Constantin Spataru, Mihaela-Claudia Spataru, Ozan Gündemir
ABSTRACT. In terrestrial species, body propulsion is mostly performed via the pelvic limbs. In semiaquatic species, both pairs of limbs are used in swimming and diving, whereas in arboreal species, the pelvic limbs are used to maintain body stability. Thus, in squirrels, the synsarcosis muscles participate in body propulsion during climbing, as they have well-developed muscular bellies. Among these, the pectoral transverse muscle, which originates along the entire sternum and is inserted on the humeral crest, stands out for its width. The cervical parts of the trapezius and rhomboideus muscles are reduced and their thoracic parts more developed. As a result, muscles such as the occipitoscapularis or atlantoscapularis coordinate forelimb protraction and neck displacement. The serratus ventralis muscle is very well developed and clearly divided into cranial (cervical) and caudal (thoracic) parts; it produces a strong adduction of the thoracic limbs when the parts contract, and when they relax, a large abduction of the forelimbs is produced, enlarging the body size during jumping.
Keywords: rodent; squirrel; muscle; scapula; flexion.
Cite
ALSE and ACS Style
Spataru, C.; Spataru, M.-C.; Gündemir, O. The morpho-functional peculiarities of the synsarcosis muscles in red squirrels. Journal of Applied Life Sciences and Environment 2022, 55(1), 85-91.
https://doi.org/10.46909/alse-551048
AMA Style
Spataru C, Spataru M-C, Gündemir O. The morpho-functional peculiarities of the synsarcosis muscles in red squirrels. Journal of Applied Life Sciences and Environment. 2022; 55(1): 85-91.
https://doi.org/10.46909/alse-551048
Chicago/Turabian Style
Spataru, Constantin, Mihaela-Claudia Spataru, and Ozan GÜNDEMIR. 2022. “The morpho-functional peculiarities of the synsarcosis muscles in red squirrels” Journal of Applied Life Sciences and Environment 55, no. 1: 85-91.
https://doi.org/10.46909/alse-551048
View full article (HTML)
The Morpho-Functional Peculiarities of the Synsarcosis Muscles in Red Squirrels
Constantin SPATARU1a, Mihaela-Claudia SPATARU1b* and Ozan GÜNDEMIR2
1 Iasi University of Life Sciences, Faculty of Veterinary Medicine, 8, Mihail Sadoveanu Alley, 700489, Iasi, Romania;
a Department of Preclinics, e-mail: cspataru@uaiasi.ro; b Department of Public Health;
2 Istanbul University-Cerrahpașa, Faculty of Veterinary Medicine, Department of Anatomy e-mail: ozan_gundemir@hotmail.com
*Correspondence: mspatarufmv@yahoo.com
Received: Sep. 19, 2022. Revised: Nov. 29, 2022. Accepted: Dec. 05, 2022. Published online: Dec. 14, 2022
ABSTRACT. In terrestrial species, body propulsion is mostly performed via the pelvic limbs. In semiaquatic species, both pairs of limbs are used in swimming and diving, whereas in arboreal species, the pelvic limbs are used to maintain body stability. Thus, in squirrels, the synsarcosis muscles participate in body propulsion during climbing, as they have well-developed muscular bellies. Among these, the pectoral transverse muscle, which originates along the entire sternum and is inserted on the humeral crest, stands out for its width. The cervical parts of the trapezius and rhomboideus muscles are reduced and their thoracic parts more developed. As a result, muscles such as the occipitoscapularis or atlantoscapularis coordinate forelimb protraction and neck displacement. The serratus ventralis muscle is very well developed and clearly divided into cranial (cervical) and caudal (thoracic) parts; it produces a strong adduction of the thoracic limbs when the parts contract, and when they relax, a large abduction of the forelimbs is produced, enlarging the body size during jumping.
Keywords: rodent; squirrel; muscle; scapula; flexion.
INTRODUCTION
Squirrels are rodents adapted to arboreal displacement (Emmons, 1980), moving by climbing or leaping (Gupta, 1966). The red squirrel (Sciurus vulgaris) is an arboreal, primarily herbivorous rodent that is ordinarily found throughout Europe and Asia (Harrison et al., 2003; Rézouki et al., 2014; Lurz et al., 2015). Through the peculiarities of their muscles and the osteo-ligamental system, the forelegs ensure the support and stability of the body in terrestrial species and propulsion in aquatic and arboreal species (Emmons, 1980; Emry and Korth, 1996). In the terrestrial environment, gravity is an important factor in muscle dynamics, in contrast to the aquatic one, where buoyancy reduces the effects of gravity (Gillis and Blob, 2001). For terrestrial species, in addition to gravity, there are other loads, such as support of the body weight and body displacement, all of which determine the intensification of muscle activity to ensure the correct joint angles (Preuschoft, 2002; Schmidt and Fischer, 2011).
Research concerning the development of the thoracic limb muscles in squirrels underlines the peculiarities concerning its way of life and movement. The forelegs are attached to the trunk by the clavicle and by muscles (synsarcosis) in species where the clavicles are well developed and only by muscles in species where the clavicles are rudimentary (Stein, 1986). The force acting in movement is directly proportional to the development of their musculature (Preuschoft, 2002; Schmidt and Fisher, 2011).
MATERIALS AND METHODS
Studies were based on dissections of 11 adult squirrel corpses (8 males and 3 females), whose deaths resulted from car or hunting accidents that occurred in city parks and leaf-bearing forests of the Iasi district. The goal of the dissections was to emphasise the muscles, as well as the shape and orientation of fibres in the muscular bellies. By identifying the insertions and the development of the bone eminences, as well as the position that they occupy related to each joint, we could establish the role of each muscle in foreleg movement. After stratigraphic dissections, photos were taken in order to describe, discuss and argue (from an anatomical point of view) the morpho-functional aspects of the synsarcosis muscles in squirrels.
RESULTS AND DISCUSSION
In red squirrels, the synsarcosis muscles are represented by muscles with massive bellies that ensure a strong connection for transmitting force from the forelimbs to the trunk (Figures 1, 2 and 4) (Ryan, 1989, Spataru et al., 2010). The 2-cm-long clavicle (Figures 1 and 2), which is shaped like a boomerang, is a useful consolidation and organizational element of the region.
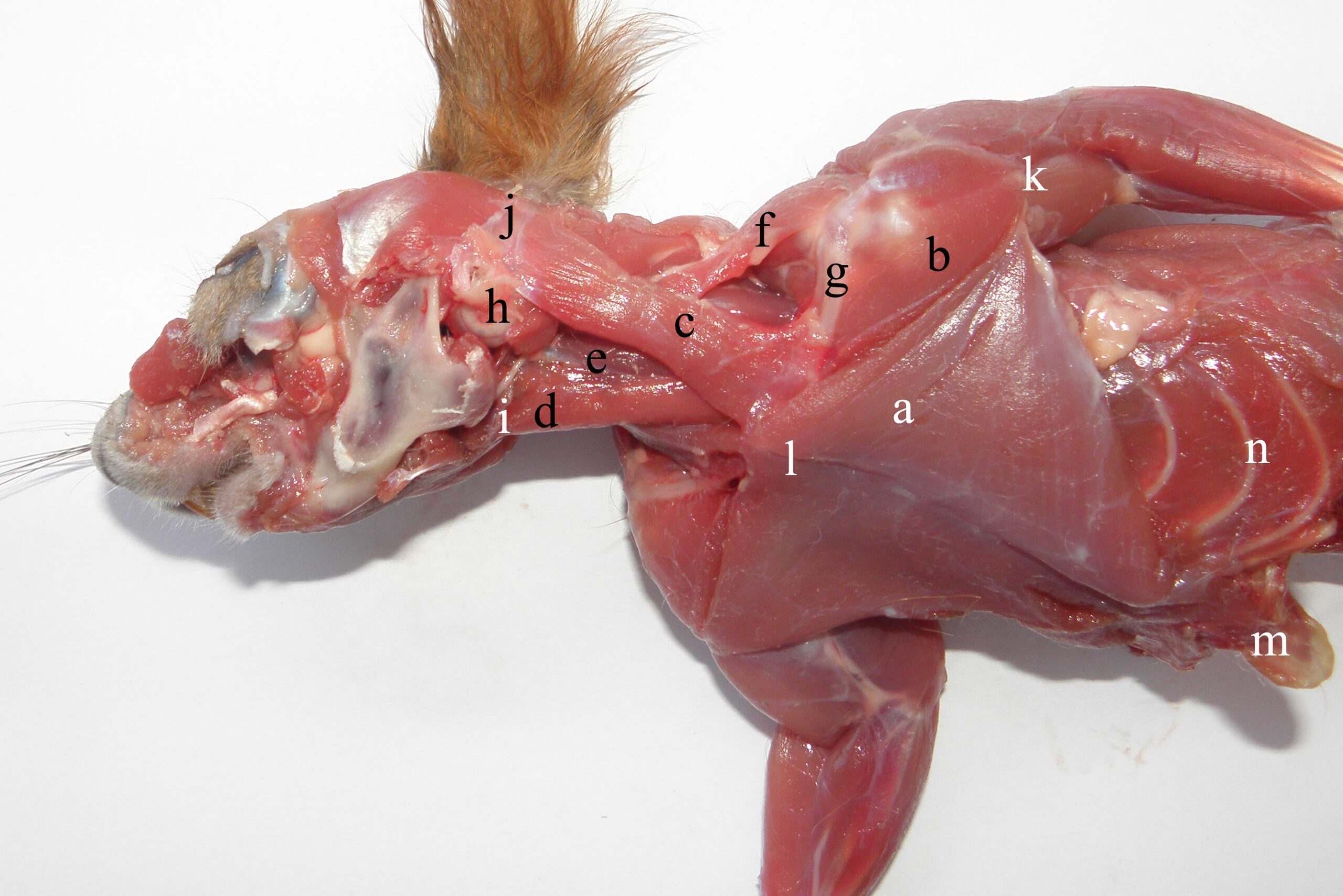
Figure 1 – The ventro-lateral aspect of the synsarcosis muscles in the red squirrel a- m. pectoralis transversus, b- m. deltoideus, pars clavicularis (m. cleidobrachialis), c- m. sternocleidomastoideus, d- m. sternohyoideus, e- m. omohyoideus, f- m. occipitoscapularis, g- clavicula, h- bulla tympanica and processus mastoideus, i- basihyoideum, j- crista occipitalis, k- crista humeralis, l- sternum, m- cartilago xiphoidea, n- arcus costalis
The clavicular insertion of the deltoideus (Figures 1-3) and the sterno-cleido-mastoideus muscle (Figures 1 and 2) affect the contraction of both muscles by acting on forces at this level, used in forelimb protraction (Thorington et al., 1997). Thus, the cleidobrachialis muscle (cleidal part of the deltoid muscle), originating on the humeral crest, has a triangular aspect, acting as an extensor when passing over the shoulder joint, and pulls the clavicle caudally (Figure 1) (Thorington et al., 1997).
The sternocleidomastoideus muscle originates on the sternum tracheal appendix (Figure 1), on the sterno-clavicle jointing capsule and on the cranial edge of the medial half of the clavicle (Figure 1 and Figure 2). Its belly passes latero-dorsally through the cervical region, inserting on the mastoid process (Figure 1 and Figure 4) and the nuchal crest up to the external occipital protuberance (Figure 1). In contraction, it produces flexion and lateral displacement of the neck. Through its cleidal insertion, it coordinates the propulsion and abduction of the foreleg.
The pectoralis transversus muscle is the most developed of the pectorals (Figure 1, Figure 2), originating on the ventral face of the sternum and inserting on the humeral crest (Figure 1 and Figure 2), together with the cleidobrachialis muscle (Figure 1). The triangular form and the insertion on the humeral crest produce adduction of forelegs, which is very useful for making a strong connection with stems and branches during climbing.
As in other rodents and carnivores, the deltoid muscle consists of three parts: scapular (Figure 3 and Figure 4), acromial (Figure 3 and Figure 4) and clavicular (Figure 1, Figure 3 and Figure 4) (Emry and Thorington, 1982). In squirrels, the scapular part is long and strong, originating along the caudal edge of the scapula (Figure 3 and Figure 4). Aided by scapular obliquity, it produces rapid flexion and efficient abduction of the leg. The acromial part is triangular and, together with the scapular part, adheres to the cleido-brachialis muscle, and the two act together as strong shoulder extensors (Spataru et al., 2010).
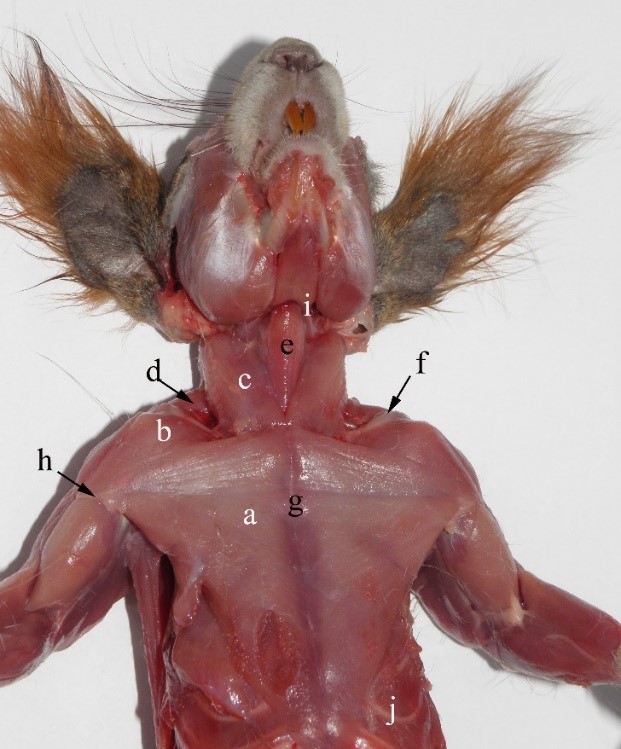
Figure 2 – The ventral muscles of the neck and trunk of the red squirrel a- m. pectoralis transversus, b- m. cleido-brahialis, c – m. sternocleidomastoideus, d- m. cleidotransversus, e- m. sternohyoideus, f- – clavicula, g- sternum, h- crista humeralis, i- bazyhioideum, j- arcus costalis
Together, the parts of the trapezius muscle form a common mass (Stein, 1986) (Figure 3, Figure 4 and Figure 5). The cervical part is reduced and thin, intertwining with the cervical fasciae of the neck and the occipitoscapular muscle (Figure 5) up to the occipital crest (Figure 4). The muscles aid in lateral movements of the head and neck rather than propulsion of the scapula.
The thoracic part (Figure 3 and Figure 5) originates on the spinous processes up to the 10th thorax vertebra (Figure 5), and the muscles are more developed, covering the entire scapular spine (Figure 3). When acting together, they function in protraction of each scapula or in foreleg abduction, in opposition to the transverse pectoral muscle.
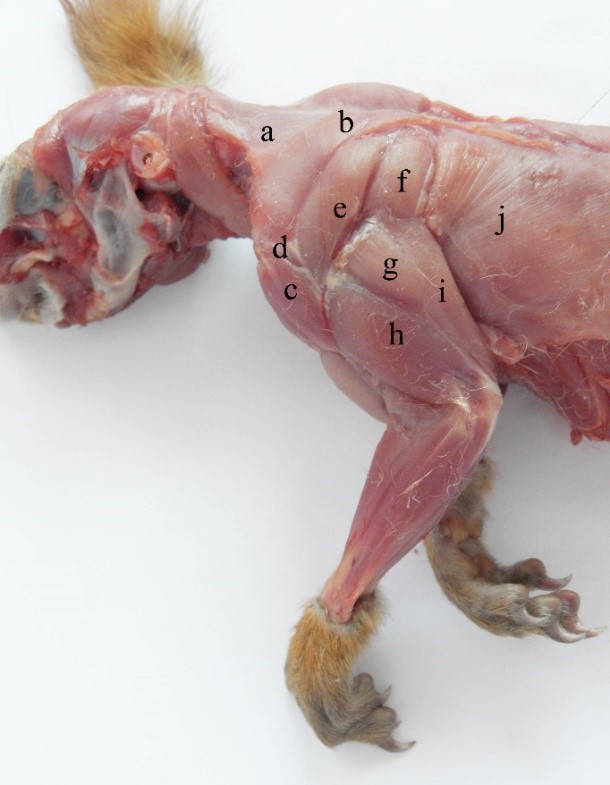
Figure 3 – The superficial muscles of the forelimb in the red squirrel a- m. trapezius pars cervicalis, b- m. trapezius pars thoracica, c- m. deltoideus, pars clavicularis d- m. deltoideus, pars acromialis, e- m. deltoideus, pars scapularis f- m. teres major, g- m. triceps brachii, pars scapularis, h- m. triceps brachii, pars lateralis, i- m. triceps brachii, pars longa, j- m. latissimus dorsi
The occipitoscapularis muscle (Figure 1, Figure 4 and Figure 5) works in concert with the atlantoscapularis ventralis and dorsalis muscles. They have a strip-like shape, inserting on the cranial edge of the processus hamatus and the scapular spine near the insertion of the trapezius muscle (Figure 4). The occipitoscapularis is a relatively powerful muscle: it represents about 7.06% of the extrinsic scapular muscle group (Lagaria and Youlatos, 2006). It contributes to scapular protraction, because of the underdevelopment of the cervical parts of the trapezius and rhomboideus muscles. It passes under the trapezius (Emry and Thorington, Jr, 1982) and the cleido-occipitalis muscle. When acting alone, it coordinates lateral movements of the neck with those of the forelegs (Ryan, 1989). When acting together with the forelimb adductor muscles, it produces body propulsion during climbing by fixing the body to the substrate in vertical displacement (Schmidt and Fisher, 2011). Due to their position on either side of the neck, the muscles induce the extension of the cervical region through their insertion on the transverse processes of the axis of the third and fourth cervical vertebrae (Figure 1).
In red squirrels, the latissimus dorsi muscle (Figure 3 and Figure 4) is a large muscle with a fan-like shape, representing about 20.8% of the extrinsic scapular muscles (Thorington et al., 1997, Lagaria and Youlatos, 2006), and, at the level of the second thoracic vertebra, the muscle passes over the thoracic angle of the scapula. In the lumbar region, the muscle becomes thinner and aponeurotic, inserting on the spinous processes of the lumbar vertebrae and the iliac crest, together with the common mass. The muscle inserts together with the teres major muscle on the distal third of the medial surface of the humerus (according to Lagaria and Youlatos (2006), it occupies about 36.2% of the humerus). From its ventral border, a muscular strip is detached to join the tendon of the biceps brachii muscle. Through contraction, it produces flexion and lateral rotation of the arm and is an efficient humeral retractor. When acting together, these muscles produce propulsion of the body via alternation of the four legs.
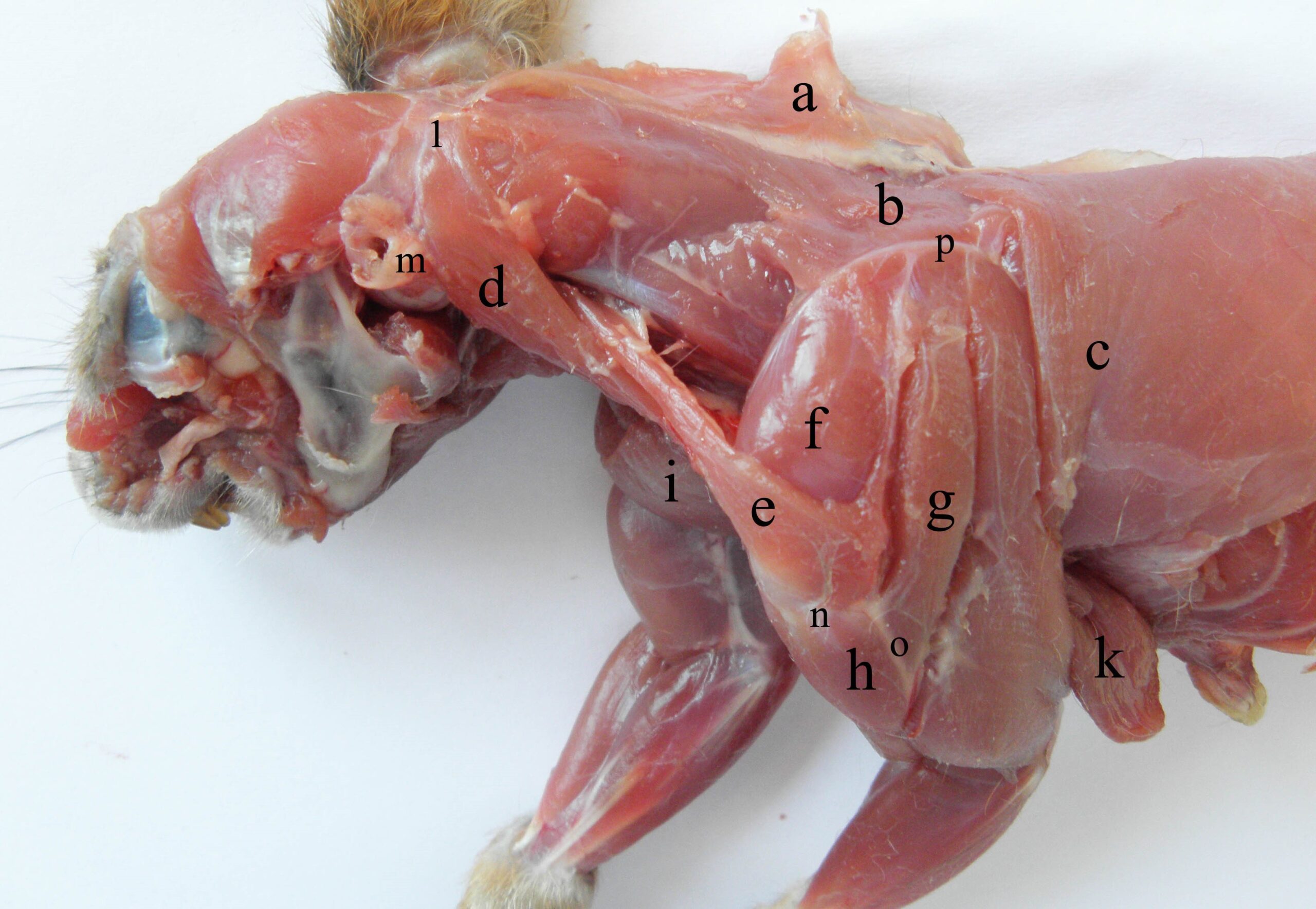
Figure 4 – The lateral muscles of the neck and arm in the red squirrel a- m. trapezius, b- m. rhomboideus pars thoracis, c- m. latissimus dorsi, d- m. sternooccioitalis, e- m. occipitoscapularis, f- m. supraspinatus, g- m. deltoideus, pars scapularis, h- m. deltoideus, pars acromialis, i- m. deltoideus, pars clavicularis, k- m. pectoralis profundus, l- crista occipitalis, m- bulla tympanica et processus mastoideus), n- processus hamatus, o- crista deltoidea, p- margo dorsalis scapulae
The caudal part of the rhomboideus muscle (Figure 4 and Figure 5) is more developed than the cervical part. Both parts of the rhomboideus muscle form a common mass, which inserts on the dorsal edge and on the caudal angle of the scapula (Figure 4) (Ryan, 1989). The fibres originate from the last for transverse processes of the cervical vertebrae up to the lateral part of the first 7–8 ribs.
The serratus ventralis muscle is clearly divided into cranial and caudal parts, originating on the transverse processes of the cervical vertebrae and the ribs and inserting on the dorsal part of the costal face of the scapula, together with the rhomboideus muscle (the serratus ventralis cervicis muscle), and on the medial crest of the scapula (serratus ventralis thoracis) (Figure 5) (Ryan, 1989). Generally, every non-articular prominence develops as a consequence of the traction forces which act on the bone (Swidersky, 1993; Benjamin and Ralphs 2001). So, in the case of red squirrels, the serratus ventralis muscles act to produce strong adduction of the forelimbs, more so than in other species, in the alternating movements of the thoracic limbs with respect to the trunk and to ensure thorax flexibility during respiration or displacement.
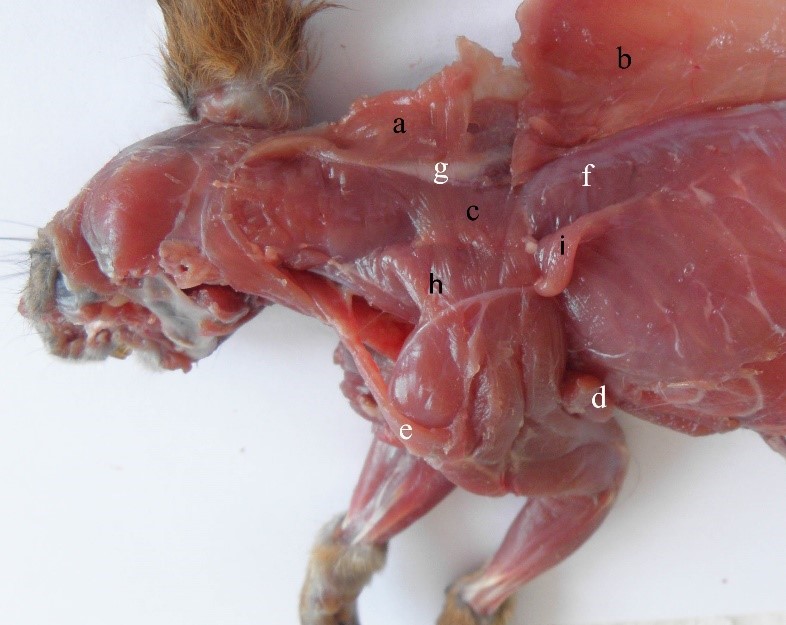
Figure 5 – The deep synsarcosis muscles in the red squirrel a- m. trapezius, pars cervicalis, b- m. trapezius, pars thoracica, c- m. rhomboideus, d- m. latissimus dorsi, e- m. occipitoscapularis, f- m. longissimus dorsi, g- ligamentus nuchae, h- m. serratus ventralis, cervicis, i- m. serratus ventralis thoracis
Relaxation of the long muscular bellies of the serratus ventralis and rhomboid muscles permits abduction of the forelegs to enlarge the body surface, which is useful during leaping (Gupta, 1966). They also have an essential role in consolidating the connection between the trunk and forelegs during propulsion, during both climbing and landing. Through the powerful muscular bellies, the muscles consolidate and disperse the force in a uniform way to the spine, at the same time diminishing the pressure on the spine through their large insertion.
CONCLUSION
In the case of red squirrels, the synsarcosis musculature is represented by muscles that form a tight connection between the body and the thoracic limbs.
The triangular form and the development of the transverse pectoral muscle, as well as their the insertion on the humeral crest, allow the forelegs to be adducted and brought together during climbing. The correlation between the head, spine and forelimb movements is controlled by strong muscles such as the occipitoscapularis, the sternoocipitalis or the serratus ventralis muscles.
Relaxation of the long muscular bellies of the serratus ventralis and rhomboideus muscles permits abduction of the forelegs to enlarge the body surface, which is very useful during leaping.
Author Contributions: Conceptualization, writing original draft preparation, C.S., M.C.S. and O.G. All authors declare that they have read and approved the publication of the manuscript in this form.
Funding: There was no external funding for this study.
Conflicts of Interest: The authors declare that there are no conflicts of interest related to this article.
REFERENCES
Emmons, L.H. Ecology and resource partitioning among nine species of African rain forest squirrels. Ecological Monographs. 1980, 50, 31-54. https://doi.org/10.2307/2937245.
Gupta, B.B. Notes on the gliding mechanism in the flying squirrel. Occasional Papers of the Museum of Zoology, University of Michigan. 1966, 645, 1-7. https://hdl.handle.net/2027.42/57081.
Harrison, R.G.; Bogdanowicz, S.M.; Hoffmann, R.S.; Yensen, E.; Sherman, P.W. Phylogeny and Evolutionary History of the Ground Squirrels (Rodentia: Marmotinae). Journal of Mammalian Evolution. 2003, 10, 249-276. https://doi.org/10.1023/ B:JOMM.0000015105.96065.f0.
Rézouki, C.; Dozières, A.; Le Cœur, C.; Thibault, S.; Pisanu, B.; Chapuis, J-L.; Baudry, E. A Viable Population of the European Red Squirrel in an Urban Park. PLoS ONE. 2014, 9, 1-7. https://doi.org/10.1371/journal.pone.0105111.
Lurz, P.W.W.; Gurnell, J.; Louise Magris, L. “Sciurus vulgaris”. Mammalian Species. 2005, 769, 1-10. https://doi.org/10.1644/1545-1410(2005)769[0001:SV]2.0.CO;2.
Emry, R.; Korth, W.W. A new genus of squirrel (Sciuridae: Rodentia) from the Chadronian of western North America. Journal of Vertebrate Paleontology. 1996, 16, 775-780. https://doi.org/10.1671/0272-4634(2007)27[693:ANGOSR]2.0.CO;2.
Gillis, G.B.; Blob, R.W. How muscles accommodate movement in different physical environments: aquatic vs. terrestrial locomotion in vertebrates. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2001, 131, 61-75. https://doi.org/10.1016/s1095-6433(01)00466-4.
Preuschoft, H. What does “arboreal locomotion” mean exactly and what are the relationships between “climbing”, environment and morphology? Anthropologischer Anzeiger. 2002, 83, 171-188. https://doi.org/10.1127/zma/ 83/2002/171.
Schmidt, A.; Fischer, M.S. The kinematic consequences of locomotion on sloped arboreal substrates in a generalized (Rattus norvegicus) and a specialized (Sciurus vulgaris) rodent. Journal of Experimental Biology. 2011, 214, 2544-2559. https://doi.org/10.1242/jeb.051086.
Stein, B.R. Comparative limb myology of four arvicolid rodent genera (Mammalia, Rodentia), Journal of Morphology. 1986, 187, 321-342. https://doi.org/10.1002/jmor.1051870305.
Ryan, J.M. Comparative myology and phylogenetic systematics of the Heteromyidae (Mammalia, Rodentia), 176. Ann Arbor, Museum of Zoology, University of Michigan, Geneva, New York, 1989, 103. https://deepblue.lib. umich.edu/bitstream/handle/2027.42/56420/MP176.pdf;sequence=1.
Spataru, C.; Spataru, M.C.; Lazăr, M.; Munteanu, A. Morpho-functional peculiarities of the thoracic limb joints at red squirrel (Sciurus vulgaris), Scientific papers – Veterinary Medicine. 2010, 3, 525-528.
Thorington Jr., R.W.; Darrow, K.; Betts, A.D. Comparative myology of the forelimb of squirrels (Sciuridae). Journal of Morphology. 1997, 234, 82-155. https://doi.org/10.1002/(SICI)1097-4687(199711)234:2<155::AID-JMOR4>3.0.CO;2-B.
Emry, R.J.; Thorington Jr, R.W. Descriptive and comparative osteology of the oldest fossil squirrel, Protosciurus (Rodentia: Sciuridae). Smithsonian Contributions to Paleobiology. 1982, 47, 1-35. https://doi.org/10.5479/si.008 10266.47.1.
Lagaria, A.; Youlatos, D. Anatomical Correlates to Scratch Digging in the Forelimb of European Ground Squirrels (Spermophilus citellus), Journal of Mammalogy. 2006, 87, 563-570. https://doi.org/10.1644/05-MAMM-A-251R1.1.
Swidersky, D.L. Morphological evolution of the scapula in tree squirrels, chipmunks, and ground squirrels (Sciuridae): An analysis using thin-plate splines. Evolution. 1993, 47, 1854-1873. https://www.jstor.org/stable/2410226.
Benjamin, M.; Ralphs, J.R. Entheses–the bony attachments of tendons and ligaments. Italian Journal of Anatomy and Embryology. 2001, 106, 151-157.
Academic Editor: Dr. Isabela Simion
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Gündemir Ozan, Spataru Constantin, Spataru Mihaela-Claudia



