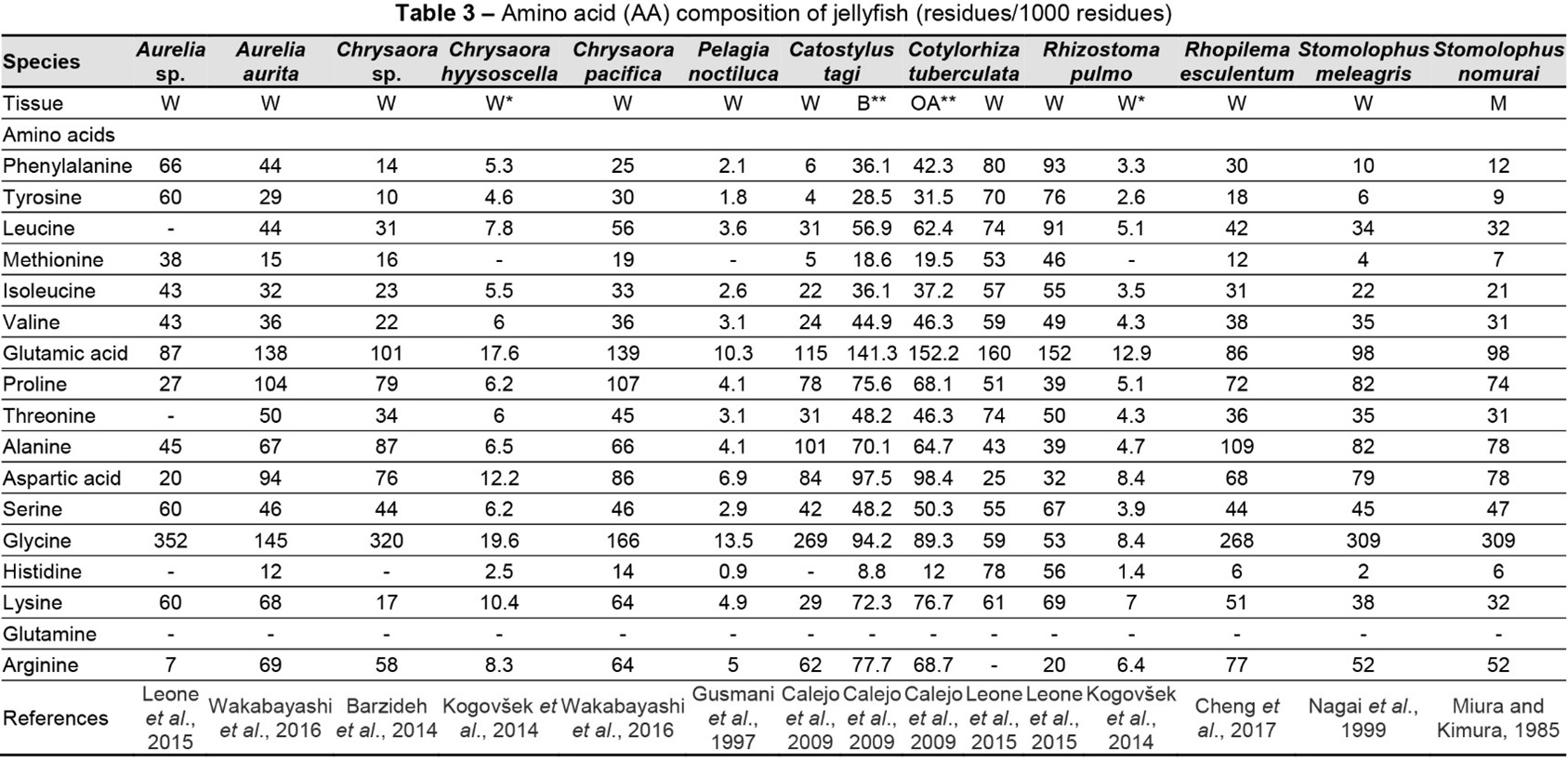
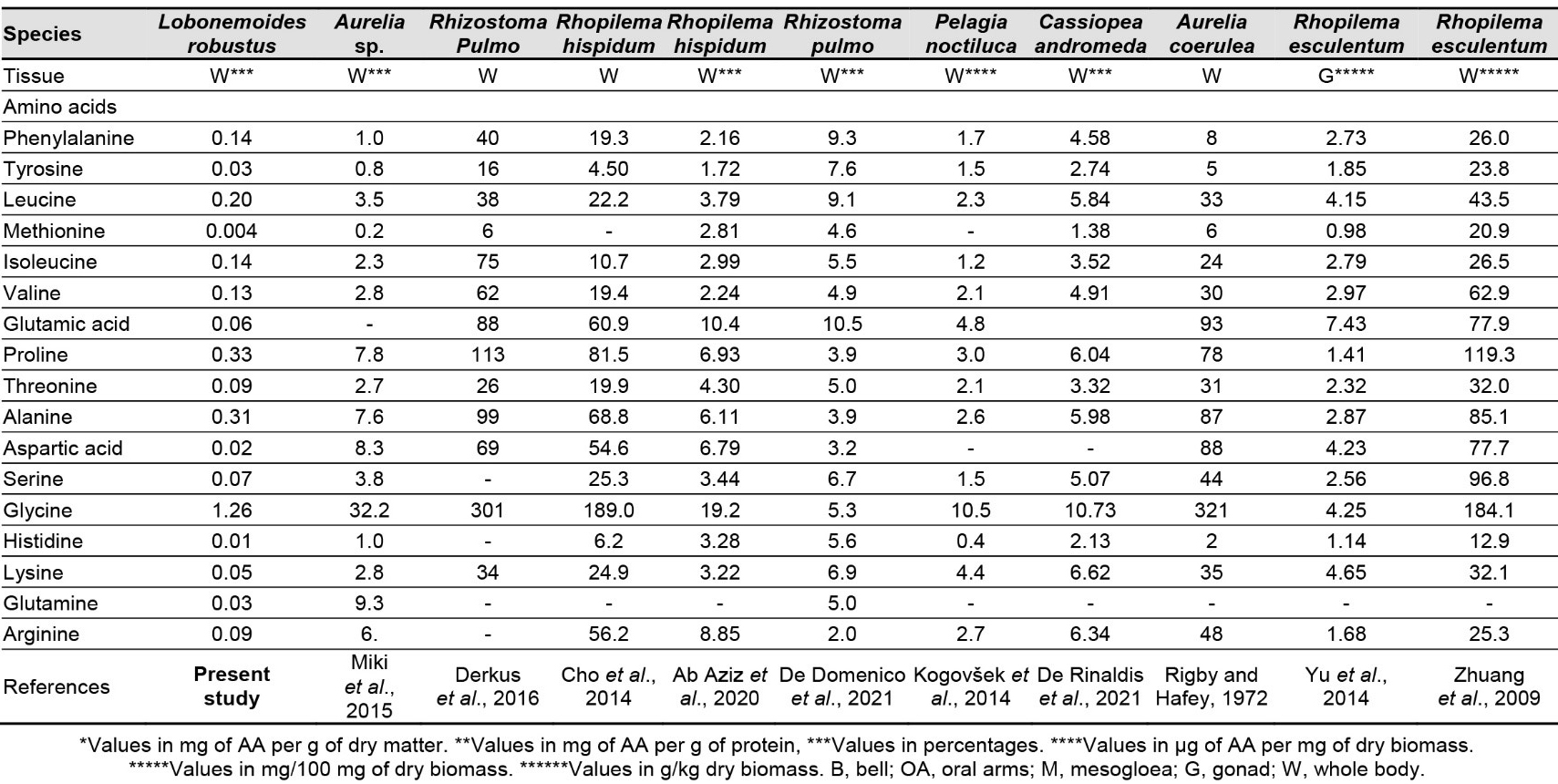
In general, L. robustus had low EAA levels. The most abundant EAA is Gly (Figure 2), followed by Glu, Asp, Thr, and Pro. The results of this investigation are consistent with those of Khong et al. (2016) and Hsieh et al. (2001). According to Khong et al. (2016), JF, regardless of the body area, contains roughly 33% EAAs, 46% conditionally EAAs, and 21% NEAAs. Kogovšek et al. (2014) reported that in JF, Asp, Lys, Arg, Gly, and Glu are the most abundant AAs per unit of dry mass, accounting for over half of the entire pool of AAs.
Consistent with our findings, Gly is the most prevalent AA in scyphomedusae. This EAA is one of the major structural units of collagen (Merquiol et al., 2019). Cheng et al. (2017) and Kittiphattanabawon et al. (2005) showed that Gly is the most prevalent AA in JF collagen. Although there were no statistically significant differences, Wakabayashi et al. (2016) reported a higher EAA content in Aurelia aurita compared with Chrysaora pacifica. Pro, Ala, Leu, Phe, Ile, and Val are other EAAs that are present in good concentrations (Table 3).
Table 1
The mass spectrometry acquisition conditions
|
Parameters |
State |
|
Run time |
22 minutes |
|
Ion polarity |
Positive ion mode |
|
Ion source |
Atmospheric pressure electrospray ionisation |
|
Capillary voltage (kV) |
4.0 |
|
Block temperature |
400°C |
|
Desolvation line temperature |
300°C |
|
CID gas |
Argon (270 kPa) |
|
Nebulising gas flow |
N2, 1.5 L/min |
|
Drying gas flow |
N2, 15.0 L/min |
|
Heating gas flow |
10 L/min |
|
Interface temperature |
300°C |
Table 2
The multiple reaction monitoring (MRM) transition events of the amino acids
|
Amino acid |
Type |
m/z |
Retention time (min) |
MRM event |
|
Serine |
Target |
106.10>60.20 |
1.707 |
7:MRM(+) |
|
Glycine |
Target |
76.00>30.00 |
1.732 |
16:MRM(+) |
|
Glutamine |
Target |
147.00>84.10 |
1.723 |
6:MRM(+) |
|
Lysine |
Target |
147.00>84.10 |
1.731 |
15:MRM(+) |
|
Aspartic acid |
Target |
134.10>73.90 |
1.720 |
3:MRM(+) |
|
Histidine |
Target |
156.10>110.10 |
1.761 |
11:MRM(+) |
|
Threonine |
Target |
120.10>74.00 |
1.758 |
8:MRM(+) |
|
Alanine |
Target |
90.10>44.10 |
1.785 |
1:MRM(+) |
|
Arginine |
Target |
175.10>70.10 |
1.786 |
2:MRM(+) |
|
Glutamic acid |
Target |
148.10>84.10 |
1.804 |
4:MRM(+) |
|
Proline |
Target |
116.10>70.10 |
1.934 |
17:MRM(+) |
|
Valine |
Target |
118.20>72.00 |
2.177 |
10:MRM(+) |
|
Methionine |
Target |
150.10>56.10 |
2.380 |
13:MRM(+) |
|
Leucine |
Target |
132.10>86.30 |
2.979 |
12:MRM(+) |
|
Isoleucine |
Target |
132.10>86.30 |
3.180 |
12:MRM(+) |
|
Tyrosine |
Target |
182.10>136.20 |
3.244 |
9:MRM(+) |
|
Phenylalanine |
Target |
166.10>120.10 |
4.583 |
14:MRM(+) |
Compared with Semaeostomeae, Rhizostomeae have more EAAs (Merquiol et al., 2019). Only Cotylorhiza tuberculata and Rhizostoma pulmo contain significant levels of His; in other scyphomedusae, this EAA is either absent or very low (Table 3). L. robustus also contains detectable amounts of Thr, Arg, Ser, Glu, and Lys (Figure 2).
Compared with the AA composition of rat tail collagen, JF had a low Pro content and higher Glu and Ala contents (Derkus et al., 2016). Rhopilema hispidum gelatine has notably high Gly (18.90%), Pro (8.15%), and hydroxyproline (13.93%) contents (Table 3) (Cho et al., 2014). Chrysaora sp. has a low concentration of Pro and hydroxyproline (Barzideh et al., 2014). According to De Rinaldis et al. (2021), the most prevalent AAs in Cassiopea andromeda are Glu, Gln, and Gly. This species contains 15.68 g of these AAs per 100 g lyophilised sample, more than twice as much as R. pulmo (6.1 ± 0.09 g per 100 g lyophilised sample) and Pelagia noctiluca (8.1 ± 0.3 g per 100 g lyophilised sample) samples analysed in parallel. De Rinaldis et al. (2021) also reported high levels of Ala and taurine in C. andromeda, namely 0.96 g per 100 g dry weight. The contents of the main AAs of wild JF gonad and cultured JF gonad – Glu, Lys, Gly, Asp, and Leu – are 51.47% and 52.52% of the total AA content, respectively. Asp and Glu are often present in enzyme-active sites and are crucial for preserving the solubility and ionic nature of proteins (Yu et al., 2014). Stabili et al. (2018) found free AAs in a gonadal extract from R. pulmo. The ovaries of this species may provide an abundant supply of AAs for pharmacological and nutraceutical purposes. Additionally, the ovaries may provide proteins needed for the creation of novel nutritional supplements intended to sustain fish.
Fatty acids (FAs) in L. robustus
FAs are components of membranes and cell structures, but they also accumulate as energy storage units in plants and animals. They can be absorbed from food or biosynthesised by the organism (Saha and Pathak, 2021). FAs do not decompose during digestion, in contrast to other complex compounds; rather, they stay mostly unaltered or barely altered. Because they often do not change as reservoirs during normal cell metabolism (Elsamadony et al., 2021), they are regarded as traditional indicators that are used in environmental research to clarify the relationships between organisms in the food chain and to ascertain the movement of organic matter from lower trophic levels to higher trophic levels (De Troch et al., 2012). In the present study, C18:3 was the most prevalent FA (0.43%) in L. robustus, followed by heptadecanoic acid (0.29%), gamma-linolenic acid (0.24%), cis-9-oleic acid (0.18%), decanoic acid (0.13%), and myristic acid (0.12%) (Figure 3).
According to De Renaldis et al. (2021), PUFAs and SFAs make up roughly 48% and 44% of all FAs in C. andromeda, respectively, but MUFAs make up only 8% of all FAs. In terms of MUFA content, the hydroalcoholic extract and the entire JF extract have similar levels of isooleic acid, oleic acid, and palmitoleic acid. Svetashev (2019) recorded different types of omega-3 FAs in A. aurita and Rhopilema esculentum, which are the principal PUFAs; R. esculentum contains 1.6% of C26 PUFAs.
Ying et al. (2012) reported high 20:4(ω-6) concentrations (>10%) and ratios of 20:5(ω-3)/20:6(ω-3) > 1 in JF. The month–diameter interaction has a substantial impact on the FA profile of Aurelia labiata, meaning that changes in the FA profile with diameter vary from month to month (Schaub et al., 2023). According to Wakabatake et al. (2016), of the five essential fatty acids, anandamide and 20:6(ω-3) are more abundant in C. pacifica than in A. aurita, while 20:5(ω-3) is more abundant in A. aurita than in C. pacifica. According to Leone et al. (2015), the zooxanthellate JF Cotylorhiza tuberculate has a considerably higher presence of ω3 and ω6 PUFAs. With a high percentage (62.7%) of unsaturated FAs, the FA profile of A. aurita from the Atlantic Ocean differs significantly from that of A. aurita from the Aegean Sea (Kariotoglou and Mastronicolis, 2001). A. aurita’s FA profile exhibits notable seasonal fluctuation, with mature medusae having the highest FA levels. Furthermore, the moon jelly contains multiple critical FAs – 20:4(ω-6), 20:5(ω-3), and 20:6(ω-3) – likely to support its essential physiological activities (Stenvers et al., 2020).
The majority of FAs in Catostylus tagi are PUFAs, followed by MUFAs and SFAs. According to Moris et al. (2009), there is a considerable increase in the concentration of ARA, EPA, 20:4ω6, DHA (about 32%) in the oral arms and umbrellas of JF (mostly 20:5ω3). There have been similar findings in Rhizostoma luteum (Prieto et al., 2018), where almost half of the FAs are PUFAs, mainly ω3 C18:3, essential ω6 C18:2, and ω6 C20:4 acids. Stabili et al. (2018) described the presence of PUFAs, diunsaturated fatty acids (DUFAs), MUFAs, and SFAs in the gonads of R. pulmo.
While the overall fatty acid concentration of P. noctiluca changes according to the body area, MUFAs and PUFAs make up 15% and 14%–19% of the total, respectively, and there are no sex differences (Costa et al., 2019). In comparison, Leone et al. (2015) discovered that in JF, SFAs (55%–70%) dominate, followed by PUFAs (25%–30%) and MUFAs (4%–15%).
The gonads of R. pulmo have ω3 PUFAs, primarily DHA and EPA, which suggests that these molecules could be extracted from them and used in the pharmaceutical industry (Stabili et al., 2018). DHA and EPA have anti-inflammatory and antioxidant properties and may be used in treatment plans for mental health issues and memory impairments brought on by neuroinflammation (Apetz et al., 2014). Additionally, considering that fish diets are typically supplemented with extra EPA and DHA, the gonads of R. pulmo may provide these necessary FAs that could be extracted and then added to the fish feed (Stabili et al., 2018).
According to Khong et al. (2016), EAAs, conditionally EAAs, and non-EAAs account for 33%, 46%, and 21% of the total amino acids (TAAs), respectively, in JF species. According to Yu et al. (2014), the TAAs in R. esculentum gonads are made up of 40.70–42.89% EAAs, 47.39%–50.12% taste AAs, and 66.55%–66.92% medicinal AAs. According to Leone et al. (2015), the proportion of EAAs of the TAA content in Aurelia sp., R. pulmo, and C. tuberculate is 31.4%, 50.8%, and 53.6%, respectively.
These findings suggest that JF may find usage as a functional food and dietary supplement (Raposo et al., 2022).
CONCLUSIONS
The search for substitute sources of bioactive chemicals to take the place of overfished resources is a pressing need for modern society. JF are important sources of AAs and FAs. In the present study, we found that L. robustus is rich in Gly. The most common FAs are linoleic acid, myristic acid, cis-9-oleic acid, gamma-linolenic acid, and heptadecanoic acid. Our data indicate that L. robustus could be a sustainable source of AAs and FAs for use in manufacturing natural nutraceutical, cosmeceutical, and biomedical products. Moreover, in Southeast Asia, L. robustus is commonly used for food. The commercially valuable L. robustus could be exported to other countries and contribute to a blue economy.
Author Contributions: Conceptualisation, Methodology, Funding acquisition, Supervision: MTI; Conceptualisation, Methodology, Statistical analysis, Writing – original draft: MSB; Literature survey, SA; Analysis, review and editing, MK, NKK; Review and editing: MSB, MK, SA. All authors have read and agreed to the published version of the manuscript.
Data availability: This study’s findings are supported by the data presented in the report.
Acknowledgments: The authors are grateful to the Bangladesh Oceanographic Research Institute for providing this research facility. Authors also express heartfelt thanks to Bangladesh Reference Institute for Chemical Measurements (BRiCM) for providing chemical analysis facilities.
Conflicts of Interest: The authors declare no competing interests.
REFERENCES
Ab Aziz, N.A.; Salim, N.; Zarei, M.; Saari, N.; Yusoff, F.M. Extraction, anti-tyrosinase, and antioxidant activities of the collagen hydrolysate derived from Rhopilema hispidum. Preparative biochemistry & biotechnology. 2021, 51, 44-53. https://doi.org/10.1080/10826068.2020.1789991
Apetz, N.; Munch, G.; Govindaraghavan, S.; Gyengesi, E. Natural compounds and plant extracts as therapeutics against chronic inflammation in Alzheimer’s disease–a translational perspective. CNS & Neurological Disorders-Drug Targets. 2014, 13, 1175-1191. https://doi.org/10.2174/1871527313666140917110635
Barzideh, Z.; Latiff, A.A.; Gan, C.Y.; Benjakul, S.; Karim, A.A. Isolation and characterisation of collagen from the ribbon jellyfish (Chrysaora sp.). International journal of food science & technology. 2014, 49, 1490-1499. https://doi.org/10.1111/ijfs.12464
Brotz, L.; Cheung, W.W.; Kleisner, K.; Pakhomov, E.; Pauly, D. Increasing jellyfish populations: trends in large marine ecosystems. In Jellyfish Blooms IV: Interactions with humans and fisheries. J. E. Purcell, H. Mianzan & J. R. Frost, 2012, 3-20.
Calejo, M.T.; Morais, Z.B.; Fernandes, A.I. Isolation and biochemical characterisation of a novel collagen from Catostylus tagi. Journal of Biomaterials Science, Polymer Edition. 2009, 20, 2073-2087.
Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, characterization and evaluation of collagen from jellyfish Rhopilema esculentum Kishinouye for use in hemostatic applications. PloS One. 2017, 12. https://doi.org/10.1371/journal.pone.0169731
Cho, S.; Ahn, J.R.; Koo, J.S.; Kim, S.B.K. Physicochemical properties of gelatin from jellyfish Rhopilema hispidum. Fisheries and aquatic sciences. 2014, 17, 299-304. https://doi.org/10.5657/FAS.2014.0299
Costa, R.; Capillo, G.; Albergamo, A.; Li Volsi, R.; Bartolomeo, G.; Bua, G.; Spanò, N. A multi-screening Evaluation of the Nutritional and Nutraceutical Potential of the Mediterranean Jellyfish Pelagia noctiluca. Marine drugs. 2019, 17, 172. https://doi.org/10.3390/md17030172
Crawford, M.A.; Sinclair, A.J.; Hall, B.; Ogundipe, E.; Wang, Y.; Bitsanis, D.; Johnson, M.R. The imperative of arachidonic acid in early human development. Progress in lipid research. 2023, https://doi.org/10.1016/j.plipres.2023.101222
Das, S.; Patel, B. Marine resources and animals in modern biotechnology. In Animal Biotechnology. Academic Press. 2020, 567-591. https://doi.org/10.1016/B978-0-12-811710-1.00027-6
Das, Y.; Karunarathne, K.D.; Roy, M.; Chowdhury, M.S.N.; Sharifuzzaman, S.M. Record of Crambionella annandalei Rao, 1931 from Bangladesh, with a review of the geographic distribution of the genus Crambionella (Cnidaria: Scyphozoa). Marine Biology Research. 2023, 19, 279-293. https://doi.org/10.1080/17451000.2023.2203500
De Domenico, S.; De Rinaldis, G.; Gallo, T.; Leone, A. Jellyfish as source of bioactive compounds with nutraceutical value. https://gojelly.eu/wp-content/uploads/2021/12/GoJelly_Nutraceuticals_SDD_ok2_AL.pdf, 2021.
De Rinaldis, G.; Leone, A.; De Domenico, S.; Bosch-Belmar, M.; Slizyte, R.; Milisenda, G.; Santucci, A.; Albano, C.; Piraino, S. Biochemical characterization of Cassiopea andromeda (Forsskål, 1775), another red sea jellyfish in the western Mediterranean Sea. Marine Drugs. 2021, 19, 498. https://doi.org/10.3390/md19090498
De Troch, M.; Boeckx, P.; Cnudde, C.; Van Gansbeke, D.; Vanreusel, A.; Vincx, M.; Caramujo, M.J. Bioconversion of fatty acids at the basis of marine food webs: insights from a compound-specific stable isotope analysis. Marine Ecology Progress Series. 2012, 465, 53-67. https://doi.org/10.3354/meps09920
Derkus, B.; Arslan, Y.E.; Bayrac, A.T.; Kantarcioglu, I.; Emregul, K.C.; Emregul, E. Development of a novel aptasensor using jellyfish collagen as matrix and thrombin detection in blood samples obtained from patients with various neurodisease. Sensors and Actuators B: Chemical. 2016, 228, 725-736. https://doi.org/10.1016/j.snb.2016.01.095
dos Santos, S.I.P.M. Re-evaluation of essential amino acids in fish by a meta-analytical approach. MSc Thesis, Universidade do Porto, Portugal, 2013.
Duarte, I.M.; Marques, S.C.; Leandro, S.M.; Calado, R. An overview of jellyfish aquaculture: for food, feed, pharma and fun. Reviews in Aquaculture. 2022, 14, 265-287. https://doi.org/10.1111/raq.12597
Elsamadony, M.; Mostafa, A.; Fujii, M.; Tawfik, A.; Pant, D. Advances towards understanding long chain fatty acids-induced inhibition and overcoming strategies for efficient anaerobic digestion process. Water Research. 2021, 190, 116732. https://doi.org/10.1016/j.watres.2020.116732
Fonseca, S.; Amaral, M.N.; Reis, C.P.; Custódio, L. Marine Natural Products as Innovative Cosmetic Ingredients. Marine Drugs. 2023, 21, 170. https://doi.org/10.3390/md21030170
Gusmani, L.; Avian, M.; Galil, B.; Patriarca, P.; Rottini, G. Biologically active polypeptides in the venom of the jellyfish Rhopilema nomadica. Toxicon. 1997, 35, 637-648. https://doi.org/10.1016/S0041-0101(96)00182-1
Haider, S.M.B.; Bhuyan, M.S.; Sharif, A.S.M.; Islam, M.T.; Kibria, A.S.M.M.; Peas, M.H. JellyFish (Lobonemoides robustus Stiasny, 1920) Bloom in the Cox’s Bazar Coast During 3-4 August 2022: Factors Identification and Minimization Approaches. Journal of Oceanography & Marine Environmental System. 2022, 6, 01-17. https://doi.org/10.5829/idosi.jomes.2022.01.17
Hsieh, Y.P.; Leong, F.M.; Rudloe, J. Jellyfish as food. In Jellyfish Blooms: Ecological and Societal Importance: Proceedings of the International Conference on Jellyfish Blooms, held in Gulf Shores, Alabama, 12–14 January Springer Netherlands, 2001, 11-17.
Kariotoglou, D.M.; Mastronicolis, S.K. Sphingophosphonolipids, phospholipids, and fatty acids from Aegean jellyfish Aurelia aurita. Lipids. 2001, 36, 1255. https://doi.org/10.1007/s11745-001-0840-3
Khong, N.M.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food chemistry. 2016, 196, 953-960. https://doi.org/10.1016/j.foodchem.2015.09.094
Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food chemistry. 2005, 89, 363-372. https://doi.org/10.1016/j.foodchem.2004.02.042
Kogovšek, T.; Tinta, T.; Klun, K.; Malej, A. Jellyfish biochemical composition: importance of standardised sample processing. Marine Ecology Progress Series. 2014, 510, 275-288.
Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The bright side of gelatinous blooms: Nutraceutical value and antioxidant properties of three Mediterranean jellyfish (Scyphozoa). Marine Drugs. 2015, 13, 4654-4681. https://doi.org/10.3390/md13084654
Merquiol, L.; Romano, G.; Ianora, A.; D’Ambra, I. Biotechnological applications of Scyphomedusae. Marine drugs. 2019, 17, 604. https://doi.org/10.3390/md17110604
Miki, A.; Inaba, S.; Baba, T.; Kihira, K.; Fukada, H.; Oda, M. Structural and physical properties of collagen extracted from moon jellyfish under neutral pH conditions. Bioscience, Biotechnology, and Biochemistry. 2015, 79, 1603-1607. https://doi.org/10.1080/09168451.2015.1046367
Miura, S.; Kimura, S. Jellyfish mesogloea collagen. Characterization of molecules as alpha 1 alpha 2 alpha 3 heterotrimers. Journal of Biological Chemistry. 1985, 260, 15352-15356. https://doi.org/10.1016/S0021-9258(18)95743-1
Monroig, Ó.; Shu-Chien, A.C.; Kabeya, N.; Tocher, D.R.; Castro, L.F.C. Desaturases and elongases involved in long-chain polyunsaturated fatty acid biosynthesis in aquatic animals: From genes to functions. Progress in lipid research. 2022, 86, 101157. https://doi.org/10.1016/j.plipres.2022.101157
Nagai, T.; Ogawa, T.; Nakamura, T.; Ito, T.; Nakagawa, H.; Fujiki, K.; Nakao, M.; Yano, T. Collagen of edible jellyfish exumbrella. Journal of the Science of Food and Agriculture. 1999, 79, 855-858. https://doi.org/10.1002/(SICI)1097-0010(19990501)79:6<855::AID-JSFA299>3.0.CO;2-N
Prieto, L.; Enrique-Navarro, A.; Li Volsi, R.; Ortega, M.J. The large jellyfish Rhizostoma luteum as sustainable a resource for antioxidant properties, nutraceutical value and biomedical applications. Marine drugs. 2018, 16, 396. https://doi.org/10.3390/md16100396
Primavera, J.H.; Friess, D.A.; Van Lavieren, H.; Lee, S.Y. Chapter 1 – The Mangrove Ecosystem. World Seas: An Environmental Evaluation (Second Edition), Academic Press. 2019, 1-34. https://doi.org/10.1016/B978-0-12-805052-1.00001-2
Raposo, A.; Alasqah, I.; Alfheeaid, H.A.; Alsharari, Z.D.; Alturki, H.A.; Raheem, D. Jellyfish as Food: A Narrative Review. Foods. 2022, 11, 2773. https://doi.org/10.3390/foods11182773
Rigby, B.J.; Hafey, M. Thermal properties of the collagen of jellyfish (Aurelia coerulea) and their relation to its thermal behaviour. Australian journal of biological sciences. 1972, 25, 1361-1364. https://doi.org/10.1071/BI9721361
Romano, G.; Almeida, M.; Varela Coelho, A.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; et al. Biomaterials and bioactive natural products from marine invertebrates: From basic research to innovative applications. Marine Drugs. 2022, 20, 219. https://doi.org/10.3390/md20040219
Saha, S.K.; Pathak, N.N. Fundamentals of animal nutrition, 1, Springer Singapore, Singapore, 2021. https://doi.org/10.1007/978-981-15-9125-9
Schaub, J.; McLaskey, A.K.; Forster, I.; Hunt, B.P. Size-based changes in trophic ecology and nutritional quality of moon jellyfish (Aurelia labiata). Ecosphere. 2023, 14, e4430. https://doi.org/10.1002/ecs2.4430
Stabili, L.; Rizzo, L.; Fanizzi, F. P.; Angilè, F.; Del Coco, L.; Girelli, C.R.; Lomartire, S.; Piraino, S.; Basso, L. The jellyfish Rhizostoma pulmo (Cnidaria): Biochemical composition of ovaries and antibacterial lysozyme-like activity of the oocyte lysate. Marine drugs. 2018, 17, 17. https://doi.org/10.3390/md17010017
Stenvers, V.; Chi, X.; Javidpour, J. Seasonal variability of the fatty acid composition in Aurelia aurita (Cnidaria: Scyphozoa): implications for gelativore food web studies. Journal of Plankton Research. 2020, 42, 440-452. https://doi.org/10.1093/plankt/fbaa026
Svetashev, V.I. Fatty acids of the medusae Aurelia aurita (Linnaeus, 1758) and Rhopilema esculentum (Kishinouye, 1891): The presence of families of polyenoic acids with 24 and 26 carbon atoms. Russian Journal of Marine Biology. 2019, 45, 113-117. https://doi.org/10.1134/S1063074019020123
Wakabayashi, K.; Sato, H.; Yoshie-Stark, Y.; Ogushi, M.; Tanaka, Y. Differences in the biochemical compositions of two dietary jellyfish species and their effects on the growth and survival of Ibacus novemdentatus phyllosomas. Aquaculture Nutrition. 2016, 22, 25-33. https://doi.org/10.1111/anu.12228
Xu, S.; Gu, M.; Wu, K.; Li, G. Unraveling the role of hydroxyproline in maintaining the thermal stability of the collagen triple helix structure using simulation. The Journal of Physical Chemistry B. 2019, 123, 7754-7763. https://doi.org/10.1021/acs.jpcb.9b05006
Yao, Y.; Ding, L.; Huang, X. Diverse functions of lipids and lipid metabolism in development. Small Methods. 2020, 4, 1900564. https://doi.org/10.1002/smtd.201900564
Ying, C.; Ying, W.; Jing, Z.; Na, W. Potential dietary influence on the stable isotopes and fatty acid compositions of jellyfishes in the Yellow Sea. Journal of the Marine Biological Association of the United Kingdom. 2012, 92, 1325-1333. https://doi.org/10.1017/S0025315412000082
Yu, H.; Li, R.; Liu, S.; Xing, R.E.; Chen, X.; Li, P. Amino acid composition and nutritional quality of gonad from jellyfish Rhopilema esculentum. Biomedicine & Preventive Nutrition. 2014, 4, 399-402. https://doi.org/10.1016/j.bionut.2014.04.007
Zhuang, Y.; Sun, L.; Zhao, X.; Wang, J.; Hou, H.; Li, B. Antioxidant and melanogenesis-inhibitory activities of collagen peptide from jellyfish (Rhopilema esculentum). Journal of the Science of Food and Agriculture. 2009, 89, 1722-1727. https://doi.org/10.1002/jsfa.3645
Akter Sumi, Bhuyan Simul, Islam Tarikul, Khan Mala, Kumer Kundu Nayan, Kunda Mrityunjoy