A. Kheloufi, L.M. Mansouri, H. Khettache
ABSTRACT. Cherimoya (Annona cherimola Mill.) has an exceptional flavor and aroma, which makes it a fruit with great potential. However, little is known about its propagation by seeds. According to the scientific literature, the germination of cherimoya seeds is affected much more by external conditions than by internal conditions. Germination of cherimoya variety ‘Concha Lisa’ were tested for germination at constant temperatures of 25, 30, 40°C, and at room temperature, varying from 20-25°C, coupled with total darkness. Seeds were sown in Petri dishes (0.8% agar water), for 25 days of incubation. The kinetics of germination was determined according to five closely related parameters, viz. final germination percentage (FGP), mean germination time (MGT), coefficient of velocity of germination (CVG), time to 50% germination (T50) and seedling length (SL). The temperature of 30°C was found optimally suitable with 70.8% FGP, 17.5 days MGT and 3.91 cm SL, while the room temperature of 20-25°C slightly improved germination with only 25% FGP. Furthermore, significant decrease in FGP and SL was observed at 25°C and 40°C of temperature in comparison to 30°C. The analysis also revealed that cherimoya seed germination, day 10-15 after seed sowing is suitable for final counts. An overview on the emergence of cherimoya seedlings, during a 12-week period in pots is presented.
Keywords: agriculture; Annonaceae; custard apple; exotic fruit; fruit tree; seed quality.
View full article (HTML)
Seed Germination and Seedling Establishment of Cherimoya (Annona Cherimola Mill.) at different Temperatures
A. Kheloufi1*, L.M. Mansouri1, H. Khettache1
1Department of Ecology and Environment, University of Batna 2, Batna 05078, Algeria
*E-mail: abdenour.kheloufi@yahoo.fr
Received: Apr. 16, 2020. Revised: May 20, 2020. Accepted: May 27, 2020. Published online: July 18, 2020
ABSTRACT. Cherimoya (Annona cherimola Mill.) has an exceptional flavor and aroma, which makes it a fruit with great potential. However, little is known about its propagation by seeds. According to the scientific literature, the germination of cherimoya seeds is affected much more by external conditions than by internal conditions. Germination of cherimoya variety ‘Concha Lisa’ were tested for germination at constant temperatures of 25, 30, 40°C, and at room temperature, varying from 20-25°C, coupled with total darkness. Seeds were sown in Petri dishes (0.8% agar water), for 25 days of incubation. The kinetics of germination was determined according to five closely related parameters, viz. final germination percentage (FGP), mean germination time (MGT), coefficient of velocity of germination (CVG), time to 50% germination (T50) and seedling length (SL). The temperature of 30°C was found optimally suitable with 70.8% FGP, 17.5 days MGT and 3.91 cm SL, while the room temperature of 20-25°C slightly improved germination with only 25% FGP. Furthermore, significant decrease in FGP and SL was observed at 25°C and 40°C of temperature in comparison to 30°C. The analysis also revealed that cherimoya seed germination, day 10-15 after seed sowing is suitable for final counts. An overview on the emergence of cherimoya seedlings, during a 12-week period in pots is presented.
Keywords: agriculture; Annonaceae; custard apple; exotic fruit; fruit tree; seed quality.
INTRODUCTION
The cherimoya (Annona cherimola Mill.) is a subtropical fruit tree of the family Annonaceae. Cherimoya has one of the highest market approval scores among all commercially produced fruits (Kader and Yahia, 2011). The flesh is smooth, delicate, sweet and fragrant with a custard-like texture. Few people, however, know of this crop because it is not produced on their continent. This fruit has a good potassium content (compared to its sodium content) and other micronutrients, such as phosphorus, calcium and magnesium (Eilers et al., 2011). Due to this composition, cherimoya concentrate is a perfect health care product (González et al., 2010). Cherimoya has been shown to have antioxidant benefits, reduce the risk of some forms of cancer and reduce the risk of certain heart and blood pressure diseases (Guruvayoorappan et al., 2015).
The growing region of cherimoya extends over many countries of Central and South America and it has been planted in most subtropical regions of the world (Pinto et al., 2005). Cherimoyas are mostly commercially cultivated in areas that are congenial to the planting of avocados (Palma et al., 1993). For several countries, cherimoya matures for late winter or early spring (Grossberger, 1999). Spain is the world’s major producer of cherimoya, and ‘Fino de Jete’ is the leading cherimoya cultivar (Padilla and Encina, 2003). There are usually two commercial varieties generally exist, a smooth skin variety (‘Concha Lisa’) and hillocky skin variety (Bronceada) (Palma et al., 1993).
Most Annona species are propagated by grafting and budding (George and Nissen, 1987; Joshi et al., 2000). With few exceptions, annonas are deciduous, even tropical species, especially when cultivated in areas with dry or cool seasons and without irrigation (Pinto et al., 2005). With the exception of a few cultivars, clonal propagation of cherimoya and Annona hybrids by cuttings or marcottage has not been very successful (Pinto et al., 2005). However, variation in rootstock yield of seedlings is a major cause of scion yield and reduction in fruit quality (George and Nissen, 1987). Propagation of cherimoya by stem cuttings was not effective either (Bourke, 1976). In addition, low and erratic germination of seeds is one of the problems in cherimoya cultivation (De Smet et al., 1999). Cherimoya seeds exhibit irregular germination distribution with germination occurring over a long period of time, nuking problems of generative propagation. This pattern of germination is possibly due to the existence of dormancy, natural defense to give seedlings a better chance of survival, by inducing germination under ideal environmental conditions, and away from the mother plant, thereby preventing competition (Lambers and Oliveira, 2019).
Germination of freshly harvested cherimoya seed may be highly variable and has been reported to range from 30 to 80% (George and Nissen, 1987). Seed scarification and stratification do not improve germination of this species (Jubes et al., 1975; Padilla and Encina, 2003). Gibberellic acid and high temperature have been shown to enhance germination of cherimoya seeds (Padilla and Encina, 2003). The particular characteristic of Annona seed is the presence of a primitive, slow-growing embryo, which is often not yet distinguished when the fruit is ripe (De Smet et al., 1999). The development of the embryo continues in the seed after harvesting the fruits (Garwood, 1995).
The objective of the present study was, firstly, to investigate some morphological characteristics of the seeds, secondly, to evaluate the effect of different temperatures and to observe the germination curves in order to determine the optimal condition to enhance and homogenize germination.
MATERIALS AND METHODS
Seed characteristics and sampling
This study focused on cherimoya, Annona cherimola var. ‘Concha Lisa’. Ten fruits of approximately 400 g weight were collected from mature commercial orchards in Granada (Spain), on November 2019. Each fruit contained 60-70 seeds. The seeds were extracted by opening the fruits and removing the pulp. Seeds were then cleaned and air dried for 10 days (Fig. 1). The seed sample for our experiment was obtained by mixing the seeds to minimize inter-genetic variation (Kheloufi et al., 2017). Seeds of length: 15.1 ± 1.25 mm, width: 9.98 ± 1.59 mm, thickness: 5.73 ± 0.61 mm, weight: 0.46 ± 0.04 g, n = 50) were then stored in a bottle glass at 4°C, for one month. The thousand-seed weights 466 g.
Germination test in Petri dishes
The research experiment was carried out at the Laboratory of the Department of Ecology and Environment‚ University of Batna 2, Algeria. The experiment was performed in January-February, 2020, at three constant temperatures of 25, 30, 40°C, and at room temperature, varying from 20-25°C. To determine the effect of temperature during germination, a total of four replicates of 12 seeds were disinfected with 1% sodium hypochlorite for one minute, rinsed with distilled water and immediately sown on 0.8% (water agar), in 9 cm Petri dishes, under aseptic conditions.
Seeds were incubated simultaneously for 25 days, in the dark, under three continuous temperature regimes (25, 30 and 40°C) and room temperature (20-25°C). Seed germination was under dark conditions because we were only interested in studying the impact of temperature on seed germination, ignoring the influence of other factors, such as light. Germination counts were performed daily, to determine germination kinetics. Seeds were considering germinating only when 2 mm radicles emerged. The parameters evaluated were as follows:
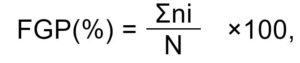
where, FGP is the final germination percentage, ni is the number of seeds germinated on the last day of testing, and N is the total number of seeds incubated per test (Côme, 1970). However, FGP only reflects the final percentage of germination attained and provides no picture of the speed or uniformity of germination.
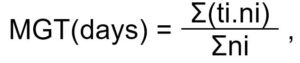
where, MGT is the mean germination time, ti is the number of days since the start of the test, ni is the number of germinated seeds recorded at time ti, and Σni is the total number of germinated seeds (Orchard, 1977).
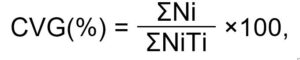
where, CVG is the coefficient of velocity of germination, Ni is the number of seeds germinated each day, Ti is the number of days from sowing corresponding to N (Jones and Sanders, 1987). The coefficient of velocity of germination gives an indication of the rapidity of germination. It increases when the number of germinated seeds increases and the time required for germination decreases. Theoretically, the highest CVG possible is 100, which is the case if all seeds germinated on the first day (Jones and Sanders, 1987).
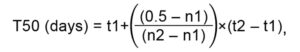
where, T50 (days) is the time up to 50% germination, n1 is the cumulative percentage of germinated seeds, which value is closest to 50% by a lower value, n2 is the cumulative percentage of germinated seeds, which value is the closer to 50% by higher value, t1 is the time necessary for the germination of n1 of seeds, t2 is the time necessary for the germination of n2 of seeds (Côme, 1970).
The lengths of eight randomly selected seedlings (SL) per thermal condition were also recorded at the end of the experimental period using a digital caliper.

Figure 1 – Cherimoya (Annona cherimola): A – Fruits; B, C – Seeds; D – Seed germination and seedling emergence after 25-day period
Statistical analysis
The experiments were conducted with four replicates of 12 seeds (n=4), for the trait seed germination, and with eight replicates (n=8), for the seedlings lengths, and the results were expressed as mean ± standard deviation (SD). All the data were subjected to one-way analysis of variance (ANOVA) and Duncan’s multiple range test (P< 0.05), using SAS Version 9.0 (Statistical Analysis System) (2002) software.
RESULTS AND DISCUSSION
Germination of cherimoya seeds
The effect of temperature on the germination kinetic of cherimoya seeds is illustrated on Fig. 2, in which three stages are indicated: a first period of latency due to the imbibition, a second exponential period where germination is accelerated and a stationary phase follows. The differential behavior of the ‘Concha Lisa’ cherimoya variety indicated that the seed germination potential was significantly (p< 0.001) influenced by temperature (Table 1). Temperature may affect the percentage and rate of germination by affecting dormancy loss and the germination process itself (Khurana and Singh, 2001; Dresch et al., 2014).
Temperature regimes and number of days to count have affected cherimoya seed germination (Fig. 2). Indeed, temperature fluctuations, number of days to count and their interactions were highly important (p< 0.001). The percentage of germination and days to germination varied according to temperature. The maximum seed germination was found on 10th day at 30°C, 15th day at 25°C, and on 20th day at 40°C and room temperature (20-25°C). The mean percentage of seed germination over temperatures ranged from 25% (room temperature) to 70.8% (30°C) (Fig. 2, Table 1).
The primary environmental factors that influence germination in all species are temperature and water supply, influencing both the rate and the final percentage of germination. A quantitative analysis of germination sensitivity to temperature would enable better prediction of the potential impacts of temperature fluctuations on this crucial stage in the plant’s life cycle (Dürr et al., 2015). Characterization of the threshold values for germination can thus define the limits to the thermal environment a species will tolerate (Lockwood, 2011; Orrù et al., 2012).
The differences in germination speed due to temperature, as shown by the position and slope of the curves in Fig. 2, were more or less as might be expected. While the first seeds germinated at 30°C as rapidly, a minor slowing at 25°C and a pronounced pause at 40°C and room temperature was observed. Nevertheless, the fact that most of the seeds remaining at the end of the experiment were still apparently sound indicates that some factor was operating to prevent their germination. Indeed, at the end of the experiment, those seeds were transplanted and a significant percentage could germinate in potting soil after a month under greenhouse condition at a temperature of 27 ± 2°C.
According to Table 1, cherimoya seeds exhibit variable behaviors (p< 0.0001) at various temperature degrees studied at several parameters, viz. the final germination percentage (FGP), and the mean germination time (MGT), the coefficient of velocity of germination (CVG) and time to 50% germination (T50). In Petri dishes, these parameters were measured over an incubation period of 25 days. These results showed temperature effect, which played a very important role in germination activity induction. Indeed, temperature of 30°C improved cherimoya seeds by higher germination rate as indicated by lower MGT (17.5 days) and T50 (15.2 days), with higher FGP (70.8%), CVG (57.1%) and SL (3.91 cm) (Table 1). On the other hand, the lowest germination was recorded in the seed batch with a 20-25°C incubation, reporting a too long MGT (20 days) and T50 (17.6 days) value with a low CVG of 49.8% and 25% FGP (Table 1).
Table 1
Final germination percentage (FGP), mean germination time (MGT), coefficient of velocity of germination (CVG), time to 50% germination (T50) (n=4) and seedling length (SL) (n=8) of Annona cherimola seeds exposed to different temperature regimes (n=4)
|
Temperatures |
FGP (%) |
MGT (days) |
CVG (%) |
T50 (days) |
SL (cm) |
|
25°C |
35,4 ± 9,4b |
17,6 ± 0,25c |
56,7 ± 0,08a |
15,1 ± 0,31c |
1.90 ± 0.45c |
|
30°C |
70,8 ± 4,8a |
17,5 ± 0,03c |
57,1 ± 0,05a |
15,2 ± 0,01c |
3.91 ± 0.39a |
|
40°C |
33,3 ± 7,7b |
18,2 ± 0,48b |
54,9 ± 0,15c |
16,2 ± 0,79b |
1.41 ± 0.18c |
|
20-25°C |
25,0 ± 8,7b |
20,1 ± 0,18a |
49,8 ± 0,04c |
17,6 ± 0,25a |
2.58 ± 0.46b |
|
F of Fisher |
16.04 |
70.77 |
60.87 |
29.81 |
39.94 |
|
P |
0.0002 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
The same alphabet along the column indicates no significance difference (Duncan Multiple Range Test).
Cherimoya embryo has been found to be small (3-4 mm) and is located inside an impermeable testa, with the micropyle being the only water entry (De Smet et al., 1999). The optimum temperature for germination of cherimoya and Annona hybrid cultivars is between 28 and 32°C (Sanewski, 1985).
Seed can germinate in three weeks under these conditions. At lower temperatures (15-20°C), germination is delayed by 3-4 months and the germination percentage decreases (George and Nissen, 1986). Seed of most Annona species rapidly loses viability and should be planted as soon as possible after removal from the fruit (George et Nissen, 1987). However, scarification of sugar apple and cherimoya seed does not improve seed germination or reduce the period of time for germination (Jubes et al., 1975; Padilla and Encina, 2003). The poor germination rate of some Annona species or cultivars can also be caused by a high proportion of infertile seed (Barnes, 1943). The propagation of most Annona species by seed is not recommended since the seedlings are genetically diverse and most are characterized by a long juvenile period, irregular bearing and
poor fruit quality (Campbell and Phillips, 1983).
The growth of cherimoya seedlings, over a 12-week period, is demonstrated in Fig 3.
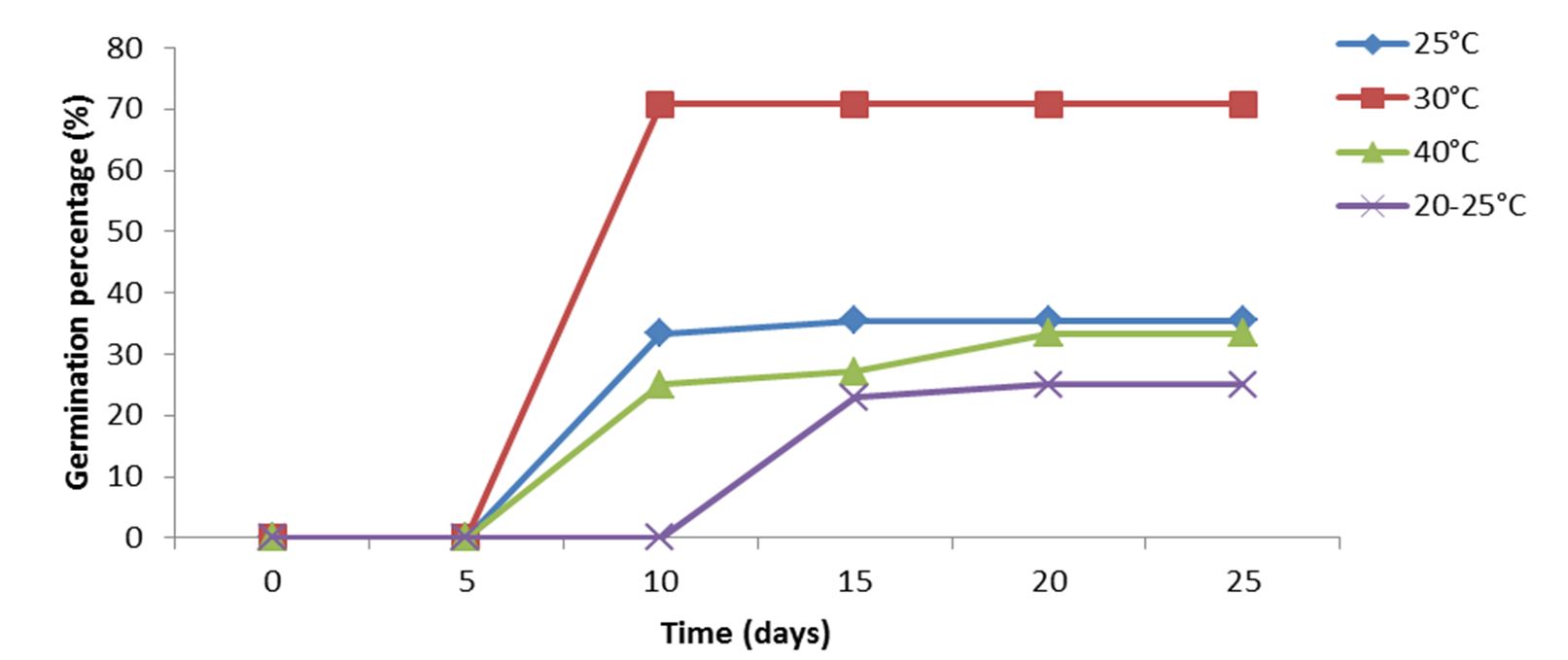
Figure 2 – Effect of temperature on seed germination of Annona cherimola in Petri dishes (0.8% Agar water) for 25 days of incubation

Figure 3 – Overview on the emergence of cherimoya seedlings during a 12-week period in pots under greenhouse conditions (27 ± 2°C temperature and 35% relative humidity)
Cherimoya seeds were directly germinated (2 cm depth) in plastic pots containing 1.0 kg of mixed substrate (two volumes of sand mixed with one volume of potting soil), under greenhouse conditions (27 ± 2°C temperature and 35% relative humidity). According to George and Nissen (1987), seedlings should be grown in root trainers as the root systems are very susceptible to twisting and strangulation and when the seedlings are 10-15 cm high, they can be transplanted into either polythene bags or nursery beds. Polythene containers are effective of handling and less disturbance to the root system at planting (Sanewski, 1985). Polybags with a capacity of 8 L and a depth of 30 cm are used to prevent root strangulation. Seedlings of the Annona hybrids and cherimoya grown in polybags are typically able to be grafted 12-18 months after transplantation. In the early autumn months of September-November, grafted plants are usually transplanted on the ground. There are significant variations in the growth rates of various Annona species seedlings (George and Nissen, 1987).
CONCLUSION
Temperature affected time to germinate and germination percent in cherimoya. Cherimoya seeds showed maximum germination at 30°C constant and controlled temperature with 70.8% of final germination rate and 17.5 days of mean germination time. This temperature appears to be the actual optimum for seed germination and the establishment of seedlings. The seeds of cherimoya are non-dormant and do not require any pre-treatment to germinate. This result could be suggested as a contribution for a crop production protocol in nurseries or for other agronomic studies.
Acknowledgements. Authors are thankful to Samir Hocini, Laboratory Engineer in Plant Biology (University of Oran, Algeria), for his great help in providing cherimoya fruit.
REFERENCES
Barnes, H. (1943). Custard apple. Queensl.Agric.J., 57: 147-149.
Bourke, D. O’D. (1976). Annona spp. In: The propagation of tropical fruit trees. R.J. Garner and S.A. Chaudhri (Eds.), Hortic.Rev., No. 4. Commonwealth Bureau of Horticulture and Plantation Crops, East Mailing,Kent, pp. 223-248.
Campbell, C.W. & Phillips, R.L. (1983). The atemoya. Fruit crops fact sheet. Florida Cooperative Extension Services, University of Florida, pp. 1-3.
Côme, D. (1970). Obstacles to germination. Masson et Cie., Paris, 162 p.
De Smet, S., Van Damme, P., Scheldeman, X. & Romero, J. (1999). Seed structure and germination of cherimoya (Annona cherimola Mill.). Acta Hortic. 497: First International Symposium on Cherimoya, pp. 269-288, DOI: 10.17660/ActaHortic.1999.497.14
Dresch, D.M., Scalon, S.P. & Masetto, T.E. (2014). Effect of storage in overcoming seed dormancy of Annona coriacea Mart. seeds. An. Acad.Bras.Ciênc., 86(4): 2077-2085, DOI: 10.1590/0001-3765201420130 276
Dürr, C., Dickie, J.B., Yang, X.Y. & Pritchard, H.W. (2015). Ranges of critical temperature and water potential values for the germination of species worldwide: contribution to a seed trait database. Agr. Forest Meteorol., 200: 222-232.
Eilers, E.J., Kremen, C., Greenleaf, S.S., Garber, A.K. & Klein, A.M. (2011). Contribution of pollinator-mediated crops to nutrients in the human food supply. PLoS ONE, 6(6): e21363, DOI: 10.1371/journal.pone.0021363
Garwood, N.C. (1995). Studies in Annonaceae. XX. Morphology and ecology of seedlings, fruits and seeds of selected Panamian species. Botanischer Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie, 117(1-2): 1-152.
George, A.P. & Nissen, R.J. (1986). Custard Apple. Biennial Report Maroochy Horticulture Research Station, 4: 46-68.
George, A.P. & Nissen, R.J. (1987). Propagation of Annona species: a review. Sci.Hortic., 33(1-2): 75-85, DOI: 10.1016/0304-4238(87)90034-3
González, J.C., Fernández, M.G. & Martín, J.J.H. (2010). Cherimoya and loquat. Soils, plant Growth and crop production. Encyclopedia of Life Support Systems (EOLSS), 33 p.
Grossberger, D. (1999). The California cherimoya industry. In: First International Symposium on Cherimoya 497, pp. 119-142, DOI: 10.17660/ActaHortic.1999.497.6
Guruvayoorappan, C., Sakthivel, K.M., Padmavathi, G., Bakliwal, V., Monisha, J. & Kunnumakkara, A.B. (2015). Cancer preventive and therapeutic properties of fruits and vegetables: an overview. In: Kunnumakkara (Ed.), Anticancer Properties of Fruits and Vegetables: A Scientific Review, World Scientific publisher, Singapore, pp. 1-52
Jones, K.W. & Sanders, D.C. (1987). The influence of soaking pepper seed in water or potassium salt solutions on germination at three temperatures. Journal of Seed Technology, 11(1): 97-102, AGR: ADL87055683
Joshi, P.S., Bhalerao, P.S., Mahorkar, V.K. & Jadhav, B.J. (2000). Studies on vegetative propagation in custard apple (Annona squamosa L.). PKV Res.J., 24(2): 103-105.
Jubes, J.T., Martinez, H., Padilla, E. & Oste, C.A. (1975). Effects of mechanical scarification, substrate, seed position and gibberellic acid on germination of cherimoya. Revista agronómica del Noroeste Argentino, 12: 1961-172.
Kader, A.A. & Yahia, E.M. (2011). Postharvest biology of tropical and subtropical fruits. In: Postharvest Biology and Technology of tropical and subtropical fruits, pp. 79-111, Woodhead Publishing, DOI: 10.1533 /9780857093622.79
Kheloufi, A., Mansouri, L.M. & Boukhatem, Z.F. (2017). Application and use of sulfuric acid to improve seed germination of three acacia species. Reforesta, 3: 1-10, DOI: 10.21750/REFOR.3.01.25
Khurana, E. & Singh, J.S. (2001). Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: a review. Environ. Conserv., 28(1): 39-52, DOI: 10.10 17/S0376892901000042
Lambers, H. & Oliveira, R.S. (2019). Life cycles: environmental influences and adaptations. In: Plant physiological ecology, pp. 451-486. Springer, Cham.
Lockwood, R. (2011). Tropical fruits. Vol. 1, 2nd ed., By R.E. Paul and O. Duarte, Wallingford, UK, pp. 400. DOI: 10.1017/S0014479711000639
Orchard, T. (1977). Estimating the parameters of plant seedling emergence. Seed Sci.Technol., 5(1): 61-69.
Orrù, M., Mattana, E., Pritchard, H.W. & Bacchetta, G. (2012). Thermal thresholds as predictors of seed dormancy release and germination timing: altitude-related risks from climate warming for the wild grapevine Vitis vinifera subsp. sylvestris. Ann.Bot., 110: 1651-1660, DOI: 10.1093/aob/mcs218
Padilla, I.M.G. & Encina, C.L. (2003). In vitro germination of cherimoya (Annona cherimola Mill.) seeds. Sci. Hortic., 97(3-4): 219-227, DOI: 10.1016/S0304-4238(02)00160-7
Palma, T., Aguilera, J.M. & Stanley, D.W. (1993). A review of postharvest events in cherimoya. Postharvest Biol.Technol., 2(3): 187-208, DOI: 10.1016/0925-5214(93)90047-7
Pinto, A.D.Q., Cordeiro, M.C.R., De Andrade, S.R.M., Ferreira, F.R., Filgueiras, H.D.C., Alves, R.E. & Kinpara, D.I. (2005). Annona species. Embrapa Cerrados-Livro científico (ALICE), Southampton: International Centre for Underutilised Crops, University of Southampton, 263 p.
Sanewski, G.M. (1985). Custard apple propagation. Queensland Department of Primary Industries Fact Sheet, Horticulture Branch, pp. 1-3.



