Liza Ogawa, Leopoldo Sussumu Matsumoto, Roberta Dos Santos Toledo, Jonatas Campos de Almeida, Victor Bittencourt Dutra Tabacow, Fernanda Maria de Oliveira Dias, Diego Resende Rodrigues, Thaís Monica Cabral, Roberta Lemos Freire, Italmar Teodorico Navarro
ABSTRACT. The purpose of this investigation was to verify the effectiveness of composting in the sanitization of sludge from urban sewage (SS). The treatments (T) used consisted of SS mixed with sugarcane bagasse (SB), tree pruning residues (TP), poultry litter (PL) or grass clipping (GC) at a carbon/nitrogen ratio of approximately 30/1 and ratios of T1 (SS+SB), T2 (SS+SB+TP), T3 (SS+SB+GC), T4 (SS+SB+PL) or T5 (SS+SB+TP+GC). Temperature was measured daily for over 280 days of composting. Every two weeks, the compounds were analyzed for thermotolerant coliforms and viable helminth eggs using the multiple-tube and Yanko techniques; and once each month to identify Cryptosporidium spp. and Giardia spp. (oo)cysts using the sucrose and zinc sulfate centrifugal flotation technique and nested PCR followed by sequencing. The maximum temperatures measured in the thermophilic phase of the compounds ranged from 55°C to 64.8°C, and at day 280, weight reductions of 27% (T1), 48% (T2), 63% (T3), 66% (T4) and 64% (T5) were observed. The absence of fecal coliforms was observed from day 98 (T4), day 126 (T1), day 196 (T3 and T5) and day 210 (T2). All treatments resulted in helminth egg inviability and the absence of protozoan (oo)cysts. Only T4 and T5 were positive for G. duodenalis according to the sequencing analysis. In conclusion, high temperatures during the composting thermophilic phase made the use of pathogens required by legislation unfeasible; therefore, it is important to investigate the viability of protozoa in compounds to ensure a safe final product for human and animal health.
Keywords: biosolids; coliforms; temperature; thermotolerant viable helminth eggs.
Cite
ALSE and ACS Style
Ogawa, L.; Matsumoto, L.S.; dos Santos Toledo, R.; Campos de Almeida, J.; Tabacow, V.B.T.; de Oliveira Dias, F.M.; Rodrigues, D.R.; Cabral, T.M.; Freire, R.L.; Navarro, I.T. The effectiveness of composting using stabilizing urban sewage sludge compounds. Journal of Applied Life Sciences and Environment 2023, 56 (2), 139-151.
https://doi.org/10.46909/alse-562091
AMA Style
Ogawa L, Matsumoto LS, dos Santos Toledo R, Campos de Almeida J, Tabacow VBT, de Oliveira Dias FM, Rodrigues DR, Cabral TM, Freire RL, Navarro IT. The effectiveness of composting using stabilizing urban sewage sludge compounds. Journal of Applied Life Sciences and Environment. 2023; 56 (2): 139-151.
https://doi.org/10.46909/alse-562091
Chicago/Turabian Style
Ogawa, Liza, Leopoldo Sussumu Matsumoto, Roberta Dos Santos Toledo, Jonatas Campos De Almeida, Victor Bittencourt Dutra Tabacow, Fernanda Maria De Oliveira Dias, Diego Resende Rodrigues, Thaís Monica Cabral, Roberta Lemos Freire, and Italmar Teodorico Navarro. 2023. “The effectiveness of composting using stabilizing urban sewage sludge compounds” Journal of Applied Life Sciences and Environment 56, no. 2: 139-151.
https://doi.org/10.46909/alse-562091
View full article (HTML)
The Effectiveness of Composting Using Stabilizing Urban Sewage Sludge Compounds
Liza OGAWA1*, Leopoldo Sussumu MATSUMOTO1, Roberta DOS SANTOS TOLEDO2, Jonatas CAMPOS DE ALMEIDA2, Victor Bittencourt Dutra TABACOW2, Fernanda Maria DE OLIVEIRA DIAS1, Diego Resende RODRIGUES1, Thaís Monica CABRAL2, Roberta Lemos FREIRE2 and Italmar Teodorico NAVARRO2
1Universidade Estadual do Norte do Paraná, Campus Luiz Meneghel. UENP/CLM. Caixa Postal 261, CEP 86360-000, Bandeirantes, Paraná, Brasil; email: leopoldo@uenp.edu.br; fernanamodias@gmail.com; diegopardal@uenp.edu.br
2Universidade Estadual de Londrina, Departamento de Medicina Veterinária Preventiva, Londrina, Paraná, Brasil; email: robertatoledo@grupointegrado.br; jonatas_campos86@hotmail.com; victor.tabacow@gmail.com; thaismonicavet@gmail.com; rlfreire@uel.br; italmar@uel.br
*Correspondence: logawa@uenp.edu.br
Received: May 08, 2023. Revised: Jun. 29, 2023. Accepted: Jul. 05, 2023. Published online: Jul. 19, 2023
ABSTRACT. The purpose of this investigation was to verify the effectiveness of composting in the sanitization of sludge from urban sewage (SS). The treatments (T) used consisted of SS mixed with sugarcane bagasse (SB), tree pruning residues (TP), poultry litter (PL) or grass clipping (GC) at a carbon/nitrogen ratio of approximately 30/1 and ratios of T1 (SS+SB), T2 (SS+SB+TP), T3 (SS+SB+GC), T4 (SS+SB+PL) or T5 (SS+SB+TP+GC). Temperature was measured daily for over 280 days of composting. Every two weeks, the compounds were analyzed for thermotolerant coliforms and viable helminth eggs using the multiple-tube and Yanko techniques; and once each month to identify Cryptosporidium spp. and Giardia spp. (oo)cysts using the sucrose and zinc sulfate centrifugal flotation technique and nested PCR followed by sequencing. The maximum temperatures measured in the thermophilic phase of the compounds ranged from 55°C to 64.8°C, and at day 280, weight reductions of 27% (T1), 48% (T2), 63% (T3), 66% (T4) and 64% (T5) were observed. The absence of fecal coliforms was observed from day 98 (T4), day 126 (T1), day 196 (T3 and T5) and day 210 (T2). All treatments resulted in helminth egg inviability and the absence of protozoan (oo)cysts. Only T4 and T5 were positive for G. duodenalis according to the sequencing analysis. In conclusion, high temperatures during the composting thermophilic phase made the use of pathogens required by legislation unfeasible; therefore, it is important to investigate the viability of protozoa in compounds to ensure a safe final product for human and animal health.
Keywords: biosolids; coliforms; temperature; thermotolerant viable helminth eggs.
INTRODUCTION
The urge to improve industrial and agricultural sectors arose with population growth and global economic expansion. Therefore, a progressive increase in waste generation occurred with time, which led researchers to search for adequate disposal locations to prevent negative effects on human, animal, and environmental health (Bentes et al., 2023; Spoti and Amaral, 2023).
In Brazil, around 50% of the population does not have access to a sewage collection and treatment system, so sewage is improperly disposed of without treatment in the environment. It is estimated that the Brazilian population will have access to and treatment of sewage by 2033 (LAW NO. 14.026, 2020; MRD, 2022). In most Brazilian cities, the residues collected from the sewage treatment system are dumped into rivers and seas, generating an important environmental impact. Therefore, environmental laws cause sanitation operators to develop alternatives for the disposal of this waste (ME, 2006; 2020).
Among these wastes, sewage sludge (SS) is produced on a large scale. Sewage companies dispose of SS in landfills, use incineration to eliminate SS and/or recycle SS by applying it to crops or using it for industry. A greener alternative to pretreated SS disposal is its application to agricultural soils that are rich in desirable nutrients for plant growth and organic matter, thus improving the soil’s physical, chemical, and biological qualities (Eid et al., 2019; Rehman and Qayyum, 2020).
However, SSs contain pathogens and heavy metals that must be sanitized before soil application. Before treatment, the heavy metal, thermotolerant coliform and viable helminth egg contents in SSs are generally above the regulated limits, which limits their use in agriculture (Roman, 2018; Chen et al., 2021).
One efficient process for removing pathogens is composting. The actions of thermophilic microbiota in organic matter degradation promote high temperatures that destroy much of the pathogenic microorganisms if maintained for several days, thus creating stable and secure final compounds (Kim et al., 2017; Gupta et al., 2021).
Composting is a process of biological decomposition and stabilization that allows the association of various urban and agro-industrial wastes and has the following advantages: favoring the economy of landfill areas (increasing their useful life), agricultural reuse of organic matter, and recycling nutrients to the soil, among others. In composting, the organic materials of the raw sludge are degraded by the action of microorganisms in an aerated environment, generating a compost of acceptable odor, easy manipulation, and with pathogenic microorganisms prevented (Kim et al., 2017; Roman, 2018).
The composting process can be carried out in windrows or piles with or without periodic turning and/or aeration with air blowing or air suction, or in a bio-reactor. In a periodic turning system, the mixture of sewage sludge and structuring residue is disposed of in piles, with aeration provided by manual or mechanical revolving and air diffusion. In the aerated static windrows’ system, the material to be composted is placed on perforated pipes that inject or suck air from the mass without stirring the windrows. In the closed system, the material is placed inside reactors in a specialized mechanical system that allows the automated control of the entire composting process (Kim et al., 2017; Chowdhury et al., 2022).
At the beginning of the composting process, mesophyllic microorganisms (optimal growth temperature between 25 and 40 °C) present in sewage sludge pure or mixed with other waste (sawdust, tree pruning, sugarcane bagasse, chicken litter, among others) degrade the organic matter, generating heat, thus gradually increasing the temperature of the medium. This increase in temperature favors the proliferation of thermophilic microorganisms (optimal temperature between 50 and 55°C), which perform the degradation (or biostabilization) of organic matter and assist in the elimination of pathogenic microorganisms. In the thermophilic phase (which can last from a few days to several months, according to the chemical characteristics of the material to be composted), the ideal is to maintain the temperature of the compost between 55 and 65 °C (Chowdhury et al., 2022).
When organic matter is largely degraded, there is a decrease in thermophiles and, consequently, a decrease in temperature, leading to the proliferation of mesophiles. The maturation process begins, lasting weeks to months, where polymerization of stable organic molecules (humification) occurs, generating a compound with no inhibitory or toxic effect on plants or soil (Gupta et al., 2021; Chowdhury et al., 2022). According to Kiehl (2012), at the end of composting, mineral salts (which contain the nutrients for plant roots) and humus (which acts as a soil conditioner) are produced.
The composting process should be monitored using physical and chemical (pH, temperature, and relation C/N, among others) measurements and by determining contaminant (potentially toxic organic substances, thermotolerant coliforms, and viable helminth eggs) contents to ensure the sanitary quality of the produced biosolids (Kim et al., 2017; Gupta et al., 2021).
The microbiological and parasitological contamination of the sludge is related to human and animal feces present in the domestic sewage, and the extent of this contamination depends on factors specific to the population, such as socioeconomic and sanitary conditions and the presence of animals, as well as the type of treatment of the sludge and effluents (Roman, 2018; Gupta et al., 2021; Chen et al., 2021).
Since sewage is not the ideal environment for pathogenic microorganisms, the tendency is for this population to gradually decrease. The sewage treatment system already decreases or eliminates most pathogens. In raw sewage, only those that have the mechanisms to do so will survive, such as the thickness of the outer membrane of a helminth egg or the resistance of the cystic or oocyst form of certain protozoa (Kim et al., 2017; Gupta et al., 2021; Chen et al., 2021).
In composting, especially in the thermophilic phase, the high temperature for several days destroys a large part of the pathogenic microorganisms and is therefore considered an effective system for sanitizing sewage sludge. The term “sanitization” of sewage sludge is defined by the legislation as the process of pathogen reduction treatment of sewage sludge or derived products, and “derived product” is every product intended for agricultural use that contains sewage sludge in its composition (ME, 2006; Paraná, 2009; Chowdhury et al., 2022).
The effectiveness of sanitization of microorganisms by temperature depends on the exposure time of pathogens to a given temperature and the uniformity of this temperature over the material to be composted, since the temperature inside a composting windrow or pile is not homogeneous (Gupta et al., 2021; Chowdhury et al., 2022).
For monitoring biosolids for agricultural use, fecal coliforms and streptococci are recommended. The group of total coliforms includes genera that are not exclusively of fecal origin, and, therefore, thermotolerant coliforms are the most commonly used to measure the sanitary quality of the sludge. Helminth egg viability has been the most accepted criterion as a limiting factor for sewage sludge recycling due to the survival of parasites in the environment (six months to seven years) and the low infective dose (Roman, 2018; Gupta et al., 2021; Chen et al., 2021).
Thus, one can suggest composting for recycling sewage sludge and other agro-industrial and urban waste, which will be of great importance for the implementation of coordinated actions in Public Health and related areas aimed at the population. The use of this organic compost with environmental and sanitary safety, obtained through microbiological, parasitological and physical-chemical monitoring, can promote improvement in crop productivity, benefiting the rural producer.
The objective of this study was to assess the effectiveness of sewage sludge composting with urban, agricultural, and industrial residues in eliminating the pathogens (thermotolerant coliforms and helminth eggs) in it.
MATERIALS AND METHODS
Preparation of sewage sludge and urban, agricultural and industrial residue treatments
Samples of raw urban SS were collected from a sewage treatment plant (STP) anaerobic stabilization lagoon located in the northern region of Paraná State.
The sewage was stored in plastic sheeting in the sun to reduce excessive moisture and to allow it to mix with other wastes. Agricultural sugarcane bagasse (SB), poultry litter (PL), urban tree pruning (TP) and grass clipping (GC) residues were used to improve the quality and efficiency of the composting process.
To determine the carbon and nitrogen contents in each residue, samples were sent to the reference laboratory in Paraná State. These values were used to calculate the proportion of the compost materials that should be mixed based on their C/N ratio. The treatments were standardized to maintain an ideal C/N ratio of 30:1 (Kiehl, 2012). After verifying the C/N ratio of the waste, the following treatments (T) were mixed manually (volume/volume):
T1: SS + SB at a ratio of 1:1.5 (C/N 29.4)
T2: SS + SB + TP at a ratio of 1:2:1.5 (C/N 30.0)
T3: SS + SB + GC at a ratio of 1:2.5:1 (C/N 29.4)
T4: SS + SB + PL at a ratio of 1:3:1 (C/N 30.3)
T5: SS + SB + TP + GC at a ratio of 1:2:0.5:0.5 (C/N 29.0)
Treatments were placed in 300-liter polypropylene boxes with external measurements of 100 x 60.5 x 58.5 cm (width x length x height) and holes in their sides and bottoms (0.3-cm holes). In addition, the boxes contained a PVC perforated tube wrapped in a fly screen to facilitate forced aeration using air compressor injection. The mixing compost materials were covered with a 10-cm layer of sugarcane bagasse to protect them from surface dryness and were moistened.
For nine months, the mixing compost materials were subjected to composting via an aerated static pile (no turning). The treatment temperature was measured at three different times (8:00 am, 1:00 pm and 4:00 pm) using a digital skewer thermometer (Multi-thermometer®).
Overall, the temperatures ranged from -50°C to +150°C with a resolution of 0.1°C.
Microbiological and parasitological analyses
Samples for microbiological and parasitological analyses were collected at the beginning of treatment and every two weeks at different points and treatment depths. These samples were combined and analyzed as a single sample to obtain a more representative result. The samples were placed in plastic bags, identified and refrigerated until the analysis was completed, which occurred within 24 hours of sampling.
The multiple-tube technique was used for thermotolerant coliform analysis according to Technical Regulation L5.202 of the Environmental Department of São Paulo State (ESTC, 1993). These results were interpreted as the logarithm of the most probable number per 100 mL (log MPN/100 mL). The helminth eggs were investigated using the Yanko (1987) technique, which was modified by Thomaz-Soccol et al. (2000). The eggs were counted in a Sedgewich-Rafter chamber (PYSER-SGI Limited, Kent, UK), and the results are expressed as the number of eggs per gram of dry matter (EPG/DM). A portion of each material subjected to this technique was incubated at 28°C for four weeks in capped test tubes to determine the viability of the helminth eggs based on the observed mobility of larvae inside the eggs.
Investigation of Cryptosporidium spp. and Giardia spp.
Materials were collected monthly for the investigation of Cryptosporidium spp. and Giardia spp. Due to the granulation of the treatments, (oo)cysts were concentrated in sucrose (Sheather, 1923) and zinc sulfate (Faust et al., 1934) using the centrifugal flotation technique. The supernatants from both concentration techniques were placed in 2-mL microcentrifuge tubes at -20°C for the molecular technique described below.
The genomic material extraction was conducted using the commercial NucleoSpin® Tissue kit (Macherey-Nagel, Düren-Germany) according to the manufacturer’s instructions.
Fragments of the 16S rRNA gene were amplified using nested PCR for Giardia spp. The first reaction primers (Invitrogen®) were Gia2029 (5’ AAGTGTGGTGCAGACGGACTC-3’) and Gia2150c (5’-CTGCTGCCGTCCTTGGATGT-3’), which amplified a 497-base pair product (Appelbee et al., 2003). In the second reaction, the primers were RH11 (5’-CATCCGGTCGATCCTGCC-3’) and RH4 (5’-AGTCGAACCCTGATTCTCC GCCAGG-3’), which generated a 292-297-base pair fragment (Hopkins et al., 1997). Both of the amplification reactions were performed in solutions containing 17.25 µL of autoclaved ultrapure water, 10 mM Tris-HCl, 50 mM KCl (pH 8.3), 200 µM dNTP, 1.5 mM MgCl2, 1 µL of the forward and reverse primers, 1.25 µL of 5% dimethyl sulfoxide (DMSO), 1.25 U of Taq DNA Polymerase and 1.5 µL of extracted DNA from each test sample (for a total volume of 25 µL). The thermal cycler parameters for both reactions included an initial cycle at 95°C for five minutes, 35 cycles at 94°C for forty five minutes (denaturation), at 58°C for forty five minutes (annealing) and 72°C for one minute (final extension). One final extension step was included at 72°C for five minutes.
To detect Cryptosporidium spp., 18S rRNA gene fragments were amplified using nested PCR. The primers (Invitrogen®) of the first reaction were 5’-TTCTAGAGCTAATACATGCG-3’ and 5’-CCCATTTCCTTCGAAACAGGA-3’, which amplified a 1325-base pair product (Xiao et al., 1999). In the second reaction, the primers were 5’-GGAAGGGTTGTATTTATTAGAT-3’ and 5’-AAGGAGTAAGGAACAACCTCCA-3’, which generated 819-825 base pair fragments (Xiao et al., 1999). The first PCR was performed in a 25-µL reaction containing 7.75 µL of autoclaved ultrapure water, 10 mM Tris-HCl, 50 mM KCl (pH 8.3), 200 µM dNTP, 2.5 mM MgCl2, 1 µL of both forward and reverse primers, 1.25 U of Taq DNA Polymerase and 2.5 µL of extracted DNA from each test sample. In the second PCR, only the volumes of autoclaved ultrapure water (9.25 µL) and the amplified PCR product (1 µL) changed. The thermal cycler parameters for both reactions included an initial denaturation at 95°C for five minutes, 35 cycles at 94°C for forty-five minutes (denaturation), 55°C for forty-five minutes (annealing) and 72°C for one minute (extension). One final extension step was conducted at 72°C for five minutes.
PCR products were subjected to electrophoresis in a 1.5% agarose gel (Ultrapure™ Agarose; Invitrogen®) containing SYBR® Safe (DNA Gel Stain; Invitrogen®) for 45 minutes for Cryptosporidium spp. and 30 minutes for Giardia spp. Band visualization was performed under UV light and documented using the LPix Image ST program (Loccus Biotecnologia®). Positive samples (with genomic materials for protozoa) from nested PCR were subjected to sequencing. The nucleotide sequences were compared with standard sequences of Cryptosporidium and Giardia deposited in GenBank using BLAST (Basic Local Alignment and Search Tool) and through manual alignment using BioEdit (Biological Sequence Alignment Editor).
RESULTS
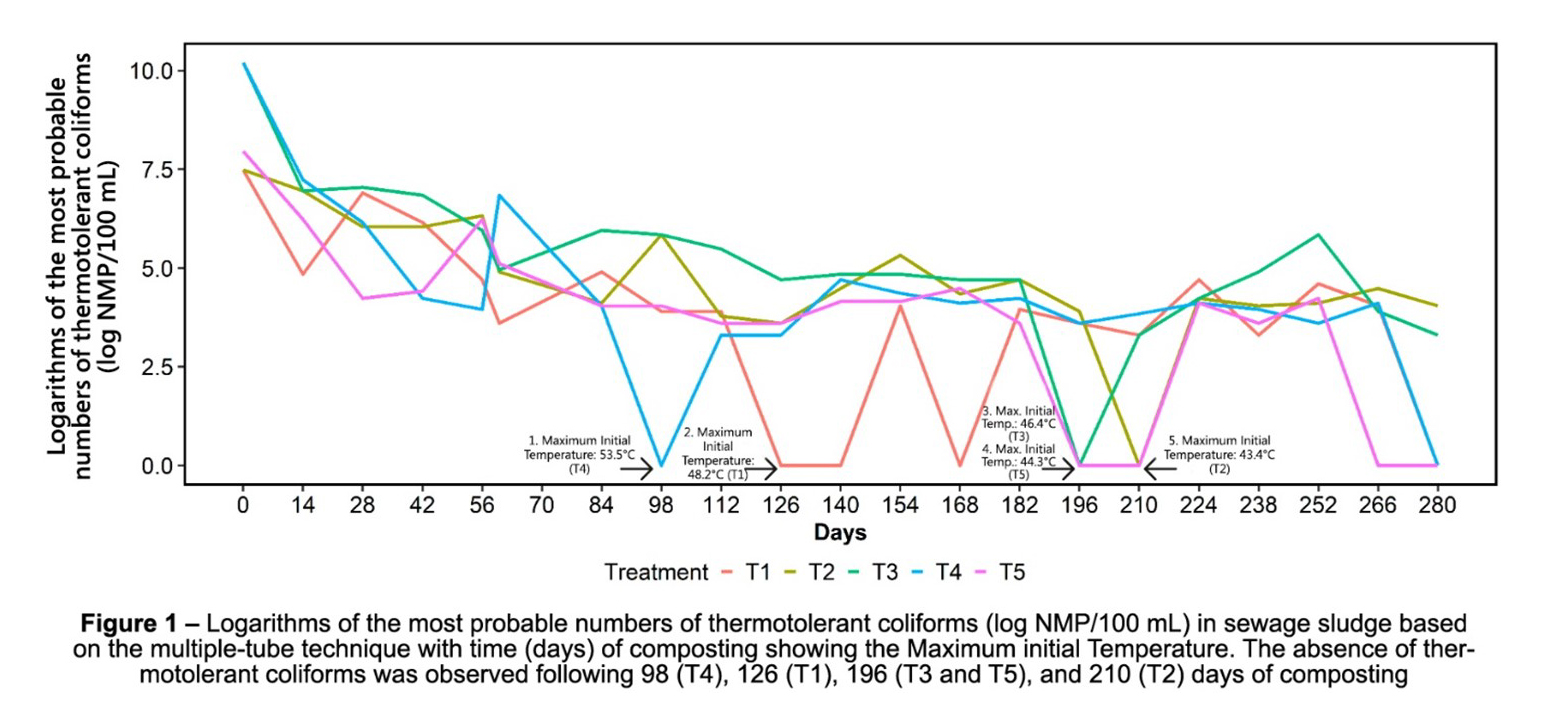
The initial weights of the mixtures were 66.8 kg (T1), 60.8 kg (T2), 50.0 kg (T3), 75.0 kg (T4) and 35.0 kg (T5). Following composting (at 280 days), mass reductions of 27% (T1), 48% (T2), 63% (T3), 66% (T4) and 64% (T5) were observed. All treatments had higher temperatures at the beginning of composting, with maximum temperatures of 48.2°C (T1), 43.4°C (T2), 46.4°C (T3), 53.5°C (T4) and 44.3°C (T5) occurring on the second day.
Microbiological analysis of the raw SS demonstrated the presence of 8.204 log NMP/100 mL total coliforms and 7.544 log NMP/100 mL thermotolerant coliforms, which exceed the legally allowed value of <3 log NMP/100 mL (Paraná, 2009). The high SS coliform count was reflected in the initial results of the treatments (Table 1).
Table 1
Logarithms of the most probable numbers of thermotolerant coliforms (log NMP/100 mL) in sewage sludge based on the multiple-tube technique with time (days) of composting
|
Days |
Treatment |
||||
|
T1 |
T2 |
T3 |
T4 |
T5 |
|
|
zero |
7.48 |
7.48 |
10.20 |
10.20 |
7.95 |
|
14 |
4.84 |
6.95 |
6.95 |
7.23 |
6.23 |
|
28 |
6.90 |
6.04 |
7.04 |
6.15 |
4.23 |
|
42 |
6.15 |
6.04 |
6.84 |
4.23 |
4.41 |
|
56 |
4.70 |
6.32 |
5.95 |
3.95 |
6.23 |
|
60 |
3.60 |
4.90 |
4.95 |
6.84 |
5.11 |
|
84 |
4.90 |
4.11 |
5.95 |
4.04 |
4.04 |
|
98 |
3.90 |
5.84 |
5.84 |
0.00 |
4.04 |
|
112 |
3.90 |
3.78 |
5.48 |
3.30 |
3.60 |
|
126 |
0.00 |
3.60 |
4.70 |
3.30 |
3.60 |
|
140 |
0.00 |
4.48 |
4.84 |
4.70 |
4.15 |
|
154 |
4.04 |
5.32 |
4.84 |
4.36 |
4.15 |
|
168 |
0.00 |
4.34 |
4.70 |
4.11 |
4.48 |
|
182 |
3.95 |
4.70 |
4.70 |
4.23 |
3.60 |
|
196 |
3.60 |
3.90 |
0.00 |
3.60 |
0.00 |
|
210 |
3.30 |
0.00 |
3.30 |
3.84 |
0.00 |
|
224 |
4.70 |
4.23 |
4.23 |
4.11 |
4.11 |
|
238 |
3.30 |
4.04 |
4.90 |
3.95 |
3.60 |
|
252 |
4.60 |
4.11 |
5.84 |
3.60 |
4.23 |
|
266 |
4.04 |
4.48 |
3.90 |
4.11 |
0.00 |
|
280 |
0.00 |
4.04 |
3.30 |
0.00 |
0.00 |
T1= SS+SB; T2= SS+SB+TP; T3= SS+SB+GC; T4= SS+SB+PL; T5= SS+SB+TP+GC
The absence of thermotolerant coliforms was observed following 98 (T4), 126 (T1), 196 (T3 and T5), and 210 (T2) days of composting (Figure 1).
Helminth eggs were detected in the compounds and included Ascaris sp., Trichuris spp., Capillaria spp., Hymenolepis nana and Toxocara canis. The quantification of the total and viable helminth eggs, expressed as the number of eggs per gram of dry matter (number of eggs g/DM), and the percentages of viability and reduction on the first and last days of composting are described in Table 2. The raw SSs contained high numbers of eggs per gram of dry matter that were above those accepted by legislation (<0.25 viable eggs g/DM).
Regarding the Cryptosporidium spp. and Giardia spp. investigation, protozoan (oo)cysts were not observed when using typical fluctuation techniques in the 102 SS and treatment samples. Giardia spp. genomic material was identified in eight samples from 154 to 280 days of composting.
DISCUSSION
The reduced density of the final compost resulted from moisture loss and the degradation of organic matter by microorganisms in proportion to each type of mixture (Chowdhury et al., 2022).
During the thermophilic phase, the temperatures ranged from 55°C to 64.8°C, and at the end of composting, the temperatures ranged from 32°C to 40°C. At the beginning of the composting stage, mesophilic microorganisms degraded the organic matter. Because this reaction was exothermic, a gradual increase in the temperature of the compounds occurred. If the biomass temperature reaches 40°C to 60°C on the second or third day of composting, the ecosystem is balanced (Fernandes and Souza, 2001).
High temperatures favor the proliferation of thermophilic microorganisms, which favor the quick degradation of organic matter and can be used to eliminate pathogens.
Table 2
Numbers and percentages of viable helminth eggs per gram of dry matter observed on day zero and 280 days after sewage sludge composting
|
Time |
Treatment |
Number of eggs |
Number of viable eggs |
Viability |
Reduction |
|||
|
(days) |
———— g.DM-1 ———— |
—————– % ————— |
||||||
|
zero |
T1 |
5.96 |
1.79 |
30 |
70 |
|||
|
T2 |
8.61 |
1.52 |
18 |
82 |
||||
|
T3 |
2.74 |
1.37 |
50 |
50 |
||||
|
T4 |
3.27 |
1.40 |
43 |
57 |
||||
|
T5 |
3.52 |
1.96 |
56 |
44 |
||||
|
|
SS |
10.13 |
2.84 |
28 |
71 |
|||
|
280 |
T1 |
0.53 |
0 |
0 |
100 |
|||
|
T2 |
0 |
0 |
0 |
100 |
||||
|
T3 |
0 |
0 |
0 |
100 |
||||
|
T4 |
0.45 |
0 |
0 |
100 |
||||
|
T5 |
0.75 |
0 |
0 |
100 |
||||
T1= SS+SB; T2= SS+SB+TP; T3= SS+SB+GC; T4= SS+SB+PL; T5= SS+SB+TP+GC
According to Chowdhury et al. (2022), the ideal temperature for maintaining compounds in the thermophilic phase is between 55°C and 65°C. When most of the organic matter is degraded, the thermophilic population and the compound temperature decrease, generating mesophiles (Kim et al., 2017; Chowdhury et al., 2022).
The accelerated elimination of these coliforms in T4 corresponded with higher temperatures in the thermophilic phase (64.8°C), rapid organic matter degradation, and significant reductions in compound density (66%). The composition of T4 (SS+SB+PL) potentially influenced these results due to the combination of SS and chicken feces from PL, which resulted in a greater microbial load and in the decomposition of the compound mass. Therefore, an increase in the C/N ratio was suggested for composting this mixture to extend the composting time, decrease the rapid degradation of the material, and obtain more humidified organic matter.
The efficient elimination or reduction of pathogens by temperature depends on the exposure time of pathogenic microorganisms to higher temperatures (55°C to 65°C) during composting and uniform temperature distributions in the compound mass. To eliminate Escherichia coli in biosolids, an exposure of 60 minutes at 60°C is needed (Gupta et al., 2021; Chen et al., 2021; Chowdhury et al., 2022). In a study of composting sewage sludge with organic household waste and tree pruning residues, Heck et al. (2013) suggested that variations in coliform counts could potentially result from contamination by feces from birds, dogs, and other animals that can access the SS through open windrows.
The pathogen content of SS reflects the health conditions of the population and varies based on the geographical area and the type and quality of the SS treatment (Thomaz-Soccol et al., 1999). Paulino et al. (2001) noted that the large number of total helminth eggs (683.4 eggs per liter) in the biosolids originated from regions with low health standards, and an anaerobic treatment (anaerobic reactors) efficiency of 75% was not sufficient for decreasing the number of helminth eggs to acceptable levels.
Treatments reached the value dictated by legislation (Paraná, 2009) at 56 (T5), 70 (T3), 112 (T4), 126 (T1) and 154 (T2) days of composting. At 168 days of composting, none of the treatments contained viable eggs, and this continued until the end of the treatment (280 days). Under these conditions, the parasitological parameters were acceptable for reuse in agriculture.
Corrêa et al. (2007) observed a reduced rate of viable eggs (from 93% to 100%) and reported that treating sewage sludge with sawdust and wood chips resulted in 0.34 viable eggs g/DM in the second mesophilic phase of sewage sludge composting and vermicomposting with sawdust, wood chips, tree pruning residues and grass clipping. The authors reported that mixtures of mud and tree pruning and grass residues resulted in no viable helminth eggs.
While examining household organic waste, tree pruning residues and sewage sludge compost from the STP in Porto Alegre city, Heck et al. (2013) found that temperature and C/N ratio parameters agreed with the beginning and maturation stages of the process. In addition, Heck et al. (2013) observed that matured compost did not contain viable helminth eggs, Salmonella sp. or enteric viruses.
According to Thomaz-Soccol et al. (1999), raw sludge or derived products may threaten population health when not properly treated because infectious doses of these protozoa are low. The presence of Giardia spp. genomic material potentially resulted from the physical disintegration of the compost as the weight and height of the treatments decreased inside the boxes, which resulted in greater concentrations and better recovery of these parasites from the biomass. The two samples that were positive for G. duodenalis were T4 and T5, which had reduced weights of 66% and 64%, respectively.
CONCLUSIONS
The high temperatures produced by the thermophilic microorganisms when composting sewage sludge from agroindustrial and urban wastes were important for eliminating pathogen indicators of contamination, such as thermotolerant coliforms and viable helminth eggs (according to the values required by Brazilian legislation). The identification of Giardia spp. genomic material did not indicate the presence of viable protozoa. Thus, the resulting biosolids could be used for agricultural applications.
Author Contributions: L.O.: project coordinator. L.S.M.: assistance in the assembly of the practical part in the field, assistance in correcting the written part of the paper. T.R.S.: assistance in molecular analysis. J.C.A: assistance in molecular analysis. V.B.D.T.: assistance in molecular analysis. F.M.O.D.: assistance in the practical part in the field. D.S.R.: assistance in the statistical analysis and writing of the paper. T.C.M.: assistance in molecular analysis, assistance in the writing and publication of the paper. R.L.F.: co-supervisor of the project, assistance in the statistical analysis and correcting the written part of the paper. I.T.N.: supervisor of the project, assistance in correcting the written part of the paper. All authors declare that they have read and approved the publication of the manuscript in this present form.
Funding: The authors would wish to express their gratitude to the Fundação Araucária, Serviço Autônomo de Água e Esgoto (Bandeirantes-PR) and Laboratório de Bionformática UENP/CLM.
Conflicts of Interest: There are no conflicts of interest.
REFERENCES
Appelbee, A.J.; Frederick, L.M.; Heitman, T.L.; Olson, M.E. Prevalence and genotyping of Giardia duodenalis from beef calves in Alberta, Canada. Veterinary Parasitology. 2003, 112, 289-94. https://doi.org/10.1016/s0304-4017(02)00422-3.
Bentes, M.F.P.; Lopes, M.C.; Guimarães, D.F.S.; Câncio, I.A.P. The environmental impacts of solid waste disposal and environmental management in the municipality of Iranduba-AM. Research, Society and Development. 2023, 12, e7012137779.
Chen, G.; Zhang, R.; Guo, X.; Wu, W.; Guo, Q.; Zhang, Y.; Yan, B. Comparative evaluation on municipal sewage sludge utilization processes for sustainable management in Tibet. Science of The Total Environment. 2021, 765, 142676. https://doi.org/10.1016/j.scitotenv.2020.142676.
Chowdhury, S.D.; Bandyopadhyay, R.; Bhunia, P. Sludge treatment: an approach toward environmental remediation. Clean Energy and Resource Recovery. 2022, 2, 355-372. https://doi.org/10.1016/B978-0-323-90178-9.00015-9.
ESTC (Environmental Sanitation Technology Company). Total and fecal coliforms – Determination by the multiple tube technique. Technical norm, L5.202, 1993.
Corrêa, R.S.; Fonseca, Y.M.F.; Corrêa, A.S. Production of agricultural biosolids through composting and vermicomposting of sewage sludge (in Portuguese). Revista Brasileira de Engenharia Agrícola e Ambiental. 2007, 11, 420-6. https://doi.org/10.1590/S1415-43662007000400012.
Eid, E.M.; Alrumman, S.A.; El-Bebany, A.F.; Fawy, K.F.; Taher, M.A.; Hesham, A.E.; El-Shaboury, G.A.; Ahmed, M.T. Evaluation of the potential of sewage sludge as a valuable fertilizer for wheat (Triticum aestivum L.) crops. Environmental Science and Pollution Research. 2019, 26, 392-401. https://doi.org/10.1007/s11356-018-3617-3.
Faust, E.C.; Sawitz, W.; Tobie, J.; Odom, V.; Peres, C.; Lincicome, D.R. Comparative efficiency of various technics for the diagnosis of protozoa and helminths in feces. The Journal of Parasitology. 1939, 25, 241-262. https://doi.org/10.2307/3272508.
Fernandes, F.; Souza, S.G. Sewage sludge stabilization (in Portuguese). In: Resíduos sólidos do saneamento: processamento, reciclagem e disposição final. Rio de Janeiro, ABES, 2001, 29-55.
Gupta, A.K.; Minj, A.; Yadav, D.; Poudel, A. Utilization of solid or liquid wastes in agriculture. Journal of Wastes and Biomass Management. 2021, 3, 9-12. https://doi.org/10.26480/jwbm.01.2021.09.12.
Heck, K.; De Marco, É.G.; Hahn, A.B.B.; Kluge, M.; Spilki, F.R.; Van Der Sand, S.T. Evaluation of degradation temperature of compounds in a composting process and microbiological quality of the compost (in Portuguese). Revista Brasileira de Engenharia Agrícola e Ambiental. 2013, 17, 54-9. https://doi.org/10.1590/S1415-43662013000100008.
Hopkins, R.M.; Meloni, B.P.; Groth, D.M.; Wetherall, J.D.; Reynoldson, J.A.; Thompson, R.A. Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. Journal of Parasitology. 1997, 83, 44-51.
Kiehl, E.J. Composting manual: compost maturation and quality (in Portuguese), 6th ed. DeGaspari, Piracicaba, 2012, 171.
Kim, H.J.; You, J.E.; Park, C.J. Review of sewage and sewage sludge treatment in Korea. International Proceedings of Chemical, Biological and Environmental Engineering. 2017, 101, 68-74.
LAW NO. 14.026. New legal framework for basic sanitation. Brazil, July 15, 2020. Available at: http://www.planalto.gov.br/ccivil_03/_ato2019-2022/2020/lei/L14026.htm.
ME (Ministry of the Environment). National Council of the Environment. Resolution no. 375, of August 30, 2006. Available at: http://www.siam.mg.gov.br/sla/download.pdf?idNorma=5956.
ME (Ministry of the Environment). National Council of the Environment. Resolution no. 498, of August 19, 2020. Defines criteria and procedures for the production and application of biosolids in soils, and makes other provisions. 2020. Available at: http://conama.mma.gov.br/index.php?option=com_sisconama&andview=atonormativoand&id=726.
MRD (Ministry of Regional Development). National Sanitation Information System. Thematic diagnosis: Water and Sewage Services – Technical Management of Sewage. Brasilia: SNIS, 2022, 31.
Paraná. Secretary of State for the Environment and Water Resources. SEMA Resolution n. 021/09. Provides for environmental licensing, establishes environmental conditions and standards and makes other arrangements for sanitation projects. Official Gazette of the State of Paraná, Curitiba. 2009, 7962, 13-16.
Paulino, R.C.; Castro, E.A. Anaerobic treatment of sewage and its efficiency in reducing the viability of helminth eggs Helminth eggs and protozoan cysts in sludge obtained by anaerobic digestion process (in Portuguese). Revista da Sociedade Brasileira de Medicina Tropical. 2001, 34, 421-8. https://doi.org/10.1590/s0037-86822001000500004.
Rehman, R.A.; Qayyum, M.F. Co-composts of sewage sludge, farm manure and rock phosphate can substitute phosphorus fertilizers in rice-wheat cropping system. Journal of Environmental Management. 2020, 259, 109700. https://doi.org/10.1016/j.jenvman.2019.109700.
Roman, N. Microbial environmental risks associated sewage sludge disposal. Journal of Microbiological Biotechnology. 2018, 3, 131. https://doi.org/10.23880/oajmb-16000131.
Sheather, A.L. The detection of intestinal protozoa and mange parasites by a floatation technique. Journal of Comparative Pathology and Therapeutics. 1923, 36, 266-75. https://doi.org/10.1016/S0368-1742(23)80052-2.
Spoti, T.B.; Amaral, C.S.T. The challenges of domestic solid waste management in Brazil. Brazilian Journal of Development. 2023, 9, 8712-8724.
Thomaz-Soccol, V.; Paulino, R.C.; Castro, E.A. Pathogens: Helminths and Protozoa in biosolid (in Portuguese). In: Andreoli C, Fernandes F. (Eds), Reciclagem de biossólidos, transformando problemas em soluções. Companhia de Saneamento do Paraná (Sanepar), Curitiba, 1999, 156-179.
Thomaz-Soccol, V.; Paulino, R.C.; Castro, E.A. Methodology for parasitological analysis in sewage sludge (in Portuguese). In: Andreoli CV, Bonnet BRP. (Eds.), Manual de métodos para análises microbiológicas e parasitológicas em reciclagem agrícola de lodo de esgoto. Prosab, Curitiba, 2000, 27-41.
Xiao, L.; Morgan, U.M.; Limor, J.; Escalante, A.; Arrowood, M.; Shulaw, W.; et al. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Applied and Environmental Microbiology. 1999, 65, 3386-91. https://doi.org/10.1128/aem.65.8.3386-3391.1999
Academic Editor: Dr. Isabela Maria Simion
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Cabral Thaís Monica, Campos de Almeida Jonatas, De Oliveira Dias Fernanda Maria, Dos Santos Toledo Roberta, Freire Roberta Lemos, Matsumoto Leopoldo Sussumu, Navarro Italmar Teodorico, Rodrigues Diego Resende, Tabacow Victor Bittencourt Dutra