Anastasia Ştefîrţă, Ion Bulhac, Lilia Brînză, Leonid Voloșciuc, Eduard Coropceanu, Maria Cocu
ABSTRACT. The effect of co-activation of the stress-memory formation potential under repeated drought of Glycine max (Merr.) L. plants was recorded using cytokinin (CK), thiourea (TH) and, especially, complex preparation Polyel. Glycine max plants (Merr.) L. of Moldovitsa, Nadejda and Magia varieties, exposed to two cycles of “drought–rehydration“ at the “first trifoliate leaf” and “flowering – pods formation” phases served as test subjects. The tolerance-inducing effect manifests itself by maintaining the content of assimilatory pigments, photosynthesis and growth processes at a significantly higher level. After the restoration of the optimal moisture background, plants pre-treated with CK, TH and the preparation Polyel, which endured moderate stress in the initial stages of ontogenesis, had restored functional processes. The information obtained in this work certainly opens the management perspective of the ability to form stress memory, adaptation and tolerance of plants to the unfavourable fluctuation of humidity and recurrent drought. The management possibilities of plant adaptation and tolerance are discussed.
Keywords: plants; adaptation; resistance; growth; photosynthesis; transpiration; productivity; cytokinin compounds; antioxidant preparation.
Cite
ALSE and ACS Style
Ștefîrță, A.; Bulhac, I.; Brînză, L.; Voloșciuc, L.; Coropceanu, E.; Cocu, M. The “photosynthesis–growth–stress memory” relationship in plants under conditions of moisture fluctuation and recurrent drought: management options. Journal of Applied Life Sciences and Environment 2022, 55 (4), 457-472.
https://doi.org/10.46909/alse-554076
AMA Style
Ștefîrță A, Bulhac I, Brînză L, Voloșciuc L, Coropceanu E, Cocu M. The “photosynthesis–growth–stress memory” relationship in plants under conditions of moisture fluctuation and recurrent drought: management options. Journal of Applied Life Sciences and Environment. 2022; 55 (4): 457-472.
https://doi.org/10.46909/alse-554076
Chicago/Turabian Style
Ştefîrţă, Anastasia, Ion Bulhac, Lilia Brînză, Leonid Voloșciuc, Eduard Coropceanu, and Maria Cocu. 2022. “The “photosynthesis–growth–stress memory” relationship in plants under conditions of moisture fluctuation and recurrent drought: management options” Journal of Applied Life Sciences and Environment 55, no. 4: 457-472.
https://doi.org/10.46909/alse-554076
View full article (HTML)
The “Photosynthesis-Growth-Stress Memory” Relationship In Plants Under Conditions of Moisture Fluctuation and Recurrent Drought: Management Options
Anastasia ŞTEFÎRŢĂ1,2, Ion BULHAC1, Lilia BRÎNZĂ1,3, Leonid VOLOȘCIUC2, Eduard COROPCEANU3 and Maria COCU1*
1Institute of Chemistry of Moldova State University, Chișinău, Republic of Moldova; e-mail: ionbulhac@yahoo.com
2Institute of Genetics, Physiology and Plant Protection of Moldova State University, Chișinău, Republic of Moldova; e-mail: anastasia.stefirta@gmail.com
3 Institute for Research, Innovation and Technology Transfer of “Ion Creangă” State Pedagogical University, Chișinău, Republic of Moldova; e-mail: coropceanu.eduard@upsc.md
*Correspondence: maria.cocu@ichem.md
Received: Feb. 15, 2023. Revised: Mar. 29, 2023. Accepted: Apr. 11, 2023. Published online: May 04, 2023
ABSTRACT. The effect of co-activation of the stress-memory formation potential under repeated drought of Glycine max (Merr.) L. plants was recorded using cytokinin (CK), thiourea (TH) and, especially, complex preparation Polyel. Glycine max plants (Merr.) L. of Moldovitsa, Nadejda and Magia varieties, exposed to two cycles of “drought–rehydration” at the “first trifoliate leaf” and “flowering – pods formation” phases served as test subjects. The tolerance-inducing effect manifests itself by maintaining the content of assimilatory pigments, photosynthesis and growth processes at a significantly higher level. After the restoration of the optimal moisture background, plants pre-treated with CK, TH and the preparation Polyel, which endured moderate stress in the initial stages of ontogenesis, had restored functional processes. The information obtained in this work certainly opens the management perspective of the ability to form stress memory, adaptation and tolerance of plants to the unfavourable fluctuation of humidity and recurrent drought. The management possibilities of plant adaptation and tolerance are discussed.
Keywords: plants; adaptation; resistance; growth; photosynthesis; transpiration; productivity; cytokinin compounds; antioxidant preparation.
INTRODUCTION
Repeated droughts caused by climate change often have catastrophic consequences for plant productivity. Urgent actions are needed to reduce the climate risk in all spheres of activity, including agriculture (FAO, 2022). Currently, the problem of crop productivity and resistance to the impact produced by climate change on Earth represents the main and most critical matter for the food security of the population (IPCC, 2022).
Water deficit is considered the strongest abiotic stress factor, limiting plant growth and productivity and threatening food security and nutrition. Data have accumulated on plant responses to stress caused by dehydration under drought conditions (Chaves et al., 2003; Kramer and Boyer, 1995; Levitt, 1986). In many physical-geographic areas on Earth, plants are subjected to recurrent episodes of drought that vary in severity and length. There is a growing interest in learning more about how plants react to repeated droughts.
Recently, it has been demonstrated that plants can “remember” past incidents from the external environment and can use these memories for an appropriate response if these events are repeated, enduring them more easily (Walter et al., 2011). Such plants form a “stress memory”. Abiotic stress that occurs at different stages of ontogenesis creates a high risk of damage, but previous stress events can provide plants with protection against subsequent stress. Multiple exposures to drought conditions provide a faster and more appropriate plant response to a new stress situation compared to plants that have not been previously exposed to drought stress. However, little is known about which mechanisms are involved in the manifestation of stress memory. From the published data (Bhattacharjee, 2005, 2012; Jacques et al., 2021; Munne-Bosch, 2013) and the results of our own investigations (Ștefîrță et al., 2021), the key systems, linked to plants reactions to recurrent drought and formation of stress memory in plants, are those coupled with self-regulation of water homeostasis (Bartoli et al., 1999; Kramer and Boyer, 1995; Ștefîrță et al., 2020), formation and neutralisation of reactive oxygen species (ROSs), and activation of the antioxidant potential (Asada, 2006; Foyer and Shigeoka, 2011; Shinozaki and Yamaguchi-Shinozaki, 1999; Ştefîrţă et al., 2021). With the creation and accumulation of numerous substances with a protective effect, such as particular proteins, amino acids, carbohydrates, etc., the modest rise in ROS level conditions the expression of protective genes and the beginning of adaptive processes (Bhattacharjee, 2012; Bruce et al., 2007). Another possibility for preserving the memory of ecological stress is the morphological changes of the plant, which remain stable for a longer time than the metabolic changes (Bartoli et al., 2013; Kalapos et al., 1996). The production and yield of plant biomass are considered the main macroscopic indicators of drought stress memory because they integrate different molecular mechanisms and physiological processes involved in the plant’s response to repeated stress (Abid et al., 2017; Perveen et al., 2015a, b).
Abid et al. (2017) consider the production and yield of plant biomass the main macroscopic indicators of drought stress memory since the plant’s reaction to repeated stress is governed by a variety of molecular pathways and physiological processes. The importance of knowing the mechanisms that ensure plant accommodation and tolerance to repeated ecological impacts over time and elucidation of the factors that induce them is indisputable. Although knowledge of the mechanisms that allow plants to tolerate drought has increased considerably in recent years (Bartoli et al., 2013; Ding et al., 2012; Kinoshita and Seki, 2014), there is relatively little information regarding the improvement of tolerance to adverse moisture fluctuation and stress caused by recurrent drought. In this context, there is a need to explore some methods or ways of inducing stress memory and tolerance to often extreme fluctuations of external factors and repeated ecological stress. One of the key ways to increase stress tolerance is the application of agents that activate native protection mechanisms. Based on these findings, it has been suggested that drought effects can be managed using plant growth regulators, phytohormonal compounds, etc. (Aimar et al., 2011; Fleta-Soriano, 2015; Liu et al., 2022; Ştefîrţă et al., 2021). An effective way to protect the seeds is to increase natural plant resistance using tolerance-enhancing compounds by applying antioxidants and phytohormones (cytokinins, auxins) as factors for intensifying antioxidant protection, stabilising growth, delaying ageing, etc. (Ştefîrţă et al., 2021). In this sense, the real mechanisms involved in increasing plant tolerance to drought are persistently studied through the exogenous application of phytohormonal compounds, knowing that they increase the ability of plant tissues to retain water, activate biosynthesis and accumulate phytomass, which ultimately ensures the formation of a plant phenotype with a wider norm of reaction to adverse conditions and reduce crop losses under repeated drought (Wahid et al., 2017).
Currently, the physiological mechanisms underlying drought stress memory are not entirely clear. We aimed to test the possibility of inducing stress memory and improving plant performance, including through changes in photosynthesis, growth and productivity under recurrent drought conditions through the exogenous application of cytokinin-type compounds.
The specific objective of this work consisted of evaluating the particularities of plant growth regulation when using cytokinin-type compounds and the exogenous induction of stress memory and plant tolerance to repeated stress caused by drought.
Working hypothesis: Polyel preparation with a positive impact on the primary non-specific reactions of the plant’s response to the action of drought – water homeostasis and antioxidant protection capacity – can induce stress tolerance in plants to unfavourable humidity fluctuations and repeated water stress through co-activation of morphophysiological mechanisms and optimisation of growth and development processes. The possibility of managing the effects of recurrent drought through the exogenous use of the new bioactive Polyel preparation with significantly expressed antioxidant properties was verified.
MATERIALS AND METHODS
Glycine max L. (Merr) cultivars Nadejda, selected in the IGFPP, Moldovitsa and Magia, selected in the Research Institute for Field Crops “Selecția”, served as study objects. Polyel preparation, obtained in the Institute of Chemistry from the Republic of Moldova (patent MD 1348 2020.02.29; patent MD 4647, 2020.04.30), is a beige powder consisting of coordinating compounds of iron(III), cobalt(III), micro- and macroelements, vitamins, and NO3– ions. Polyel represents a complex of active substances, including nicotinamide (vitamin PP) and micro- and macroelements (Ștefîrță et al., 2020), possesses significant antioxidant properties (Ştefîrţă et al., 2021), and regulates water homeostasis in plant tissues exposed to unfavourable humidity conditions (Ştefîrţă et al., 2021). The evaluation of the compensatory reactions induced by cytokinin, thiourea and Polyel was carried out under conditions of controlled soil water content and repeated water stress.
The plants were grown in Mitcherlich vegetation pots with a capacity of 40 kg, pre-treated with cytokinin, thiourea and Polyel and exposed during ontogeny to drought-moisture recovery cycles, according with Scheme for “first trifoliate leaf” and “flowering – pods formation”.
In the periods of Critical Water Needs, plants were subjected to drought cycles of 7 days each, followed by recovery periods. At the end of each cycle of stress and, respectively, of restoring the optimal humidity, new, fully developed leaves were harvested and analysed.
The intensity of transpiration and photosynthesis as well as the stomatal conductance were measured with the LCpro-SD portable analyzer. The net rate of these three parameters was established in accordance with the scheme of experiences at photosynthetically active radiation (PhAR) from 1000 μmol photon flux density m-2s-1. While leaf temperature, air humidity and CO2 concentration were recorded according to their values from the environment. All measurements were made in the morning between 8 and 11. The photosynthetic pigment content was determined using the spectrophotometric method in 80% acetone extracts. The determination of growth processes was performed every 3 days, both during the stress action period and during the post-stress period.
The statistical analysis of the obtained data was done in the “Statistics 7” environment. The degree of change in the assimilatory pigment content, growth speed and photosynthesis intensity were used as indicators to quantify the stress intensity and the effect of the compounds used on the formation of plant stress memory and tolerance to humidity fluctuations and to the action of repeated moderate drought.
RESULTS
Drought at the “first trifoliate leaf” phase conditioned the reduction of chlorophyll content by 17.75% and chlorophyll b by 15.56% in the leaves of Moldovitsa and by 24.29 and 17.01%, respectively, in the leaves of Magia (Table 1). Thereafter, the total content of the assimilatory pigments was reduced. The destruction of chloroplasts and the significant reduction of assimilation pigments inevitably led to the inhibition of the photosynthesis process.
Scheme of the experiment at the phase “first trifoliate leaf”
|
Variant |
Plants pre-treatment |
Soil moisture (% TWC) |
|
I |
untreated |
70 |
|
II |
untreated |
40 (moderate drought) |
|
III |
Cytokinin |
|
|
IV |
Thiourea |
|
|
V |
Polyel |
Scheme of the experiment at the phase “flowering – pods formation”
|
Variant |
Plants pre-treatment |
Soil moisture (% TWC) |
Drought cycles |
|
I |
untreated |
70 |
– |
|
II |
untreated |
70 – 40 |
First cycle |
|
III |
Cytokinin |
70 – 40 – 70 – 40 |
2 |
|
IV |
Thiourea |
||
|
V |
Polyel |
Note: TWC- total water capacity of soil
Table 1
The content of the assimilation pigments (mg/100 g fr. m.*) in the leaves of soybean plants exposed to recurrent drought conditions
|
Variety |
Variants, Moisture, % TWC** |
Chlorophyll a |
Chlorophyll b |
Chlorophyll a + b |
|||
|
M ± m |
Δ, %M |
M ± m |
Δ, % M |
M ± m |
Δ, %M |
||
|
at the “first trifoliate leaf” phase |
|||||||
|
Moldovitsa |
Control, 70 |
138.22±3.21 |
|
58.47±1.01 |
|
196.68±2.73 |
|
|
Drought, 70-40 |
113.68±2.18 |
-17.8 |
49.37±0.89 |
-15.6 |
163.05±3. 08 |
-17.1 |
|
|
Magia |
Control, 70 |
95.28±1.07 |
|
39.28±0.65 |
|
134.56±1.70 |
|
|
Drought, 70-40 |
72.14±1.98 |
-24.3 |
32.60±0.54 |
-17.0 |
104.73±2.42 |
-22.2 |
|
|
at the “flowering – pods formation” phase |
|||||||
|
Moldovitsa |
Control, 70 |
165.03±2.98 |
|
77.31±1.62 |
|
242.34±3.18 |
|
|
Drought, first cycle |
137.48±1.89 |
-16.7 |
64.49±1.12 |
-16.6 |
201.97±3.02 |
-16.7 |
|
|
Drought, second cycle |
153.78±2.13 |
-6.8 |
70.02±0.98 |
-9.4 |
223.80±1.9 |
-7.6 |
|
|
Magia |
Control, 70 |
158.34±2.02 |
|
71.96±0.96 |
|
230.29±3.02 |
|
|
Drought, first cycle |
122.92±1.87 |
-22.4 |
57.19±0.75 |
-20.5 |
180.08±2.52 |
-21.8 |
|
|
Drought, second cycle |
138.12±2.75 |
-12.8 |
61.94±0.81 |
-13.9 |
200.06±3.01 |
-13.1 |
|
Notes: *- fresh mass; **- total water capacity of the soil
Water stress at the “first trifoliate leaf” phase caused a decrease in the photosynthesis intensity of Moldovitsa and Magia plants by 29.37 and 47.35%, respectively, compared to the intensity of the assimilation process under optimal humidity conditions (Table 2). A negative influence was recorded in plants exposed to drought for the first time in the “flowering – pods formation” phase. The assimilation process in representatives of the Moldovitsa variety was reduced 2.7 times and in Magia by 3.6 times. Previously stressed plants withstood the repeated stress more adequately; repeated drought conditioned the reduction of photosynthesis by 45.11% in the Moldovitsa variety and by 47.5% in the Magia variety. Strong inhibition of carbon dioxide assimilation in leaves under insufficient moisture was a result not only of the decrease in the pigment content but also of the reduction in stomatal conductance, leaf dehydration and transpiration intensity (Table 2).
The stomatal resistance against CO2 diffusion under conditions of insufficient humidity increased in Moldovitsa and Magia plants by 2.1 and 3.15 times, respectively, at the “first trifoliate leaf” phase and by 1.8 and 2.6 times, respectively, at the “flowering – pods formation” phase. Consequently, the intensity of transpiration in the twice-stressed plants of both varieties constituted 40.4–44.3% of the value of the process recorded in the plants from control variant and 53.1–60.2% in those exposed to drought for the first time at this development phase.
Table 2
The intensity of photosynthesis, transpiration and stomatal conductance in Glycine max (Merr.) L. plants exposed to recurrent drought conditions
|
Variety |
Variants, Moisture % TWC |
Stomatal conductance, mmol·m-2·sec-1 |
Transpiration, µmol·m-2·sec-1 |
Photosynthesis, µmol·m-2·sec-1 |
|||
|
M ± m |
Δ, % C |
M ± m |
Δ, % C |
M ± m |
Δ, % C |
||
|
at the “first trifoliate leaf” phase |
|||||||
|
Moldovitsa |
Control, 70 |
0.063±0.002 |
|
1.66±0.04 |
|
2.69±0.12 |
|
|
Drought, 70-40 |
0.030±0.001 |
-52.4 |
0.67±0.01 |
-59.6 |
1.90±0.09 |
-29.4 |
|
|
Magia |
Control, 70 |
0.050±0.001 |
|
1.55±0.06 |
|
2.45±0.08 |
|
|
Drought, 70-40 |
0.020±0.001 |
-60.0 |
0.59±0.01 |
-61.9 |
1.29±0.07 |
-47.4 |
|
|
at the “flowering – pods formation” phase |
|||||||
|
Moldovitsa |
Control, 70 |
0.081±0.003 |
|
1.88±0.04 |
|
4.70±0.19 |
|
|
Drought, first cycle |
0.044±0.001 |
-54.3 |
0.88±0.01 |
-53.2 |
1.76±0.07 |
-62.6 |
|
|
Drought, second cycle |
0.067±0.002 |
-17.3 |
1.12±0.05 |
-40.4 |
2.68±0.09 |
-45.1 |
|
|
Magia, |
Control, 70 |
0.072±0.002 |
|
1.76±0.07 |
|
3.73±0.15 |
|
|
Drought, first cycle |
0.028±0.001 |
-61.4 |
0.70±0.02 |
-60.2 |
1.05±0.03 |
-71.9 |
|
|
Drought, second cycle |
0.049±0.002 |
-31.9 |
0.98±0.05 |
-44.3 |
1.96±0.02 |
-47.5 |
|
Plant resilience to recurrent droughts increased under the influence of substances having cytokinin activity. In these plants, stress memory manifests itself more obviously by minimising or mitigating the negative impact of subsequent stress on the assimilatory pigment content (Table 3) and photosynthesis intensity (Table 4). Under repeated conditions of humidity deficiency, the total chlorophyll content in the leaves of the plants from control variant was kept at a higher level compared to the plants from control variant exposed to water stress for the first time in the “flowering – pods formation” phase. Pre-treatment of plants with cytokinin, thiourea and Polyel ensured the maintenance of chlorophyll a + b content at a correspondingly higher level by 7.89, 13.48 and 17.83% under conditions of water content fluctuation and repeated water stress compared to the pigment content in the leaves of control plants exposed to repeated drought. Destruction of chloroplasts and significant reduction of assimilation pigments inevitably led to the inhibition of the photosynthesis process (Table 4). A particularly negative influence was recorded in plants exposed to drought for the first time in the “flowering – pods formation” phase.
In the control (untreated) plants exposed to a second cycle of drought, the process of carbon dioxide assimilation was preserved at a higher level by 18.2% compared to the plants exposed to the action of water stress for the first time at the “flowering – pods formation” phase. In twice-stressed and pre-treated plants, stomatal conductance and photosynthesis intensity decreased by 1.27 and 1.65 times, respectively, while in plants exposed to the first stress at the “flowering – pods formation” phase, stomatal conductance and photosynthesis intensity suffered an impact of 1.73 and 1.95 times, respectively. The transpiration intensity was 54.8 and 60.45% of the value of the process recorded in the control plants under an optimal humidity background.
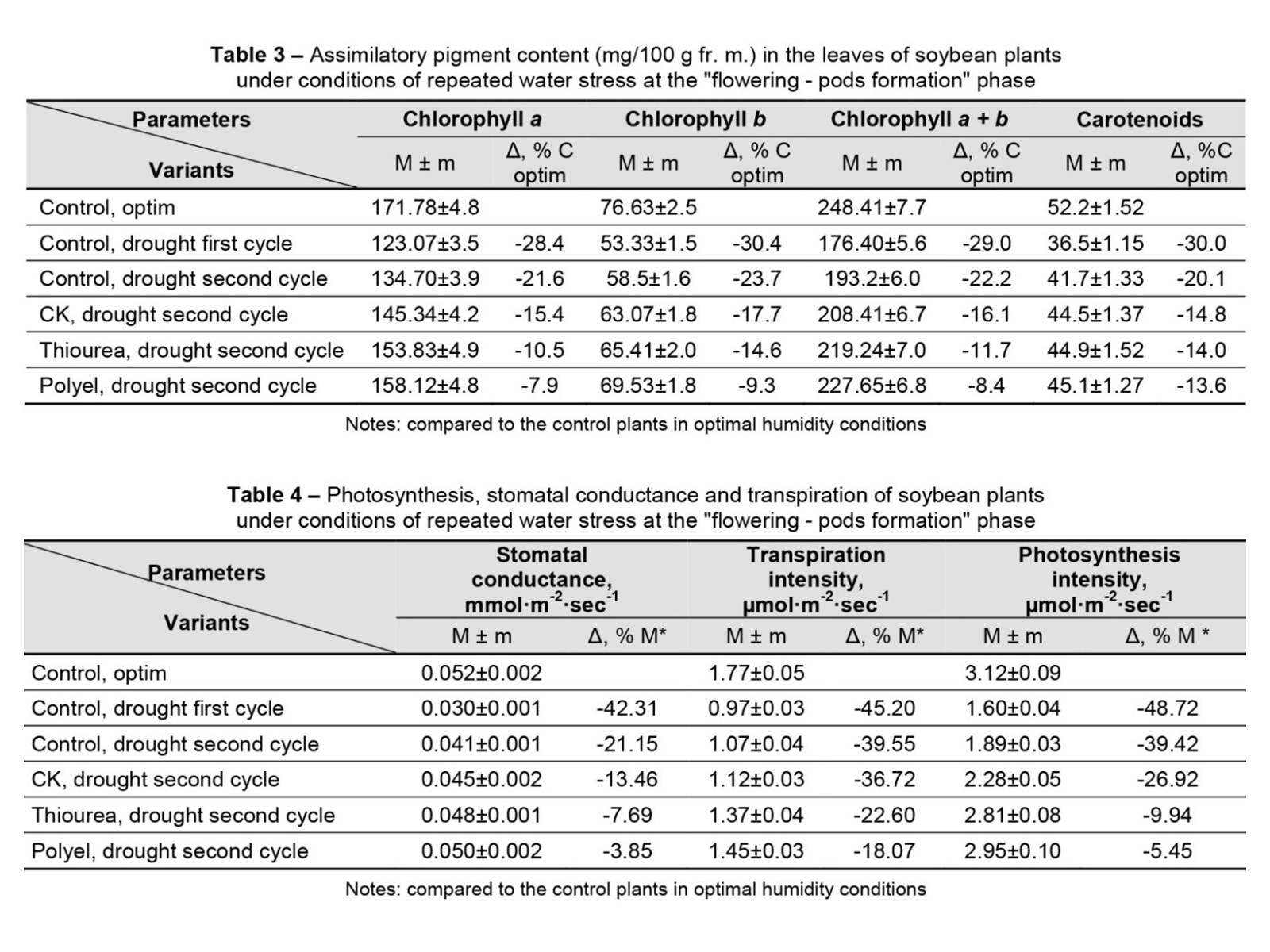
Growth maintenance under conditions of insufficient moisture represents an adaptive reaction associated with the organism’s resistance to drought. In this work, the growth of the plant as a whole was strongly marked by humidity conditions (Figure 1). After 3 days of moderate moisture deficiency (40% TWC), the growth speed of these plants was reduced by 37.9% compared to the growth speed of control plants under an optimal moisture background. The impact produced by drought on the pre-treated plants manifested itself in a reduction of the diurnal growth rate by 28.4, 19.8 and 13.12% (Figure 1).
The improvement of humidity conditions ensured a significant intensification of the growth processes of the plants preventively exposed to water stress (Figure 1 and Figure 2). It should be noted that the ability to restore growth processes in plants pre-treated with the investigated physiologically active substances (PHAS) manifests itself significantly better than in untreated plants.
On the fourth day after restoring optimal levels of soil moisture, the growth rate of untreated plants was 32.64%, those pre-treated with cytokinin – 30.48% and those treated with thiourea and Polyel – with 22.23 and 8.35%, respectively lower than in control plants (Figure 2). Plants pre-treated with this preparation restored their growth processes more quickly. After 10 days under improved levels of soil moisture, the growth rate of these plants was restored to the values of the control plants, which were not exposed to drought. The growth rate of untreated plants remained inhibited even after 10 days of moisture improvement. In the current investigation, a tendency to accelerate the growth speed of plants exposed to the action of a new cycle of drought at the “flowering – pods formation” phase compared to plants exposed for the first time to drought at this phase of development was recorded (Figure 3).
The degree of inhibition of the growth rate of plants exposed to the first cycle of drought at the “flowering – pods formation” phase was 37.4–54.9%. The growth speed of plants repeatedly exposed to drought was 35.6–54.1% higher compared to that of control plants. Repeated drought had a significantly smaller impact on the degree of change in the growth rate of plants pre-treated with CK, Thiourea and Polyel. In these plants, the ability to restore growth processes was mean significantly higher than in the untreated plants with the studied compounds. The results of the investigations led to the conclusion that CK, Thiourea and Polyel had the effect of intensifying the formation of stress-memory and increasing the tolerance of plants to repeated drought conditions caused by the fluctuation of soil moisture, which was manifested by maintaining growth processes at a higher level compared to untreated plants.
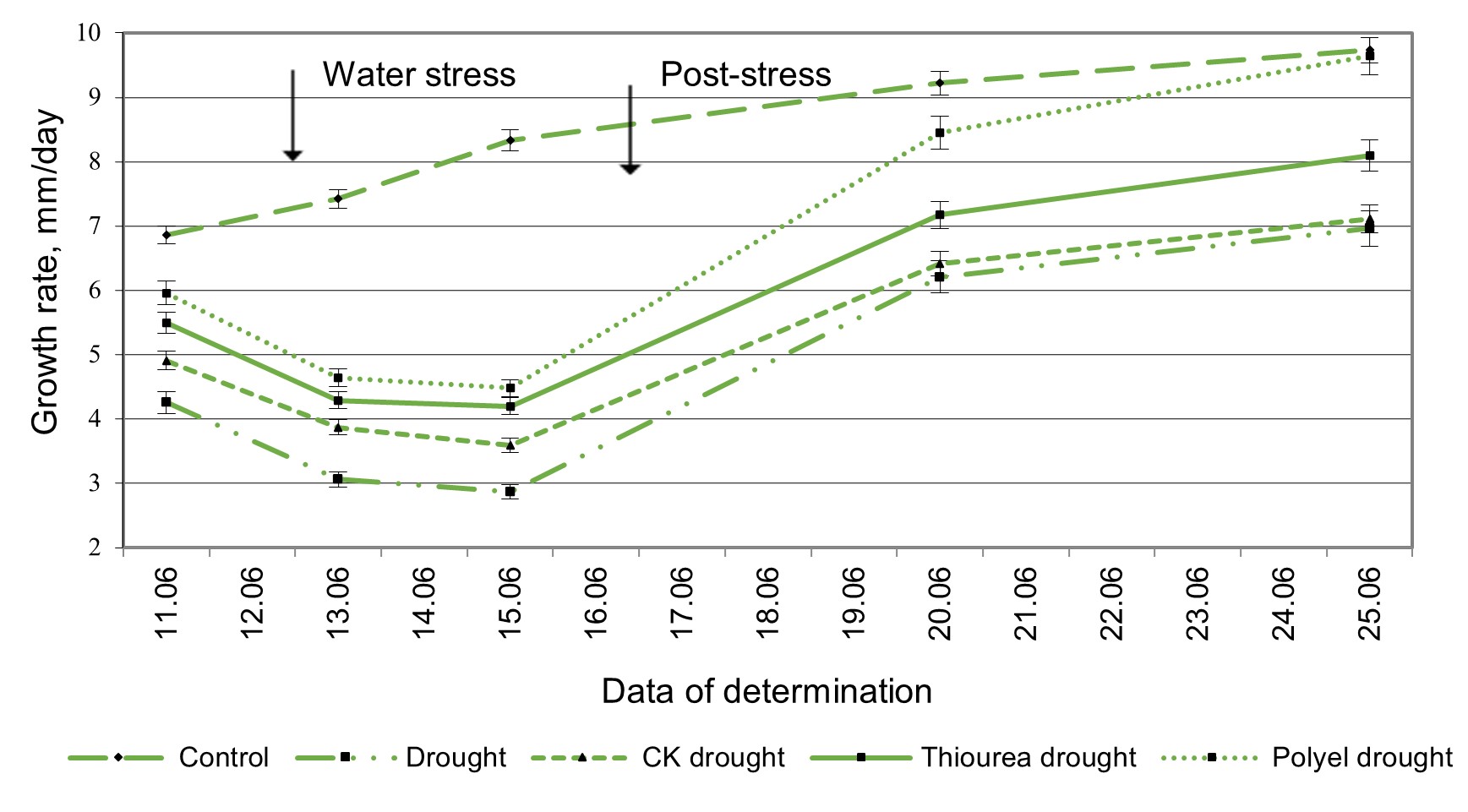
Figure 1 – The growth dynamics of soybean plants, cv. Moldovitsa, pre-treated with cytokinin-type compounds, and exposed to moderate drought at the “first trifoliate leaf” phase
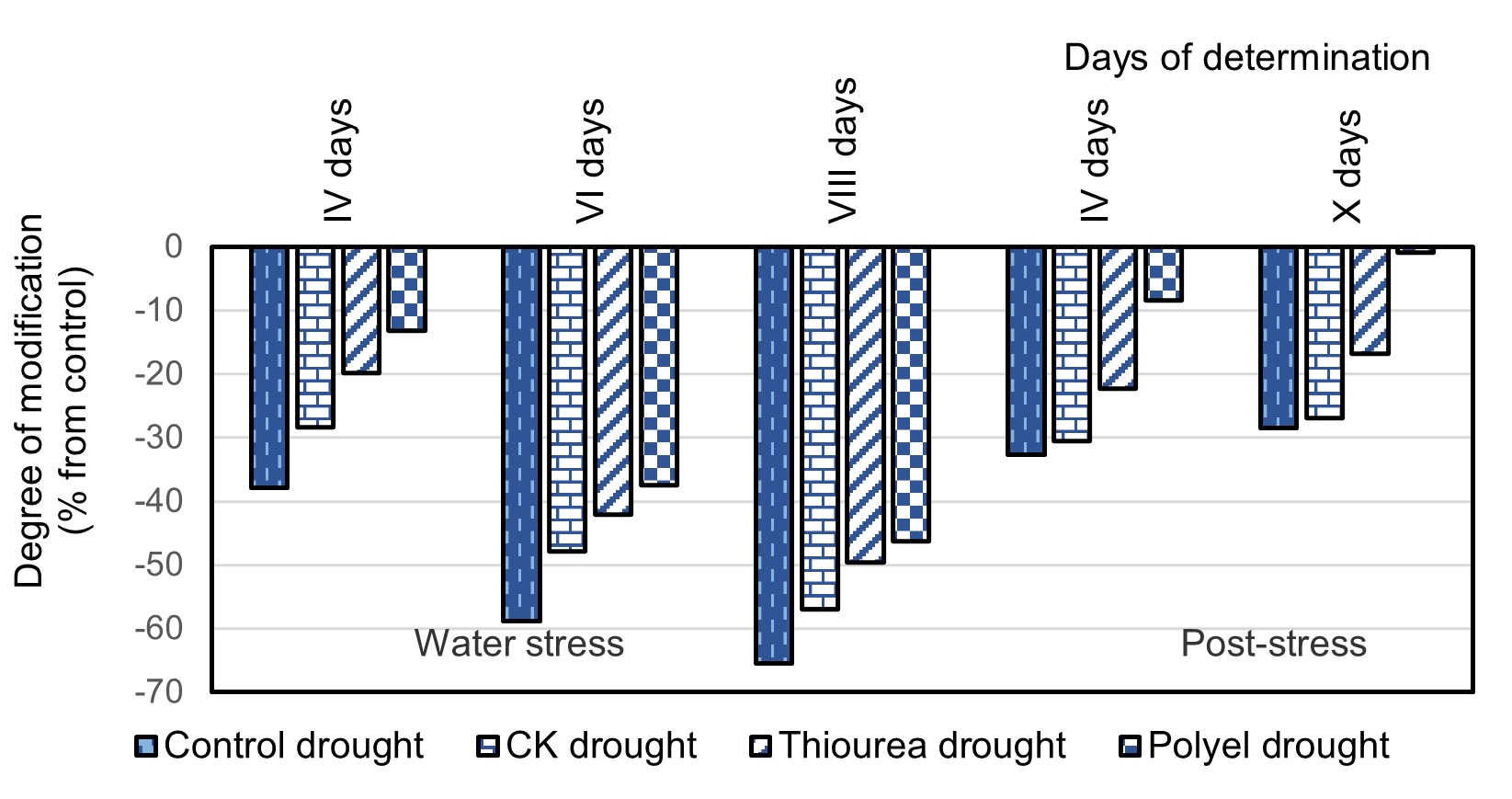
Figure 2 – The degree of modification (% from control) of the plant growth rate of Glycine max (Merr) L. variety Nadejda pre-treated with cytokinin-type compounds and exposed to the moderate drought at the “first trifoliate leaf” phase
Figure 3 – The growth dynamics of soybean plants pre-treated with cytokinin-type compounds and exposed to moderate drought at the “flowering – pods formation” phase
DISCUSSION
Resistance is a genetically determined hereditary trait that does not manifest itself under optimal conditions but is expressed only when humidity and temperature reach a certain threshold level. Resistance as a characteristic is formed in the first stages of leaf development and its increase coincides in time with the period of leaf growth through cell division. As mentioned previously, the change in water status and accelerated generation of ROSs is one of the common primary responses to different stress factors (Asada, 2006; Bartoli et al., 1999; Foyer and Shigeoka, 2011; Kramer and Boyer, 1995; Shinozaki and Yamaguchi-Shinozaki, 1999; Ştefîrţă, 2020). On the one hand, ROSs serve as signalling molecules and activators of protection systems; on the other hand, dehydration and ROSs cause chlorophyll degradation, peroxidic oxidation of membrane phospholipids, chloroplast destruction and reduction of the carbon fixation rate with a direct impact on plant growth and productivity. The degree of change in the assimilatory pigment content under the influence of repeated stress confirms the conclusion about the formation/existence of stress memory in plants. The impact of the second drought cycle at the “flowering –pods formation” phase was truly weaker compared to the degree of decrease in the content of assimilatory pigments in plants exposed to drought of the same duration and intensity for the first time at this phase of development (Table 1). The chlorophyll content in the leaves of plants exposed to repeated stress (70-40-70-40% TWC) was maintained at a higher level compared to the pigment content in the leaves of plants exposed to drought for the first time (70-40% TWC). In the Magia variety, the changes in the pigment content in the leaves were more significant compared to the plants of the Moldovitsa variety (Table 1), which demonstrates a less pronounced manifestation of stress memory and a less significant adaptation effect.
The destruction of chloroplasts and the significant reduction of assimilation pigments inevitably led to the inhibition of the photosynthesis process (Table 2). Repeated drought caused changes in the photosynthesis process of the same characteristic but was quantitatively less significant compared to carbon assimilation in plants exposed to stress for the first time during generative development. Changes in photosynthesis have been highlighted in several studies regarding water stress-induced memory (Leufen et al., 2020). Kim et al. (2020) demonstrated that a single incidence of water stress at the initial stages of ontogeny in Glycine max plants can improve the plant’s response to subsequent stress by mitigating its impact on photosynthesis, thus sustaining the state of carbon assimilation at a higher level.
Therefore, in plants that have endured moderate stress at the beginning of vegetation, a “stress memory” is formed – the ability to react adequately to subsequent stress. The first incidence of stress leaves an imprint that influences the plant’s response to the subsequent occurrence of unfavourable conditions. The dependence of stress memory formation on genotype-specific characteristics is specified.
Tissue dehydration under drought conditions greatly affects stomatal conductance and constrains gas exchange and plant growth (Scoffoni et al., 2012). Stomatal closure reduces water loss through transpiration but inevitably leads to the deceleration of photosynthesis because of the chlorophyll content decline and the access of carbon dioxide at the chloroplast level. Some of these reactions are directly induced by changes in the water status of the cells, while others are conditioned by changes in endogenous phytohormone content. A lack of moisture inevitably affects the transport of phytohormones through the phloem and xylem and disrupts the growth and development processes. Dehydration conditions the decrease in phytohormone levels of the growth stimulator class, namely indolyl acetic acid (IAA), gibberellin (GA), and cytokinin (CK), and the accumulation of “stress hormones”, namely ethylene (ET) and abscisic acid (ABA) (Dobra et al., 2010). Adverse conditions of the external environment, especially drought, inhibit the delivery of CK from roots to aerial organs. As a result, shoot growth is reduced, and the leaves fall prematurely. CK influences a wide spectrum of physiological and biochemical processes and has a positive effect on the production process: formation and operation of the photosynthetic apparatus, transport and distribution of assimilates, growth, development, storage of reserve substances, etc. (Davies et al., 2005). Together with the water flow, cytokinins are also transferred from roots to shoots due to which the stomata’s state, and, consequently, water consumption, the process of assimilation of carbon dioxide, as well as the growth by elongation of cells, were regulated. Cytokinins increase their water attraction capacity towards leaves and growing organs.
Their action is explained by the phytohormones’ ability to open the stomata’s osteols, intensify transpiration, and stimulate growth processes and biosynthesis in cells. It has been demonstrated that the exogenous use of cytokinins restrains the premature senescence of leaves and intensifies photosynthesis and plant growth under drought conditions (Ștefîrță et al., 2021). Stress memory and faster and more efficient plant responses to subsequent stress can be induced in the absence of primary stress by the application of phytohormones, of abscisic (ABA), jasmonic (JA), salicylic (SA) and beta aminobutyric acids (Aimar et al., 2011; Fleta-Soriano et al., 2015; Fleta-Soriano and Munné-Bosch, 2016; Liu et al., 2022; Ştefîrţă et al., 2021). Phytohormones have been shown to be a dominant factor in mediating plant adaptation and stress memory formation. Plant resilience to recurrent droughts increased under the influence of substances having cytokinin activity.
Plant pre-treatment with solutions of cytokinin-type compounds diminishes the negative impact of water stress and amplifies the formation/activation of plant stress memory under recurrent drought. Plants previously stressed and pre-treated with CK, Tu and Polyel endured repeated stress significantly better than untreated plants. The large gap between the decrease in photosynthetic pigment content and inhibition of photosynthesis demonstrates that strong inhibition of carbon dioxide assimilation in leaves under conditions of moisture deficit is a result not only of the decrease in the pigment content but also of the reduction in stomatal conductance and leaf dehydration. Stomatal closure reduces water loss through transpiration, but it inevitably leads to a reduction in photosynthesis because of the decrease in the content of chlorophyll and the access of carbon dioxide at the chloroplast level. Significantly smaller changes were recorded in plants pre-treated with cytokinin, thiourea and, especially, Polyel.
In summary, the information in the specialised literature and the results of our own investigations show that the mechanisms coupled with self-regulation of water homeostasis, formation and neutralisation of ROSs, and intensification/stabilisation of the synthesis of compounds with regulatory and protective functions are correlated with the formation of plant stress memory and have tangents to the regulation of morphogenesis, growth and development. The effect of co-activation of the stress memory formation potential under repeated drought of Glycine max (Merr.) L. plants were recorded using cytokinin, thiourea and, especially, the complex preparation of Polyel. The tolerance-inducing effect manifests itself by maintaining the content of assimilatory pigments, photosynthesis and growth processes at a mean significantly veridical higher level. After the restoration of the optimal moisture background, plants pre-treated with CK, Tu and, especially, the preparation Polyel, which endured moderate stress in the initial stages of ontogenesis, restored their functional processes. The information obtained in the work certainly opens the management perspective of the ability to form stress memory, adaptation and tolerance of plants to the unfavourable fluctuation of humidity and recurrent drought.
CONCLUSIONS
Plant exposure to moderate moisture deficit, as well as pre-treatment with cytokinin-type compounds at the initial stages of ontogenesis, induces the formation of stress memory, which ensures a more adequate reaction and tolerance to the repeated occurrence of drought at the stages of generative development.
Stress memory and plant adaptation to fluctuating humidity and recurrent drought involve adjusting the content of assimilatory pigments, photosynthesis and plant growth.
Pre-treatment of plants with cytokinin-type compounds significantly reduced the harmful effects of recurrent drought on photosynthesis, growth and biomass accumulation both during the action of the repeated stress factor and in post action after the improvement of moisture conditions. Moderate and short-term water stress also lessens the harmful effects of repeated drought conditions.
After restoring the optimal moisture background, plants pre-treated with CK, Tu and, especially, with Polyel preparation, which endured moderate stress in the initial stages of ontogenesis, had restored their functional processes more thoroughly.
Plant exposure to moderate water deficit, as well as pre-treatment with cytokinin-type compounds at the initial stages of ontogenesis, present options for stress memory management, adaptation and tolerance of plants to conditions of unfavourable moisture fluctuations and recurrent drought.
Author Contributions: conceptualisation (A.Ș.); Polyel synthesis (I.B., E.C., M.C.); methodology (A.Ș., I.B.); investigation and analysis (A.Ș., L.B.); data curation (A.Ș.); writing A.Ș; review (A.Ș., L.B., I.B.). All authors declare that they have read and approved the publication of the manuscript in the present form.
Acknowledgement: The research was carried out within the projects of State Programme 20.80009.5007.28 “Elaboration of new multifunctional materials and efficient technologies for agriculture, medicine, technics and educational system based on the “s” and “d” metals complexes with polydentate ligands” and 20.80009.7007.16 “Synergism between natural factors and microbiological, ecologically harmless means of regulating the density of populations of harmful organisms for the protection of agricultural crops in conventional and ecological agriculture”, financed by the National Agency for Research and Development of Republic of Moldova.
Conflicts of Interest: The authors declare the absence of conflicts of interest.
REFERENCES
Abid, M.; Shao, Y.; Liu, S.; Wang, F.; Gao, J.; Jiang, D.; Tian, Z.; Dai, T. Pre-drought priming sustains grain de-velopment under post-anthesis drought stress by regulating the growth hormones in winter wheat (Triticum aes-tivum L.). Planta. 2017, 246, 509-524. https://doi.org/10.1007/s00425-017-2698-4.
Aimar, D.; Calafat, M.; Andrade, A.; Carassay, L.; Abdala, G.I.; Molas, M.L. Drought Tolerance and Stress Hormones: From Model Organisms to Forage Crops. Plants and Environment. 2011, 137-164. https://doi.org/10.5772/24279.
Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplast and Their Functions. Plant Physiology. 2006,141, 391-396. https://doi.org/10.1104/pp.106.082040.
Bartoli, C.G.; Simontacchi, M.; Tambussi, E.; Beltrano, J.; Montaldi, E.; Puntarulo, S. Drought and water-ing-dependent oxidative stress: effect on antioxidant content in Triticum aestivum L. leaves. Journal of Experimental Botany. 1999, 50, 375-383. https://doi.org/10.1093/jxb/50.332.375.
Bartoli, C.G.; Casalongué, C.A.; Simontacchia, M.; Marquez-Garcia, B.; Foyer, C.H. Interactions between hor-mone and redox signaling pathways in the control of growth and cross tolerance to stress. Journal of Experimental Botany. 2013, 94, 73-88. https://doi.org/10.1016/j.envexpbot.2012.05.003.
Bhattacharjee, S. Reactive oxygen species and oxidative burst: role in stress, senescence and signal transduction in plants. Current Science. 2005, 89, 1113-1121.
Bhattacharjee, S. The Language of Reactive Oxygen Species Signaling in Plants. Journal of Experimental Botany. 2012, 985298. http://dx.doi.org/10.1155/2012/985298.
Bruce, T.J.A.; Matthes, M.C.; Napier, J.A.; Pickett, J.A.T. Stressful memories of plants: evidence and possible mechanisms. Plant Science. 2007, 173, 603-608. https://doi.org/10.1016/j.plantsci.2007.09.002.
Chaves, M.; Maroco, J.; Pereira, J. Understating plant response to drought: from genes to the whole plant. Functional Plant Biology. 2003, 30, 239-264. http://dx.doi.org/10.1071/FP02076.
IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [H.-O. Pörtner, D.C. Roberts, M. Tignor, et al. (eds.)]. Cambridge University Press. Cambridge University Press, Cambridge, UK and New York, NY, USA, 2022, 3056 pp. 10.1017/9781009325844.
Davies, W.; Kudoyarova, G.; Hartung, W. Long-distance ABA signaling and relation to other signaling pathways in the detection of soil drying and the mediation of the plant’s responses to drought. Journal of Plant Growth Regulation. 2005, 24, 285-295. http://dx.doi.org/10.1007/s00344-005-0103-1.
Ding, Y.; Fromm, M.; Avramova, Z. Multiple exposures to drought train transcriptional responses in Arabidopsis. Nature Communications. 2012, 3, 740. https://doi.org/10.1038/ncomms1732.
Dobra, P.; Motyka, V.; Dobrev, P. et al. Comparison of hormonal response to heat, drought and combined stress in tobacco plants with elevated proline content. Journal Plant Physiology. 2010, 167, 1360-1370. https://doi.org/10.1016/j.jplph.2010.05.013.
FAO. FAO Strategy on Climate Change 2022–2031. Food and Agriculture Organization of the United Nations, Rome. https://www.fao.org/3/cc2274en/cc2274en.pdf (accesed on 20 December 2022).
Fleta-Soriano, E.; Pintó-Marijuan, M.; Munné-Bosch, S. Evidence of drought stress memory in the facultative CAM, Aptenia cordifolia: possible role of phytohormones. PLoS One. 2015, 10, e0135391. https://doi.org/10.1371/journal.pone.0135391.
Fleta-Soriano, E.; Munné-Bosch, S. Stress Memory and the Inevitable Effects of Drought: A Physiological Pers-pective. Frontiers in Plant Science. 2016, 7. https://doi.org/10.3389/fpls.2016.00143.
Foyer, C.H.; Shigeoka, S. Understanding Oxidative Stress and Antioxidant Functions to Enhance Photosynthesis. Plant Physiology. 2011, 155, 93-100. https://doi.org/10.1104/pp.110.166181.
Jacques, C.; Salon, Ch.; Romain, L.; Vernoud, V.; Prudent, M. Drought Stress Memory at the Plant Cycle Level: A Review. Plants. 2021, 10, 1873. https://doi.org/10.3390/plants10091873.
Kalapos, T.; van den Boogaard, R.; Lambers, H. Effect of soil drying on growth, biomass allocation and leaf gas exchange of two annual grass species. Plant and Soil. 1996, 185, 137-149. http://dx.doi.org/10.1007/BF02257570.
Kim, Y.K.; Chae, S.; Oh N-L.; Hoai, N.; Cheong, J.-J. Recurrent drought conditions enhance the induction of drought stress memory genes in Glycine max L. Front. Genet. 2020, 11. https://doi.org/10.3389/fgene.2020.576086.
Kinoshita, T.; Seki, M. Epigenetic memory for stress response and adaptation in plants. Plant and Cell Physiology. 2014, 55, 1859-1863. https://doi.org/10.1093/pcp/pcu125.
Kramer, P.J.; Boyer, J.S. Water relations of plant and soil. San Diego: Academic Press, 1995, 489.
Leufen, G.; Noga, G.; Hunsche, M. Drought Stress Memory in Sugar Beet: Mismatch Between Biochemical and Physiological Parameters. Journal of Plant Growth Regulation. 2016, 35, 680-689. https://doi.org/10.1007/s00344-016-9571-8.
Levitt, J. Recovery of turgor by wilted, excised cabbage leaves in the absence of water uptake: a new factor in drought acclimation. Plant Physiology. 1986, 82, 147-153. https://doi.org/10.1104/pp.82.1.147.
Liu, H.; Able, A.J.; Jason, A.A. Priming crops for the future: rewiring stress-memory. Trends in Plant Science. 2022, 27, 699-716. https://doi.org/10.1016/j.tplants.2021.11.015.
Munne-Bosch, S.; Queval, G.; Foyer, Ch. The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiology. 2013, 161, 5-19. https://doi.org/10.1104/pp.112.205690.
Perveen A.; Wahid A.; Mahmood, S.; Hussain I.; Rasheed, R. Possible mechanism of root-applied thiourea in improving growth, gas exchange and photosynthetic pigments in cadmium stressed maize (Zea mays). Brazilian Journal of Botany. 2015a, 38, 71-79. http://dx.doi.org/10.1007/s40415-014-0124-8.
Perveen, S; Farooq, R.; Shahbaz, M. Thiourea-induced metabolic changes in two mung bean [Vigna radiata (L.) Wilczek] (Fabaceae) varieties under salt stress. Brazilian Journal of Botany. 2015b, 39, 41-54. http://dx.doi.org/10.1007/s40415-015-0209-z.
Scoffoni, Ch.; NcKown, A.D.; Rawls, M.; Sack, L. Dynamics of leaf hydraulic conductance with water status: quantification and analysis of species differences under steady state. Journal of Experimental Botany. 2012, 63, 643-658. http://dx.doi.org/10.1093/jxb/err270.
Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular Responses to Cold, Drought Heat and Salt Stress in Higher plants. Editor: Landes Bioscience, Austin, Texas. U.S.A, 1999, 178.
Ştefîrţă, A.; Brînză, L.; Bulhac, I.; Coropceanu, E.; Buceaceaia, S.; Ionaşcu, A.; Covaci, O. Cultivation process of crop plants (in Romanian). Patent of invention MD 1348 Z 2020.02.29.
Ştefîrţă, A.; Bulhac, I.; Coropceanu, E.; Brînză, L. Polyel – compound with antioxidant properties. Journal of Applied Life Sciences and Environment. 2021, 54, 146-155. https://doi.org/10.46909/journalalse-2021-014.
Wahid, A.; Basra, S.M.A.; Farooq, M. Thiourea: a molecule with immense biological significance for plants. International Journal of Agriculture and Biology. 2017, 19, 911-920. https://doi:10.17957/IJAB/15.0464.
Walter, J.; Nagy, L.; Hein, R.; Rascher, U.; Beierkuhlein, C.; Willner, E.; Jentsch, A. Do plants remember drought? Hints towards a drought-memory in grasses. Environmental and Experimental Botany. 2011, 71, 34-40. https://doi.org/10.1016/j.envexpbot.2010.10.020.
Academic Editor: Dr. Iuliana Motrescu
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Brînză Lilia, Bulhac Ion, Cocu Maria, Coropceanu Eduard, Ștefîrță Anastasia, Voloșciuc Leonid