Daniel Cocan, Vioara Mireşan, Camelia Răducu, Paul Uiuiu, Alexandru Giurgiu, Tudor Păpuc, Radu Constantinescu, Călin Laţiu
ABSTRACT. In this study, we aimed to highlight the influence of anticoagulants on erythrocyte morphometry in different vertebrate species. Anticoagulants are a category of substances that inhibit blood clotting through various mechanisms. Due to this property, they are used to collect blood samples for a wide range of laboratory tests. The literature mentions that the use of anticoagulants produces morphological changes of erythrocytes, thus influencing results. Blood samples were collected from three warm-blooded vertebrate species (horse, rabbit, and chicken) and one lower vertebrate species with nucleated erythrocytes (fish) in vacutainers with Heparin and EDTA (ethylenediaminetetraacetic acid), in a normal concentration and a double concentration. At the time of harvesting, control smears were performed. In order to be able to compare the effects produced by anticoagulants on the morphology of erythrocytes, they were evaluated morphometrically at intervals of 3, 6, and 24 hrs. after harvest. The following features were evaluated using the Toup View software: length, width, surface and perimeter of erythrocytes for species with anucleated erythrocytes. The same characteristics were evaluated in the nucleus for species with nucleated erythrocytes. The data obtained were processed with statistical programs to highlight changes in erythrocyte morphology produced by anticoagulants.
Keywords: blood cells; length-width ratio; cell surface; shape; vacutainer.
Cite
ALSE and ACS Style
Cocan, D.; Mireşan, V.; Răducu, C.; Uiuiu, P.; Giurgiu, A.; Păpuc, T.; Constantinescu, R.; Laţiu, C. The impact of two anticoagulants on erythrocytes morphology in different vertebrate species. Journal of Applied Life Sciences and Environment 2021, 54(3), 264-272.
https://doi.org/10.46909/journalalse-2021-023
AMA Style
Cocan D, Mireşan V, Răducu C, Uiuiu P, Giurgiu A, Păpuc T, Constantinescu R, Laţiu C. The impact of two anticoagulants on erythrocytes morphology in different vertebrate species. Journal of Applied Life Sciences and Environment. 2021; 54(3): 264-272.
https://doi.org/10.46909/journalalse-2021-023
Chicago/Turabian Style
Cocan, Daniel, Vioara Mireşan, Camelia Răducu, Paul Uiuiu, Alexandru Giurgiu, Tudor Păpuc, Radu Constantinescu, and Călin Laţiu. 2021. “The impact of two anticoagulants on erythrocytes morphology in different vertebrate species” Journal of Applied Life Sciences and Environment 54, no. 3: 264-272.
https://doi.org/10.46909/journalalse-2021-023
View full article (HTML)
The Impact of Two Anticoagulants on Erythrocytes Morphology in Different Vertebrate Species
Daniel Cocan1, Vioara Mireşan1, Camelia Răducu1, Paul Uiuiu1, Alexandru Giurgiu1, Tudor Păpuc1, Radu Constantinescu1, Călin Laţiu1*
1Faculty of Animal Science and Biotechnologies, University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca, Cluj-Napoca, Romania
*E-mail: calin.latiu@usamvcluj.ro
Received: Nov. 26, 2021. Revised: Jan. 10, 2022. Accepted: Jan. 14, 2022. Published online: Jan. 27, 2022
ABSTRACT. In this study, we aimed to highlight the influence of anticoagulants on erythrocyte morphometry in different vertebrate species. Anticoagulants are a category of substances that inhibit blood clotting through various mechanisms. Due to this property, they are used to collect blood samples for a wide range of laboratory tests. The literature mentions that the use of anticoagulants produces morphological changes of erythrocytes, thus influencing results. Blood samples were collected from three warm-blooded vertebrate species (horse, rabbit, and chicken) and one lower vertebrate species with nucleated erythrocytes (fish) in vacutainers with Heparin and EDTA (ethylenediaminetetraacetic acid), in a normal concentration and a double concentration. At the time of harvesting, control smears were performed. In order to be able to compare the effects produced by anticoagulants on the morphology of erythrocytes, they were evaluated morphometrically at intervals of 3, 6, and 24 hrs. after harvest. The following features were evaluated using the Toup View software: length, width, surface and perimeter of erythrocytes for species with anucleated erythrocytes. The same characteristics were evaluated in the nucleus for species with nucleated erythrocytes. The data obtained were processed with statistical programs to highlight changes in erythrocyte morphology produced by anticoagulants.
Keywords: blood cells; length-width ratio; cell surface; shape; vacutainer.
INTRODUCTION
Blood sampling is useful in the diagnosis of clinical diseases and also in the routine management of animal welfare (Harikrishnan et al., 2018). Research in the field of haematology and blood biochemistry in animals often involves collecting samples in anticoagulant media to maintain the liquid state of blood for a longer period (Barrelet and Ricketts, 2002). Anticoagulant use, according to some studies, frequently results in erroneous results, particularly in terms of erythrocyte indices, due to the morphological alterations that anticoagulants cause at the cellular level (Walencik and Witeska, 2007).
Usually, anticoagulants intervene and stop a certain stage of the coagulation process, but some anticoagulants stop two or more stages depending on their nature, which is why we have different types of anticoagulants depending on the investigations to be performed in hematology (Mireşan et al., 2003).
Anticoagulants are substances that inhibit the clotting process of blood or plasma, both in vivo and in vitro.
Blood samples can be drawn into tubes with a variety of preservatives, anticoagulants, and other chemicals, and then stored at room temperature, refrigerated, or frozen (World Health Organization, 2002). The goal is to keep the sample, as well as the drug or its metabolites of interest, in a stable state from the moment of collection through analysis (Kulkarni et al., 2016).
Sodium citrate, EDTA or heparin or even a gel whose density is between that of the blood cells and blood plasma are examples of additions.
Furthermore, some tubes contain additives that can maintain some blood components or chemicals, such as glucose (Kulkarni et al., 2016).
Erythrocytes are the most abundant elements in the composition of blood (Nemkov et al., 2018). Their number is expressed in units of 106/mm3 and shows variations depending on the species (5×106/mm3 in humans, 5.5×106/mm3 in cattle, 8×106/mm3 in horses, 4×106/mm3 in chickens, 14×106/mm3 in sheep, and 7×106/mm3 in pigs) (Mireşan et al., 2003). There are small variations in these values, given the age, gestational status, sex of the individual, and altitude, and may be differences between different populations of the same species. Deviations from normal values occur only in pathological cases (Mohri and Rezapoor, 2009; Ahyayauch et al., 2013). Erythrocytes transport and exchange oxygen and carbon dioxide between the respiratory system and other tissues.
Endothelial cells are activated to synthesize nitric oxide (NO).
Because the oxygenation of erythrocytes in the pulmonary capillaries favours the discharge of carbon dioxide. This induces the contraction and release of ATP and the activation of the synthesis capacity of nitric oxide by endothelial cells and its complexation with haemoglobin.
In the process of deoxygenation, the content of nitric oxide in the peripheral tissues decreases and at the same time the content of carbon dioxide increases. This causes erythrocyte swelling and vasodilation (Premont et al., 2021). The diameter of the erythrocyte is measured in μm and has the following values depending on the species: 8.3 μm in humans, 7-8 μm in rabbits, 3.5 μm in goats, 5-6 μm in equine, and 7.5 μm in chicken (Mireşan et al., 2003).
All mammals’ erythrocytes are anucleated, and the majority of them are in the shape of biconcave discs termed discocytes and have a life span of approximately 120 days (Barrelet and Ricketts, 2002). Erythrocytes in fish are nucleated ellipsoidal cells with varying volumes and have a life span from 13 to 500 days. Environmental conditions and especially, water temperature and its dissolved oxygen content, seasonal changes and fish activity affect the number of erythrocytes (Witeska, 2013; Cocan et al., 2018; Uiuiu et al., 2021).
Therefore, our research aimed to highlight the influence of anticoagulants on erythrocyte morphology in different vertebrate species: two species with anucleated erythrocytes (horse and rabbit) and two species with nucleated erythrocytes (chickens and fish).
MATERIALS AND METHODS
Blood samples were collected from different vertebrate species: horse (Equus caballus, Linnaeus 1758) – adult male, rabbit (Lepus europaeus, Pallas 1778) – adult male, black bullhead (Ameiurus melas, Rafinesque 1820) – adult female, and domestic chicken (Gallus gallus domesticus, Linnaeus 1758) – adult female (one specimen/species). Collection methods were as follows: in horses from the jugular vein, in rabbits from the ear veins, in fish by puncturing the caudal vein, and in chickens from the ulnar veins.
After extraction, the blood samples were transferred to vacutainers where the anticoagulant is also located. For this experiment, the anticoagulants LiHep (Lithium heparin) and K3 EDTA (ethylenediaminetetraacetic acid) were used. The vacutainers were filled with blood as follows: normal anticoagulant concentration (up to the black marker found on the label of the vacutainer) and double anticoagulant concentration (up to half of the vacutainer). During blood harvesting procedures, there are situations where the operator is unable to harvest the recommended blood volume specified by the producer on the vacutainer due to age, size, and physiological status of the animal. Investigations on the effect of double anticoagulant concentration were performed to avoid situations where, at the time of blood sampling, the amount of blood indicated by the vacutainer manufacturer is not observed. The control smears were made on the spot and on a blade, a drop of blood was inserted, which was spread with the help of a blunt blade then stained with a Diakit Panoptic quick staining kit with the three constituents: Reag-Fix Panoptic (fixator), Reag-Red Panoptic (dye with eosinophilic action), and Reag-Blue Panoptic (dye with basophilic action) to highlight the figurative elements and their fixation. From the anticoagulant vacutainers, smears were made at intervals of 3, 6, and 24 hrs. The used method was identical to that of the control samples. The effect of anticoagulants on erythrocyte morphology was evaluated at 3, 6, and 24 hrs. after blood collection. Using the ToupView software, the following characteristics were evaluated: cell surface and length-width ratio. Cell surface and cell length-width ratio may show the deformation of the blood cells (RBC) over time under the action of different concentrations and different anticoagulants, which could result in the biased interpretation of the haematological panel.
The smears were examined with a Nikon Eclipse 50i microscope with a 60×objective. The microscope is equipped with a Nikon DS-Fi1 camera, and with the help of NIS-Elements Viewer 3.0, software images were taken with the following settings: fast focus 1280×960, quality capture 2560×1920, auto exposure, AECompensation +1.0EV, and contrast antireflex.
For each smear, 10 images were taken and 10 erythrocytes were measured from each image. A total of 15600 measurements were performed in total. The collected data were processed in Microsoft Excel and GraphPad Prism 8 software. ANOVA one-way test and Dunnett’s multiple comparisons test were computed to determine the differences between the control group data and each experimental treatment.
RESULTS AND DISCUSSION
Based on the conducted experiments, it can be seen that the erythrocyte surface in horses has similar values when collected on EDTA in the recommended (normal) dose and when analysed after 24 hrs. from sampling. A similar situation is encountered when analysing the surface of erythrocytes in the blood collected on a double concentration of EDTA analysed 6 hrs. after sampling. The other treatments show significant differences compared to the values of the control group (Table 1).
Heparin in double concentration, regardless of storage time (3, 6, 24 hrs.), did not lead to changes in the length-width ratio of horse erythrocytes. A similar situation was obtained with the double dose of EDTA after 24 hrs. of storage.
Similar values to those of the control group of rabbit erythrocyte surface were observed for the following treatments: 3 hrs. after sample, blood obtained on EDTA in normal concentration was analysed and 6 hrs. later, blood collected on EDTA in double concentration was analysed, and blood collected on heparin in normal concentration, for both 3 and 24 hrs. after sampling (Table 2).
Table 1
Dunnett’s multiple comparisons test: RBC surface and RBC length-width ratio in horses
|
Control vs. |
Mean Difference |
95.00% CI of diff. |
Significant? |
Summary |
Adjusted P-value |
|
Horse (cell surface – μm2) |
|||||
|
EDTA-24h-Normal |
-0.3988 |
-0.9388 to 0.1413 |
No |
ns |
0.272 |
|
EDTA-6h-Double |
0.04657 |
-0.4935 to 0.5866 |
No |
ns |
0.9997 |
|
EDTA-3h-Normal |
1.307 |
0.7673 to 1.847 |
Yes |
**** |
< 0.0001 |
|
EDTA-6h-Normal |
0.9765 |
0.4364 to 1.516 |
Yes |
**** |
< 0.0001 |
|
EDTA-3h-Double |
2.31 |
1.770 to 2.850 |
Yes |
**** |
< 0.0001 |
|
Heparin-3h-Normal |
1.187 |
0.6466 to 1.727 |
Yes |
**** |
< 0.0001 |
|
Heparin-6h-Normal |
2.996 |
2.456 to 3.536 |
Yes |
**** |
< 0.0001 |
|
Heparin-24h-Normal |
1.986 |
1.446 to 2.526 |
Yes |
**** |
< 0.0001 |
|
Heparin-24h-Double |
1.149 |
0.6087 to 1.689 |
Yes |
**** |
< 0.0001 |
|
EDTA-24h-Double |
0.866 |
0.3260 to 1.406 |
Yes |
*** |
0.0001 |
|
Heparin-3h-Double |
0.8243 |
0.2842 to 1.364 |
Yes |
*** |
0.0003 |
|
Heparin-6h-Double |
0.6169 |
0.07685 to 1.157 |
Yes |
* |
0.0155 |
|
Horse (cell length-width ratio) |
|||||
|
EDTA-24h-Double |
0.007671 |
-0.02379 to 0.03913 |
No |
ns |
0.9958 |
|
Heparin-3h-Double |
-0.0157 |
-0.04716 to 0.01576 |
No |
ns |
0.7364 |
|
Heparin-6h-Double |
-0.01027 |
-0.04173 to 0.02119 |
No |
ns |
0.9758 |
|
Heparin-24h-Double |
-0.03089 |
-0.06235 to 0.0005767 |
No |
ns |
0.0574 |
|
EDTA-3h-Normal |
-0.2366 |
-0.2680 to -0.2051 |
Yes |
**** |
< 0.0001 |
|
EDTA-6h-Normal |
-0.1431 |
-0.1746 to -0.1116 |
Yes |
**** |
< 0.0001 |
|
EDTA-24h-Normal |
-0.1545 |
-0.1859 to -0.1230 |
Yes |
**** |
< 0.0001 |
|
EDTA-3h-Double |
-0.09063 |
-0.1221 to -0.05917 |
Yes |
**** |
< 0.0001 |
|
Heparin-6h-Normal |
-0.06586 |
-0.09733 to -0.03440 |
Yes |
**** |
< 0.0001 |
|
Heparin-24h-Normal |
-0.04309 |
-0.07455 to -0.01163 |
Yes |
** |
0.0017 |
|
EDTA-6h-Double |
-0.03591 |
-0.06737 to -0.004449 |
Yes |
* |
0.0156 |
|
Heparin-3h-Normal |
-0.03456 |
-0.06602 to -0.003099 |
Yes |
* |
0.0226 |
Legend: ns – P-value ≥ 0.05 (not significant); * – P-value from 0.01 to 0.05 (significant); ** – P-value from 0.001 to 0.01 (very significant); *** – P-value from 0.0001 to 0.001 (extremely significant); **** – P-value < 0.0001 (extremely significant)
Regarding the length-width ratio of rabbit erythrocytes, similar values with those of the control group were observed for the treatment on EDTA in double concentration, analysed 24 hrs. after sampling. The same situation was observed in the case of heparin treatments in normal concentration after 3 and 24 hrs., and in the case of double heparin concentration in 24 hrs.
Table 2
Dunnett’s multiple comparisons test: RBC surface and RBC length-width ratio in brown hares
|
Control vs. |
Mean Difference |
95.00% CI of diff. |
Signi- ficant? |
Summary |
Adjusted P-value |
|
Brown Hare (cell surface – μm2) |
|||||
|
EDTA-3h-Normal |
0.1671 |
-0.3931 to 0.7273 |
No |
ns |
0.9879 |
|
EDTA-6h-Double |
0.03512 |
-0.5251 to 0.5953 |
No |
ns |
0.9997 |
|
Heparin-3h-Normal |
-0.5017 |
-1.062 to 0.05851 |
No |
ns |
0.1057 |
|
Heparin-24h-Normal |
-0.3999 |
-0.9601 to 0.1603 |
No |
ns |
0.3094 |
|
EDTA-24h-Normal |
-1.143 |
-1.703 to -0.5826 |
Yes |
**** |
< 0.0001 |
|
EDTA-24h-Double |
-1.658 |
-2.219 to -1.098 |
Yes |
**** |
< 0.0001 |
|
Heparin-6h-Normal |
1.081 |
0.5208 to 1.641 |
Yes |
**** |
< 0.0001 |
|
Heparin-3h-Double |
-1.11 |
-1.670 to -0.5500 |
Yes |
**** |
< 0.0001 |
|
Heparin-6h-Double |
-1.166 |
-1.726 to -0.6056 |
Yes |
**** |
< 0.0001 |
|
EDTA-3h-Double |
-0.8908 |
-1.451 to -0.3306 |
Yes |
*** |
0.0001 |
|
Heparin-24h-Double |
-0.8978 |
-1.458 to -0.3376 |
Yes |
*** |
0.0001 |
|
EDTA-6h-Normal |
-0.74 |
-1.300 to -0.1798 |
Yes |
** |
0.0028 |
|
Brown Hare (cell length-width ratio) |
|||||
|
EDTA-24h-Double |
-0.00312 |
-0.03108 to 0.02483 |
No |
ns |
0.9996 |
|
Heparin-3h-Normal |
0.008082 |
-0.01987 to 0.03603 |
No |
ns |
0.9896 |
|
Heparin-24h-Normal |
-0.0175 |
-0.04545 to 0.01045 |
No |
ns |
0.467 |
|
Heparin-24h-Double |
-0.00782 |
-0.03578 to 0.02013 |
No |
ns |
0.9907 |
|
EDTA-3h-Normal |
-0.09635 |
-0.1243 to -0.06840 |
Yes |
**** |
< 0.0001 |
|
EDTA-6h-Normal |
-0.1529 |
-0.1808 to -0.1249 |
Yes |
**** |
< 0.0001 |
|
EDTA-24h-Normal |
-0.09613 |
-0.1241 to -0.06818 |
Yes |
**** |
< 0.0001 |
|
EDTA-3h-Double |
-0.07722 |
-0.1052 to -0.04926 |
Yes |
**** |
< 0.0001 |
|
EDTA-6h-Double |
-0.05241 |
-0.08036 to -0.02446 |
Yes |
**** |
< 0.0001 |
|
Heparin-3h-Double |
-0.04061 |
-0.06856 to -0.01265 |
Yes |
*** |
0.0007 |
|
Heparin-6h-Normal |
-0.03689 |
-0.06484 to -0.008935 |
Yes |
** |
0.0028 |
|
Heparin-6h-Double |
-0.03545 |
-0.06340 to -0.007494 |
Yes |
** |
0.0048 |
The symbols used have the same meaning as in Table 1
As can be seen in Table 3, for chicken erythrocytes, similar cell surface values to those of the control group were observed in the following treatments: EDTA in normal concentration at 3 and 24 hrs. after sampling, EDTA in double concentration at 24 hrs. after sampling, and in the case of heparin in normal concentration, 3 hrs. after sampling. Regarding the length-width ratio, all treatments showed extremely significant differences, compared to the control group.
For black bullhead (Table 4), the erythrocyte surface had values similar to those of the control group when the blood was collected on heparin in normal concentration and analysed 6 hrs. after sampling and in double heparin concentration, analysed 3 hrs. after sampling. Regarding the length-width ratio of erythrocytes in black bullhead, we obtained values similar to those of the control group in the following treatments: blood collected on EDTA in double concentration and analysed 24 hrs. after sampling, and for blood collected on heparin in double concentration and analysed 3, 6, and 24 hrs. after sampling.
Table 3
Dunnett’s multiple comparisons test: RBC surface and RBC length-width ratio in chickens
|
Control vs. |
Mean Difference |
95.00% CI of diff. |
Signifi- cant? |
Summary |
Adjusted P-value |
|
Chicken (cell surface – μm2) |
|||||
|
EDTA-3h-Normal |
0.3882 |
-0.3809 to 1.157 |
No |
ns |
0.7243 |
|
EDTA-24h-Normal |
0.5168 |
-0.2523 to 1.286 |
No |
ns |
0.3799 |
|
EDTA-24h-Double |
-0.5377 |
-1.307 to 0.2314 |
No |
ns |
0.333 |
|
Heparin-3h-Normal |
-0.7686 |
-1.538 to 0.0004981 |
No |
ns |
0.0503 |
|
EDTA-6h-Double |
1.401 |
0.6321 to 2.170 |
Yes |
**** |
< 0.0001 |
|
Heparin-3h-Double |
-5.744 |
-6.513 to -4.975 |
Yes |
**** |
< 0.0001 |
|
Heparin-24h-Double |
-1.662 |
-2.431 to -0.8930 |
Yes |
**** |
< 0.0001 |
|
Heparin-24h-Normal |
-1.126 |
-1.895 to -0.3566 |
Yes |
*** |
0.0006 |
|
Heparin-6h-Double |
-1.14 |
-1.909 to -0.3712 |
Yes |
*** |
0.0005 |
|
EDTA-6h-Normal |
-0.9388 |
-1.708 to -0.1697 |
Yes |
** |
0.0076 |
|
EDTA-3h-Double |
1.05 |
0.2814 to 1.820 |
Yes |
** |
0.0018 |
|
Heparin-6h-Normal |
-0.9569 |
-1.726 to -0.1878 |
Yes |
** |
0.006 |
|
Chicken (cell length-width ratio) |
|||||
|
EDTA-3h-Normal |
0.2393 |
0.1820 to 0.2965 |
Yes |
**** |
< 0.0001 |
|
EDTA-6h-Normal |
0.2627 |
0.2054 to 0.3200 |
Yes |
**** |
< 0.0001 |
|
EDTA-24h-Normal |
0.2812 |
0.2239 to 0.3385 |
Yes |
**** |
< 0.0001 |
|
EDTA-3h-Double |
0.1736 |
0.1163 to 0.2308 |
Yes |
**** |
< 0.0001 |
|
EDTA-6h-Double |
0.136 |
0.07878 to 0.1933 |
Yes |
**** |
< 0.0001 |
|
EDTA-24h-Double |
0.1963 |
0.1390 to 0.2536 |
Yes |
**** |
< 0.0001 |
|
Heparin-3h-Normal |
0.1654 |
0.1082 to 0.2227 |
Yes |
**** |
< 0.0001 |
|
Heparin-6h-Normal |
0.1947 |
0.1375 to 0.2520 |
Yes |
**** |
< 0.0001 |
|
Heparin-24h-Normal |
0.3381 |
0.2808 to 0.3953 |
Yes |
**** |
< 0.0001 |
|
Heparin-3h-Double |
0.4164 |
0.3591 to 0.4737 |
Yes |
**** |
< 0.0001 |
|
Heparin-6h-Double |
0.2171 |
0.1599 to 0.2744 |
Yes |
**** |
< 0.0001 |
|
Heparin-24h-Double |
0.1595 |
0.1022 to 0.2168 |
Yes |
**** |
< 0.0001 |
The symbols used have the same meaning as in Table 1
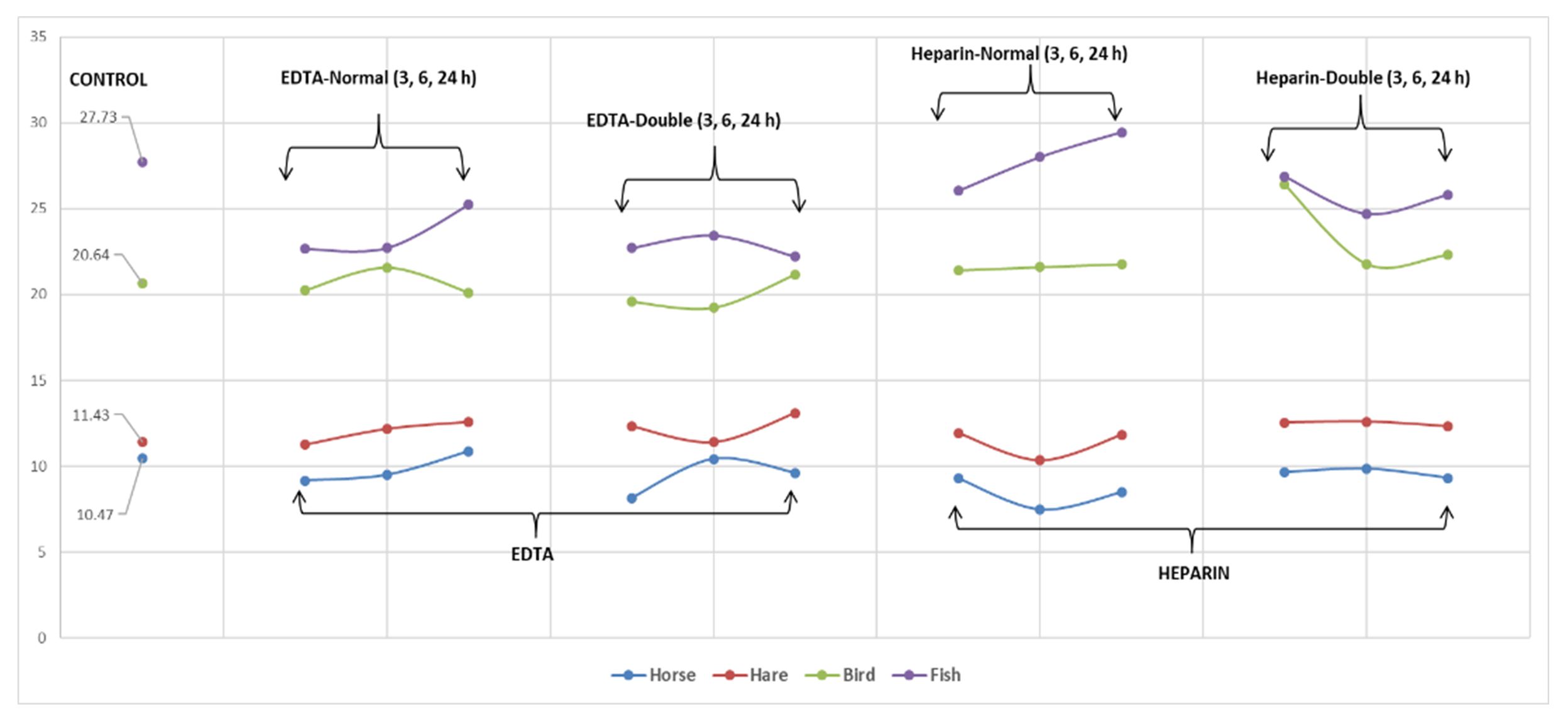
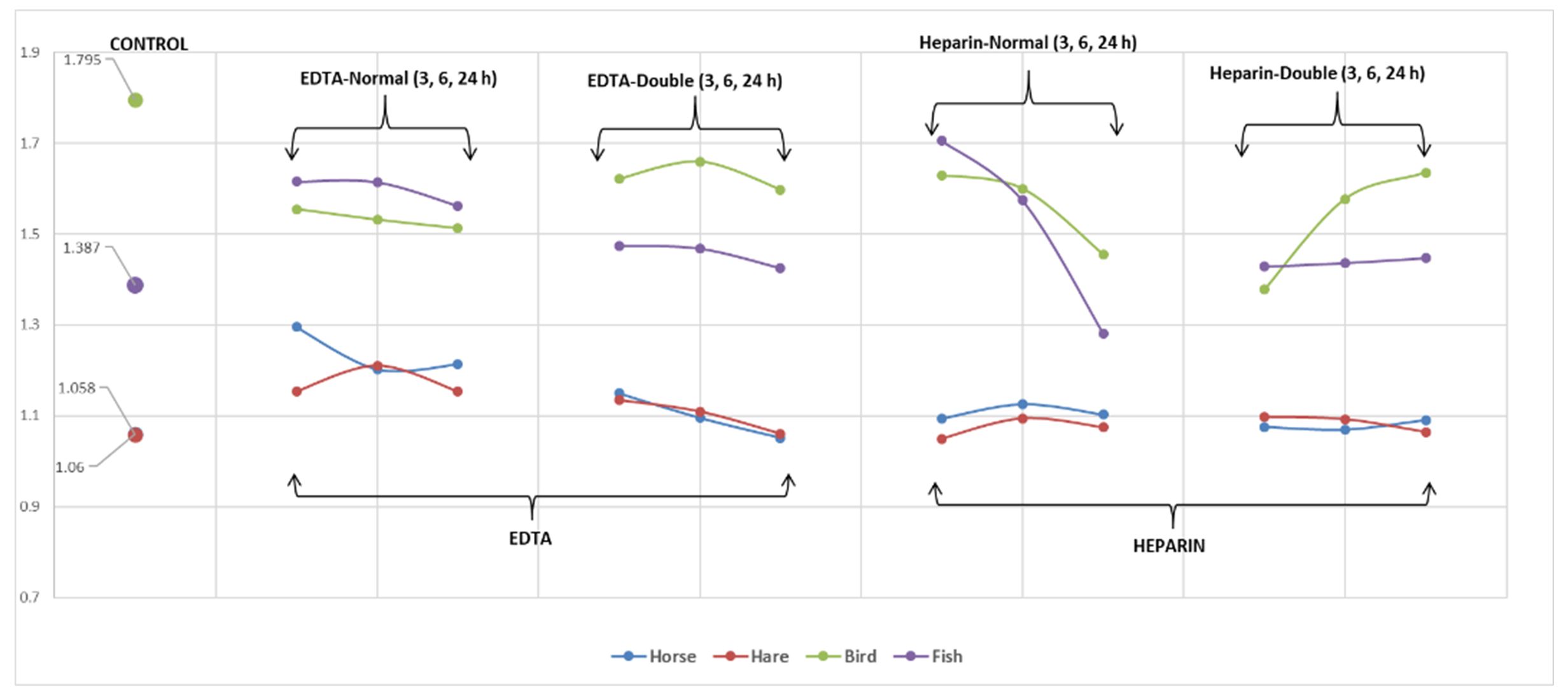
Fig. 1 and Fig. 2 show the mean values of surfaces and the length-width ratios of erythrocytes for the analysed species (Fig. 1) vertical axis shows mean values of erytrocite surfaces and Fig. 2 shows mean values of length-width ratio of erytrocites). Changes in these parameters can be observed depending on the anticoagulant used, its concentration, and the time of blood storage in the vacutainer. Due to the gradient differences between the internal medium of erythrocytes and anticoagulants, fluctuations of the studied parameters were observed.
Other previous studies (Sanchez-Migallon et al., 2008) analysed the impact of different anticoagulants on haematological values. Our results come in addition to the literature with quantitative data on erythrocyte morphology.
Table 4
Dunnett’s multiple comparisons test: RBC surface and RBC length-width ratio in black bullheads
|
Control vs. |
Mean Difference |
95.00% CI of diff. |
Signifi- cant? |
Summary |
Adjusted P-value |
|
Black bullhead (cell surface – μm2) |
|||||
|
Heparin-6h-Normal |
-0.2708 |
-1.362 to 0.8200 |
No |
ns |
0.9957 |
|
Heparin-3h-Double |
0.8569 |
-0.2339 to 1.948 |
No |
ns |
0.2095 |
|
EDTA-3h-Normal |
5.064 |
3.974 to 6.155 |
Yes |
**** |
< 0.0001 |
|
EDTA-6h-Normal |
5.006 |
3.915 to 6.097 |
Yes |
**** |
< 0.0001 |
|
EDTA-24h-Normal |
2.498 |
1.407 to 3.589 |
Yes |
**** |
< 0.0001 |
|
EDTA-3h-Double |
5.014 |
3.923 to 6.105 |
Yes |
**** |
< 0.0001 |
|
EDTA-6h-Double |
4.284 |
3.193 to 5.374 |
Yes |
**** |
< 0.0001 |
|
EDTA-24h-Double |
5.516 |
4.426 to 6.607 |
Yes |
**** |
< 0.0001 |
|
Heparin-6h-Double |
3.016 |
1.925 to 4.107 |
Yes |
**** |
< 0.0001 |
|
Heparin-24h-Double |
1.915 |
0.8241 to 3.006 |
Yes |
**** |
< 0.0001 |
|
Heparin-3h-Normal |
1.689 |
0.5985 to 2.780 |
Yes |
*** |
0.0002 |
|
Heparin-24h-Normal |
-1.725 |
-2.816 to -0.6344 |
Yes |
*** |
0.0002 |
|
Black bullhead (cell length-width ratio) |
|||||
|
EDTA-24h-Double |
-0.03833 |
-0.1172 to 0.04054 |
No |
ns |
0.7629 |
|
Heparin-3h-Double |
-0.04164 |
-0.1205 to 0.03723 |
No |
ns |
0.675 |
|
Heparin-6h-Double |
-0.04954 |
-0.1284 to 0.02933 |
No |
ns |
0.4631 |
|
Heparin-24h-Double |
-0.06006 |
-0.1389 to 0.01881 |
No |
ns |
0.24 |
|
EDTA-3h-Normal |
-0.2287 |
-0.3075 to -0.1498 |
Yes |
**** |
< 0.0001 |
|
EDTA-6h-Normal |
-0.2272 |
-0.3061 to -0.1484 |
Yes |
**** |
< 0.0001 |
|
EDTA-24h-Normal |
-0.1757 |
-0.2546 to -0.09686 |
Yes |
**** |
< 0.0001 |
|
Heparin-3h-Normal |
-0.3179 |
-0.3968 to -0.2390 |
Yes |
**** |
< 0.0001 |
|
Heparin-6h-Normal |
-0.1875 |
-0.2664 to -0.1086 |
Yes |
**** |
< 0.0001 |
|
Heparin-24h-Normal |
0.1046 |
0.02575 to 0.1835 |
Yes |
** |
0.0026 |
|
EDTA-3h-Double |
-0.08738 |
-0.1663 to -0.008513 |
Yes |
* |
0.0209 |
|
EDTA-6h-Double |
-0.08084 |
-0.1597 to -0.001969 |
Yes |
* |
0.0412 |
The symbols used have the same meaning as in Table 1
CONCLUSIONS
The anticoagulant type, its concentration, and the exposure time of the blood to the anticoagulant are factors that can influence the quality and accuracy of blood analyses. In the tables and figures presented, the recommended concentrations for both types of anticoagulants and the optimal blood analysis time marks can be identified. Failure to observe the recommended concentrations for different types of anticoagulants and the time of exposure of erythrocytes to them may lead to morphological changes. These changes from the increase or decrease in volume can negatively influence certain haematological parameters, in particular haematocrit values. Anticoagulants are isotonic with the internal medium of mammals. In the case of lower vertebrates (birds, fish, reptiles, amphibians), anticoagulants may induce morphological changes in erythrocytes, as the latter have a lower concentration in the internal medium.
REFERENCES
Ahyayauch, H., Sansar, W., Rendon-Ramirez, A., Goñi, F.M., Bennouna, M. & Gamarani, H. (2013). Effects of chronic and acute lead treatments on the biophysical properties of erythrocyte membranes and a comparison with model membranes. FEBS Open Bio, 3: 212-217, DOI: 10.1016/j.fob.2013.04. 001.
Barrelet, A. & Ricketts, S. (2002). Haematology and blood biochemistry in the horse: a guide to interpretation. In: Practice, 24: 318-327, DOI: 10.1136/ inpract.24.6.318.
Cocan, D., Popescu, F., Laţiu, C., Uiuiu, P., Coroian, A., Răducu, C., Coroian, C., Mireşan, V., Kokkinakis, A. & Constantinescu, R. (2018). Effects of thermal stress on hematological and metabolic profiles in brown bullhead, Ameiurus nebulosus (Lesueur, 1819). AgroLife Sci. J., 7(1): 33-41.
Guzman, D.S., Mitchell, M.A., Gaunt, S.D., Beaufrère, H. & Tully, T.N. (2008). Comparison of hematologic values in blood samples with lithium heparin or dipotassium ethylenediaminetetraacetic acid anticoagulants in Hispaniolan Amazon parrots (Amazona ventralis). J. Avian Med. Surg., 22(2): 108-113, DOI: 10.1647/2007-011.1.
Harikrishnan, V.S., Hansen, A.K., Abelson, K.S.P. & Sørensen, D.B. (2018). A comparison of various methods of blood sampling in mice and rats: Effects on animal welfare. Lab. Anim., 52(3): 253-264, DOI: 10.1177/0023677 217741332.
Kulkarni, P., Karanam, A., Gurjar, M., Dhoble, S., Naik, A.B., Vidhun, B.H. & Gota, V. (2016). Effect of various anticoagulants on the bioanalysis of drugs in rat blood: implication for pharmacokinetic studies of anticancer drugs. SpringerPlus, 5(1): 2102, DOI: 10.1186/s40064-016-3770-4.
Lulijwa, R., Alfaro, A.C., Young, T., Venter, L., Decker, P., Merien, F. & Meyer, J. (2021). Effect of anticoagulants on farmed giant kokopu, Galaxias argenteus (Gmelin, 1789) haematological parameters and erythrocyte fragility. J. Fish Biol., 99(2): 684-689, DOI: 10.1111/jfb.14746.
Mireşan, V., Eresk, A. & Răducu, C. (2003). Physiology of domestic animals (In Romanian). Cluj-Napoca, Ed. Risoprint.
Mohri, M. & Rezapoor, H. (2009). Effects of heparin, citrate, and EDTA on plasma biochemistry of sheep: Comparison with serum. Res. Vet. Sci., 86(1): 111-114, DOI: 10.1016/j.rvsc.2008.05.010.
Nemkov, T., Reisz, J.A., Xia, Y., Zimring, J.C. & D’Alessandro, A., (2018). Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport. Expert Rev. Proteomic., 15(11), 855-864. DOI: 10.1080/14789450.2018.153 1710.
Premont, R.T., Reynolds, J.D., Zhang, R. & Stamler, J.S. (2021). Red blood cell-mediated S-nitrosohemoglobin-dependent vasodilation: Lessons learned from a β-globin Cys93 knock-in mouse. Antioxid. Redox Signal., 34(12): 936-961, DOI: 10.1089/ars.2020.8153.
Uiuiu, P., Lațiu, C., Păpuc, T., Craioveanu, C., Ihuț, A., Sava, A., Răducu, C., Șonea, C., Constantinescu, R., Cocan, D. & Mireșan, V. (2021). Multi-approach assessment for stress evaluation in rainbow trout females, Oncorhynchus mykiss (Walbaum, 1792) from three different farms during the summer season. Animals, 11(6): 1810, DOI: 10.3390/ani11061810.
Walencik, J. & Witeska, M. (2007). The effects of anticoagulants on hematological indices and blood cell morphology of common carp (Cyprinus carpio L.). Comp. Biochem. Physiol. C Toxicol. Pharmacol., 146(3): 331-335, DOI: 10.1016/j.cbpc.2007.04.004.
Witeska, M. (2013). Erythrocytes in teleost fishes: a review. Zool. Ecol., 23(4): 275-281, DOI: 10.1080/21658005.2013.846 963.
World Health Organization (2002). Use of anticoagulants in diagnostic laboratory investigations. Geneva, 62 p
Cocan Daniel, Constantinescu Radu, Giurgiu Alexandru, Laţiu Călin, Mireşan Vioara, Păpuc Tudor, Răducu Camelia, Uiuiu Paul