F. Moradinezhad, M. Mehregan, M. Jahani
ABSTRACT. The loss of chemical characteristics and quality of the fresh seedless barberry fruit during storage and qualitative losses of its dried fruit are the most important postharvest challenges in barberry industry and its exports. The fresh harvested fruit samples were dried using an electrical drier at 50°C to 50% moisture content. Thereafter, the effects of hot water alone (65°C for 45 sec), and hot water + 2% calcium chloride were carried out on the quality maintenance and chemicals during the cold storage of seedless barberry. The results showed that the samples treated with calcium chloride stored at 2°C had the highest TSS over time, whereas the titratable acidity of barberry fruits was not significantly affected by postharvest treatments. Hot water alone or in combination with calcium chloride treatment increased redness and chroma values result in better appearance quality than control. In addition, the treatments reduced the variable L* and thereby enhanced fruit lightness. The highest antioxidant content (% 77.92) was observed in hot water treated samples and the lowest (% 54.28) was obtained on control. Also, the highest amount of anthocyanins and antioxidants were obtained from samples treated with hot water. Only calcium chloride treatment had a significant effect on Ca content of the samples. The results revealed that postharvest application of hot water and calcium chloride treatments improved the appearance quality and nutritional values of fresh seedless barberry fruit, as well as extend the cold storage life, likely due to reduced pathogen contamination.
Keywords: anthocyanins; antioxidants; hot water treatment; quality; postharvest.
View full article (HTML)
Physicochemical traits of seedless barberry (Berberis Vulgaris L.) fruits stored under refrigeration as affected by heat and calcium chloride treatments
F. Moradinezhad1*, M. Mehregan1, M. Jahani2
1Department of Horticultural Science, College of Agriculture, University of Birjand, Birjand, Iran
2Department of Plant Protection, College of Agriculture, University of Birjand, Birjand, Iran
*E-mail: fmoradinezhad@birjand.ac.ir
Received: June 05, 2018. Revised: Oct. 11, 2018. Accepted: Oct. 31, 2018. Published online: Oct. 3, 2019
ABSTRACT. The loss of chemical characteristics and quality of the fresh seedless barberry fruit during storage and qualitative losses of its dried fruit are the most important postharvest challenges in barberry industry and its exports. The fresh harvested fruit samples were dried using an electrical drier at 50°C to 50% moisture content. Thereafter, the effects of hot water alone (65°C for 45 sec), and hot water + 2% calcium chloride were carried out on the quality maintenance and chemicals during the cold storage of seedless barberry. The results showed that the samples treated with calcium chloride stored at 2°C had the highest TSS over time, whereas the titratable acidity of barberry fruits was not significantly affected by postharvest treatments. Hot water alone or in combination with calcium chloride treatment increased redness and chroma values result in better appearance quality than control. In addition, the treatments reduced the variable L* and thereby enhanced fruit lightness. The highest antioxidant content (% 77.92) was observed in hot water treated samples and the lowest (% 54.28) was obtained on control. Also, the highest amount of anthocyanins and antioxidants were obtained from samples treated with hot water. Only calcium chloride treatment had a significant effect on Ca content of the samples. The results revealed that postharvest application of hot water and calcium chloride treatments improved the appearance quality and nutritional values of fresh seedless barberry fruit, as well as extend the cold storage life, likely due to reduced pathogen contamination.
Keywords: anthocyanins; antioxidants; hot water treatment; quality; postharvest.
INTRODUCTION
Seedless barberry tree (Berberis vulgaris L.) belongs to the family of Berberidaceae (Zargari, 1993).
Barberry is one of the important fruits owing to its extensive medicinal properties, its resistance to a wide range of adverse water and soil conditions, its role in the industry, and its environmental impact in Iran. Fresh barberry fruits have high water content (about 80%) at harvest time. Therefore, normally, the main part of the harvested fruit is dried to reduce microbial contamination leading to postharvest losses along the supply chain. In this respect, the main problems for the barberry industry are related to its traditional drying methods and its improper handling and packaging (Kafi et al., 2002). The drawbacks of traditional methods of barberry processing, including the extensive reduction of the fruit moisture for its longevity and the long duration of its drying by traditional methods, reduce its postharvest quality and safety. In addition, demand for fresh small fruits, like barberry and cranberry, has been raised in the market during the last decade because of consumers awareness about the high nutritional value of fresh produce, needed for human well-being. Hence, postharvest treatments are essential to minimize microbial spoilage and reduce the risk of pathogen contamination for fresh fruits.
Calcium chloride (CaCl2) is widely applied to fruits and vegetables, in either full or cut forms, as a preservative and tissue hardening agent (Martin-Diana et al., 2007). It is known that aging rate depends on the tissue Ca content, so that higher Ca content influences the parameters underpinning senescence, such as res-piration, protein content, chlorophyll, and membrane fluidity (Poovaiah, 1986). A study on the effect of Ca on mechanical properties of potatoes and the cell wall reported that Ca application alleviated the stress of degradation of potato tissue and cell wall (Cybulska et al., 2011). Similarly, Moradinezhad and Khayyat (2014) reported that the treatments with hot water, 2% CaCl2 and 2 mM salicylic acid (SA), extended the shelf life and inhibited the decay of pomegranates, compared to the control. It has been shown that post-harvest Ca treatment extended the shelf life of strawberries, so that the dipping of strawberries in 1% CaCl2 solution increased Ca content of the fruit tissue, maintained fruit firmness, and controlled postharvest decay of the fruits (Garcia et al., 1996). Heat treatment has been applied in different studies, as an alternative to chemical treatments for harvested fresh fruits and vegetables (Mahajan et al., 2014). Heat treatment, especially in the form of hot water, to remove pests and fungal diseases in plants has commenced being commercially applied since the early 20th century (Escribano and Mitcham, 2014).
Then, interests aroused to this safe technique, that merely uses heat as an alternative to chemical treatments to control postharvest decay of the harvested fresh fruits (Fallik, 2004). The heat treatment may be applied in several ways to fresh fruits and vegetables, but hot water dip is most preferred because it is more efficient than air in heat transfer (Fallik, 2004).
A recent study focused on the effect of pre-storage treatment, including the immersion of stone fruits in water with various temperature (24-70°C) on the control of brown rot and found that immersion in 60°C hot water for 60 sec. reduced the disease by as high as 73% in peach and nectarine fruits during cold storage (Karabulut et al., 2010).
Previous studies have revealed that hot water dip is adequately effective on maintaining the quality of some freshly cut crops, like lettuces (Murata et al., 2004; Moreira et al., 2006), eggplants (Barbagallo et al., 2012), and onions (Siddiq et al., 2013).
As the literature shows, there is little information about the effects of hot water dip and CaCl2 treatments and their combination on the postharvest response of fresh seedless barberry fruits held at cold storage.
Postharvest treatments may make it possible to maintain the nutritional quality of barberry, even when it has higher moisture content because fresh fruits possess the highest chemical quality, but they can be stored only for a short time or in frozen form.
Therefore, in this report, we examined the effect of hot water and CaCl2 treatments on semi-moist barberry fruit in order to reduce losses and to extend storage life, as well as to improve fruit quality.
MATERIAL AND METHODS
Preparation of plant material and treatments
Uniform branches from determined trees were harvested from Research Orchard of the University of Birjand, Birjand, Iran, and transferred to the physiology laboratory on October 23, 2015. Fresh fruits were then separated from the branches and stored at 10°C before treatment.
The fresh fruit samples were dried using an electrical drier (Suzuki Food Dehydrator, ZFD-1200, China) at 50°C to 50% moisture content. Thereafter, fruits were immersed either in a solution of 65°C hot water + 2% CaCl2 or in hot water (65°C), alone for 45 sec. The control samples were immersed in distilled water at 25°C and for 45 sec. Afterward, they were packed in LDPE bags (10 × 15 cm, 0.02 mm thickness) with normal atmosphere and were stored in a refrigerator at 2±0.5°C.
The physical traits (color compo-nents) and chemical traits (total soluble solids, titratable acidity, anthocyanin, antioxidant activity, phenolic compounds, and fresh fruit Ca content) were measured after 45 days of cold storage.
Color measurement of the samples
The following color components of the fruits were determined with a color-meter (TES-135 A, Taiwan), after three weeks of cold storage: L* (lightness), a* (-greenness to + redness) and b* (-blueness to + yellowness). The indicator C (Chroma) and h˚ (Hue) was determined by equations (1) and (2), respectively (Hosseini and Moradinezhad, 2018).
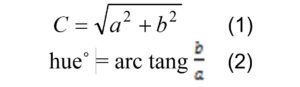
Measurement of total soluble solids
The fruit samples were oven-dried at 50°C and were then ground. Thereafter, 25 g was taken from each sample and mixed with 100 mL water and was extracted by a cloth filter with tiny mesh. The total soluble solids of the extract was measured with a hand-held refractometer (RF 10, 0-32%, Extech Co., USA) at 25°C, and expressed as °Brix.
Titratable acidity
To measure titratable acidity (TA), 5 mL of the extract was poured into a volumetric flask and was adjusted to 100 mL, by adding distilled water.
Then, the diluted extract was titrated with 0.1 N NaOH in the presence of phe-nolphthalein as an indicator (2-3 drops). Titration was terminated when a light pink color emerged for few seconds.
Fruit total acidity (in mg 100 mL-1 fruit juice) was calculated by the following equation (3) and was reported on the basis of malic acid (the dominant acid in barberries).

Total anthocyanin
Total anthocyanin content was quantified spectophotometrically by the pH differential method. A volume of 2 mL of the fruit extract was added with 25 mL of buffer solution (pH = 0.1), that contained a mixture of 0.2 M potassium chloride and 0.2 M hydrochloric acid. Then, another 2 mL of the plant extract was adjusted to 25 mL, by adding buffer solution (pH = 4.5), containing a mixture of 1 M sodium acetate and 1 M hydro-chloric acid.
The absorption by the samples was recorded at 510 nM (Rapisarda et al., 2000). Anthocyanin concentration was determined by equation (4).
C mg L–1 = (abs pH 2 – abs pH 4.5) × 484.82 × 1000/24825 × DF (4)
Measurement of antioxidant activity
Antioxidant activity (%) was determined by DPPH (2, 2-diphenyl-1-picrylhydrazyl) method. Briefly, a quantity of 1 g of the dried sample was separated and mixed with 10 ml of 70% methanol. Then, it was placed on a shaker for 10-15 min and was centrifuged at 150 rpm, after 20 min. The supernatant was collec-ted and refrigerated for 24 hrs. Afterward, 500 μL of the extract was mixed with 1.5 ml of DPPH solution, and was placed in darkness for 60 min, and recorded at 517 nm. The following equation (5) was used for the calculation.
|
Antioxidant activity (%) |
= |
absorbance of control – sample of absorbance × 100 |
(5) |
|
absorbance of control |
Measurement of phenolic compounds
Total phenolic compounds (TPH) were measured by Folin-Ciocalteau’s (FC) assay (Makkar et al., 1993). The absorbance was measured at 725 nm, using a spectrophotometer (Biospec 1601, Shimadzu, Tokyo, Japan). A calibration curve was also prepared using gallic acid (Merck KGaA, Darmstadt, Germany) and results were expressed as mg gallic acid per 100 g dry matter.
Measurement of calcium content
Calcium (Ca) content of the extracts was measured by atomic absorbance device (Li et al., 2007).
Statistical design and data analysis
The study was laid on a Randomized Complete Block Design with four replications. Data were analyzed by the GenStat (version 12.1, VSN, International, Ltd., UK, 2009) statistical program. Means were compared by Fisher’s LSD test at the 5% level.
RESULTS AND DISCUSSION
Color properties
Analysis of variance for fruit color components (a*, b*, C, and L*) revealed that the effect of treatments was significant (p< 0.01) on lightness (L*), redness (a*), and color intensity (C).
According to means comparison, the treatments of hot water alone and hot water + CaCl2 did not differ significantly and both improved color components in the stored fruits, compared to the control (Table 1). Accordingly, both treatments reduced the variable L* and thereby enhanced fruit lightness (Table 1) after 45 days of cold storage. Also, hot water increased redness, yellowness, and color intensity of the fruits by increasing a*, b*, and C, respectively (Table 1). It has been reported that the L* and hue values of cherries gradually decreased in storage, but Ca treatment postponed this loss for four weeks (Wang et al., 2015).
Brambilla et al. (2011) also found that the redness (a*) of blueberry fruits was increased when they were treated with hot water, that was in consistence with the presented results in our study. However, in a study on the immersion of freshly-cut papaya in 0.1% cinnamaldehyde and 0.75% calcium chloride, it was reported that L* and hue values were increased in compared to control (Albertini et al., 2016). Also, after six days of storage, Chroma in samples treated with 0.75% calcium chloride was higher than those of control and it was constant until the end of storage, whereas it was decreased in other treatments (Albertini et al., 2016), that was in agreement with our results.
Table 1
Effect of immersion treatments on the studied color components of fresh seedless barberries
|
Postharvest treatments |
Color components |
||||
|
L* |
a* |
b* |
C |
h˚ |
|
|
Control |
27.49 a |
35 b |
8.03 a |
36.02 b |
14.22 a |
|
Hot water |
24.09 b |
43.6 a |
9.12 a |
44.62 a |
11.74 a |
|
Hot water + 2% CaCl2 |
23.01 b |
40.4 a |
8.87 a |
41.44 ab |
12.56 a |
Similar letters in each column show insignificant differences at p< 0.05 level.
Total soluble solids
Analysis of variance indicated that total soluble solids (TSS) were influenced by immersion significantly (p<0.01). Means comparison of postharvest treatments showed that the treatment with hot water + 2% CaCl2 significantly changed TSS, compared to untreated control. The highest TSS (6.84 °Brix) was observed in treated fruits with hot water + 2% CaCl2 (Fig. 1).
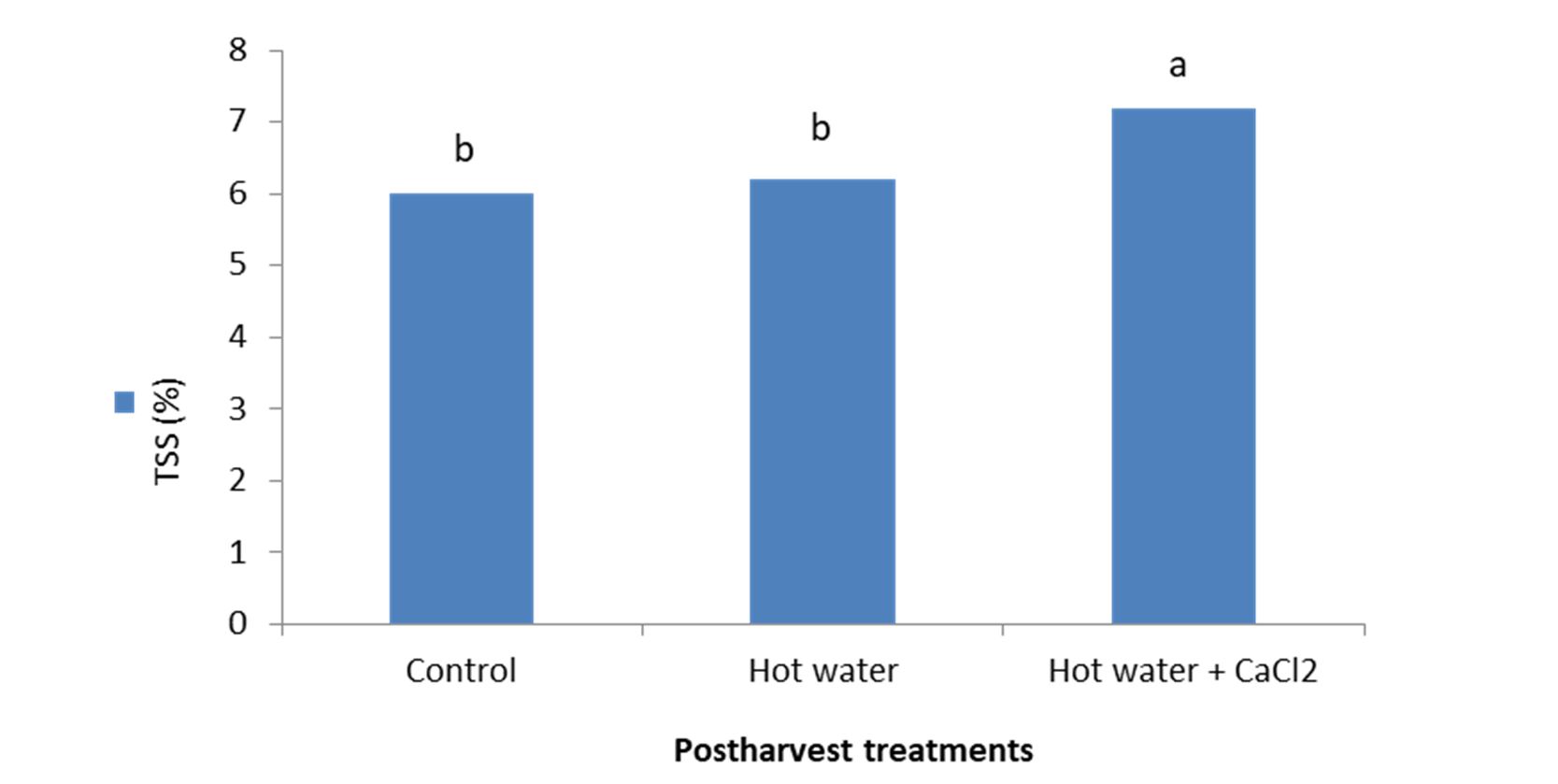
Figure 1 – Effect of hot water alone and hot water + 2% CaCl2 treatments on total soluble solids of fresh seedless barberry fruit stored at 2°C for 45 days
The increase in sugar content during storage can be partially related to the loss of fruit juice and the increased concentration of fruit juice contents (Sayari et al., 2009). Since sugar and acids act as the main substrate for respiratory metabolism, the loss of TSS during storage is mainly due to the respiration and the conversion of sugars to carbon dioxide and water. There is a report showing that the treatment with hot water (50°C for 3 min) and hot water + 2% CaCl2 improved TDS in pome-granate fruits ‘Shishe-Kab’, as com-pared to control (Moradinezhad et al., 2014).
Titratable acidity (TA)
According to the results of analysis of variance, the titratable acidity of barberry fruits was not significantly affected by postharvest treatments.
The fruits lose their acids over the storage due to the decomposition of organic acids (Rabiei and Rahmani, 2014). Organic acids may be mostly respired, as a carbon source during the tricarboxylic acid cycle. Thus, TA concentration is reduced during storage (Kays et al., 2004). The loss of cherry taste during the loss of fruit acids impairs the potential shelf life of this fruit. Therefore, inhibition of the loss of fruit acid is the main goal to extend the shelf life and its marketability (Mattheis et al., 1997). In contrary to our results, in previous studies on fig and pomegranate fruits, the TA significantly increased in treated fruit with CaCl2. It is reported that TA of fig fruits was increased during storage when they were treated with calcium chloride and calcium lactate (Irfan et al., 2013). Similarly, a study on the foliar application of different rates of calcium chloride and urea on pomegranate fruits showed that 2% CaCl2 had the highest impact on increasing the TA of the fruits (Ramezanian et al., 2009).
Moradinezhad et al. (2014) re-ported that the treatment with hot water (50°C for 3 min) and hot water + 2% CaCl2 enhanced the TA of pomegranate fruits ‘Shishe-Kab’ cultivar, as compared to control.
Anthocyanin
Analysis of variance revealed the significant impact of postharvest immersion treatments on the antho-cyanin content of the samples. Means comparison indicated that the highest anthocyanin (0.39 mg L-1) was observed in treated fruit with hot water and the lowest (0.24 mg L-1) value was obtained on control (Fig. 2).
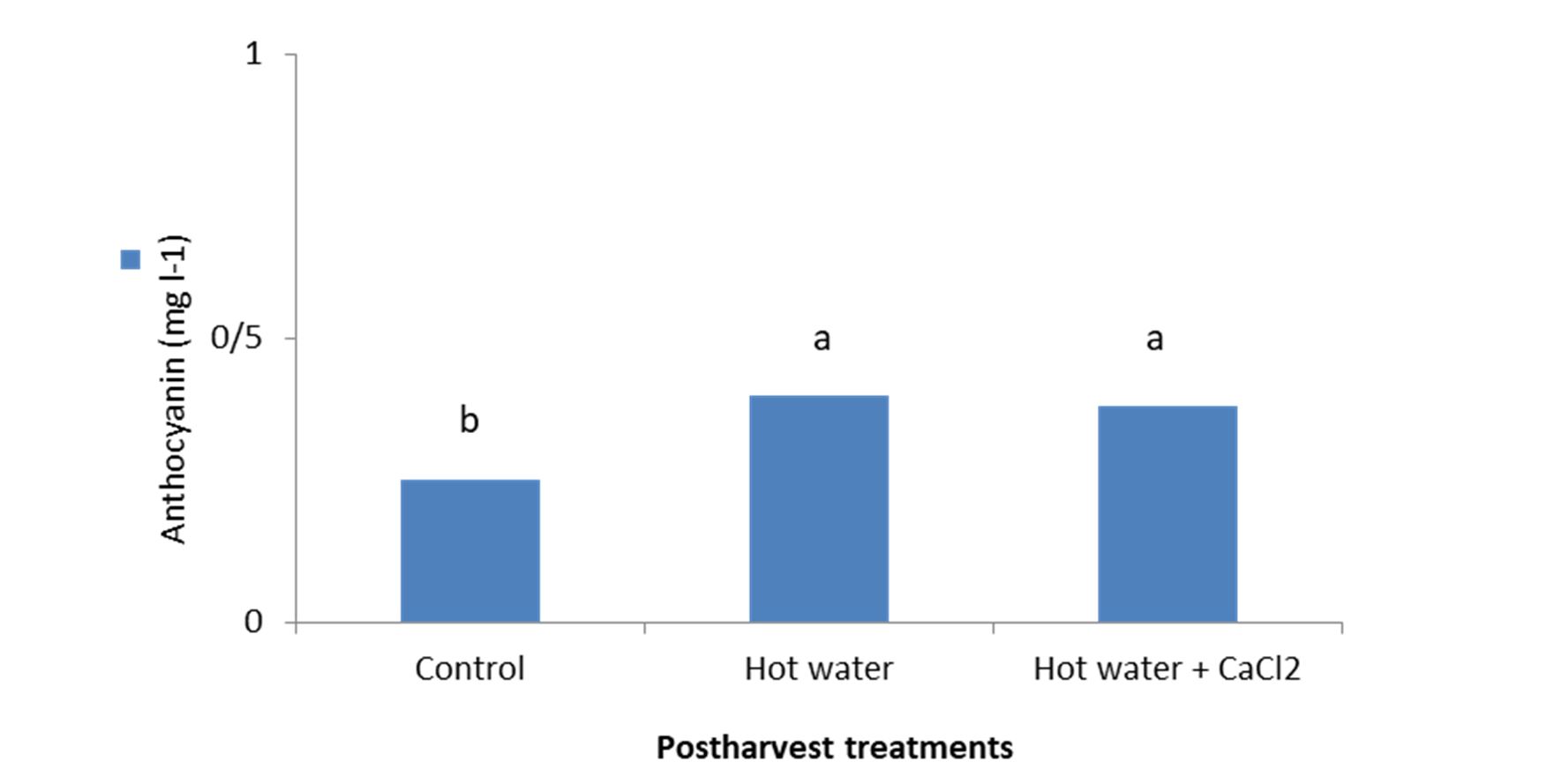
Figure 2 – Effect of hot water alone and hot water + 2% CaCl2 treatments on anthocyanin content of fresh seedless barberry fruit stored at 2°C for 45 days
Anthocyanins are highly instable molecules in foods whose color stability is strongly determined by metal ions and sugars, pH, tempe-rature, structure, oxygen, light, enzymes, and other accompanying compounds (Rein, 2005).
Like most reactions, the decomposition of the anthocyanins is considerably accelerated with tempe-rature rise. So, the storage tempe-rature impacts anthocyanin content remarkably, so that lower storage temperature increases anthocyanin content of Arbutus unedo L. fruits (Guerreiro et al., 2013).
Aghdam et al. (2013) reported that CaCl2 at various rates enhanced anthocyanin content of cornelian cherry (Cornus mas) fruits. In addi-tion, Giovanelli et al. (2012) conduc-ted a similar study on blueberries and reported that hot water treatment increased fruit anthocyanin content slightly, which is consistent with our findings. In contrast, Brambilla et al. (2011) reported the loss of anthocyanin content in blueberry by postharvest hot water treatment.
Antioxidant
As the analysis of variance showed, antioxidant was influenced significantly (p< 0.01) by the hot water alone, and hot water + 2% CaCl2 treatments. The highest antioxidant content (% 77.92) was observed in hot water treated samples and the lowest (% 54.28) was obtained on control (Fig. 3).
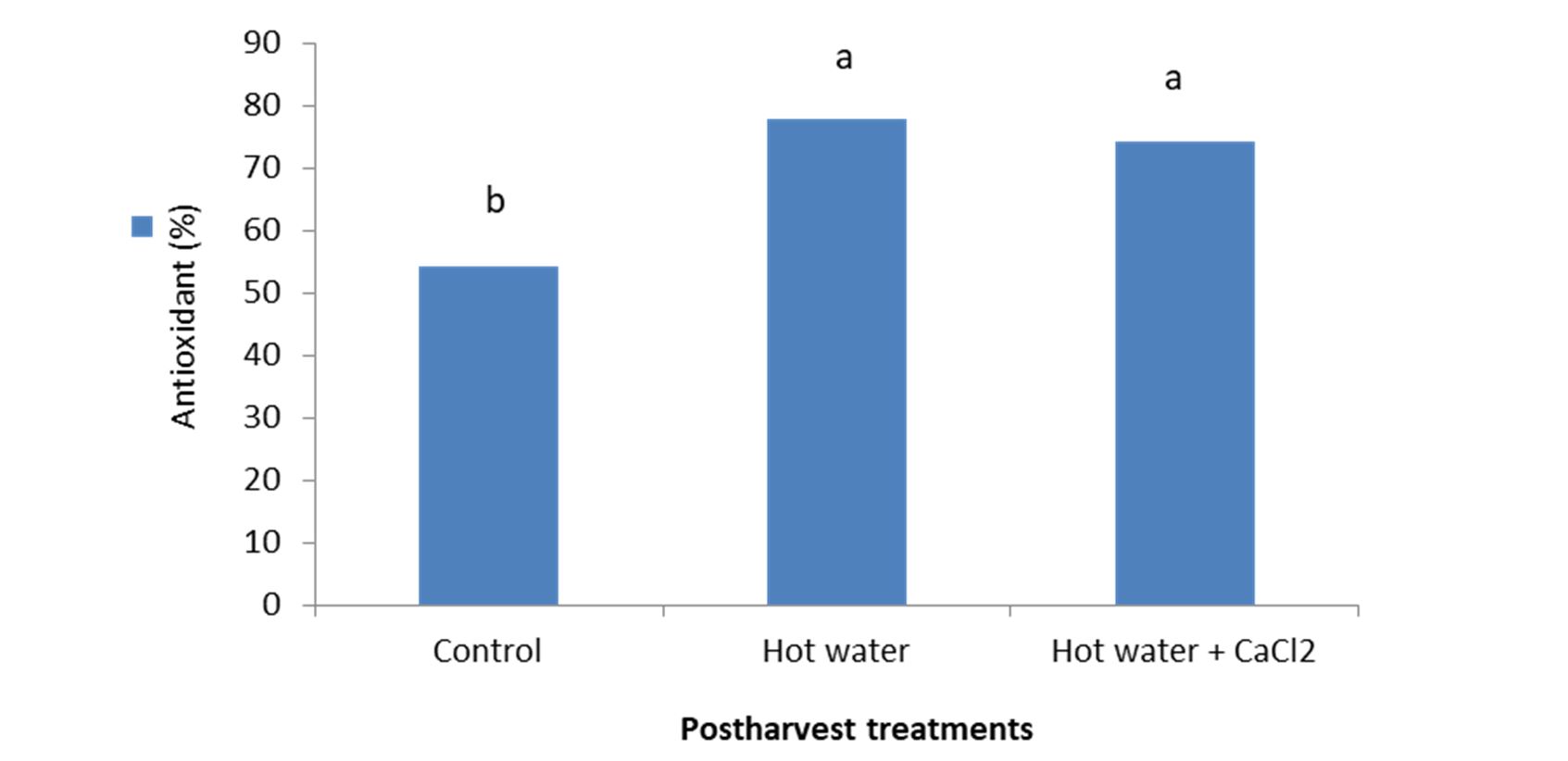
Figure 3 – Effect of hot water alone and hot water + 2% CaCl2 treatments on antioxidant content of fresh seedless barberry fruit stored at 2°C for 45 days
The loss of antioxidant capacity at higher temperatures may be partially related to the decrease in ascorbic acid content as a non-enzy-matic antioxidant factor (Javanmardi and Kubota, 2006).
Lower antioxidant capacity at higher temperatures and during storage is likely to associate with the fact that a great part of antioxidant compounds is consumed to defend free radicals (Gulen and Eris, 2004). Different rates of CaCl2 increased considerably the antioxidant capacity of comelian cherry fruits, compared to control (Aghdam et al., 2013). It has been reported that Ca in carrots augments antioxidant activity in response to the increased capacity of free radicals (Jacobo-Velázquez et al., 2011), which is similar to our results.
Total phenolic compounds
The results show that postharvest dipping treatments affected signifi-cantly total phenolic compounds of fruits, compared to control. The highest phenol content (4.1 mg g DW) was observed in fruits treated with hot water + 2% CaCl2, and the lowest (3.88 mg g DW) was obtained with hot water alone treatment (Fig. 4). Phenols are representative of a large group of secondary compounds (flavornoids, anthocyanins, tannins, and lignins) in plant tissues (Grace and Logan, 2000). The antioxidant property of polyphenols is associated with their high responsiveness as electron or hydrogen donor, the establishment of stability and the removal of paired electrons due to their chemical structure, their capability in the transport of metallic ions, the capability of flavonoids in changing peroxidation trend by changing the fats, and the reduction of membrane fluidity (Arora et al., 2000). These changes can inhibit the penetration of free radicals and limit peroxidase reactions. These com-pounds are strong antioxidants in stressful plant tissues and this feature is associated with the skeleton structure of a phenolic group of these metabolites. Aromatic ring-binding hydroxyl groups can scavenge free radicals and mitigate oxidative damages, thereby protecting cell structure against the adverse effects of stress (Al-Amier and Craker, 2007). Similar results showed that CaCl2 at various rates increased total phenols in comelian cherry fruits during storage and the samples treated with higher CaCl2 rates displayed a more remarkable increase in fruit phenol content (Aghdam et al., 2013). In addition, it has been reported that treatment of raspberry and strawberry fruits with 2% CaCl2 dip had a positive effect on the retention of the total phenolic contents, during the storage period (Turmanidze et al., 2017). Hot water treatment is known to have desirable impacts on the maintenance of polyphenol compo-nents in fruits, maybe because of the inactivation of endogenous polyphe-nol oxidase enzyme of the fruits (Rossi et al., 2003; Sablani et al., 2010; Skrede et al., 2000). However, postharvest hot water treatment of blueberries resulted in the loss of total phenols of the fruits over the storage (Giovanelli et al., 2012). Similarly, Brambilla et al. (2011) reported that total phenol compounds of blueberries were decreased after the application of hot water.
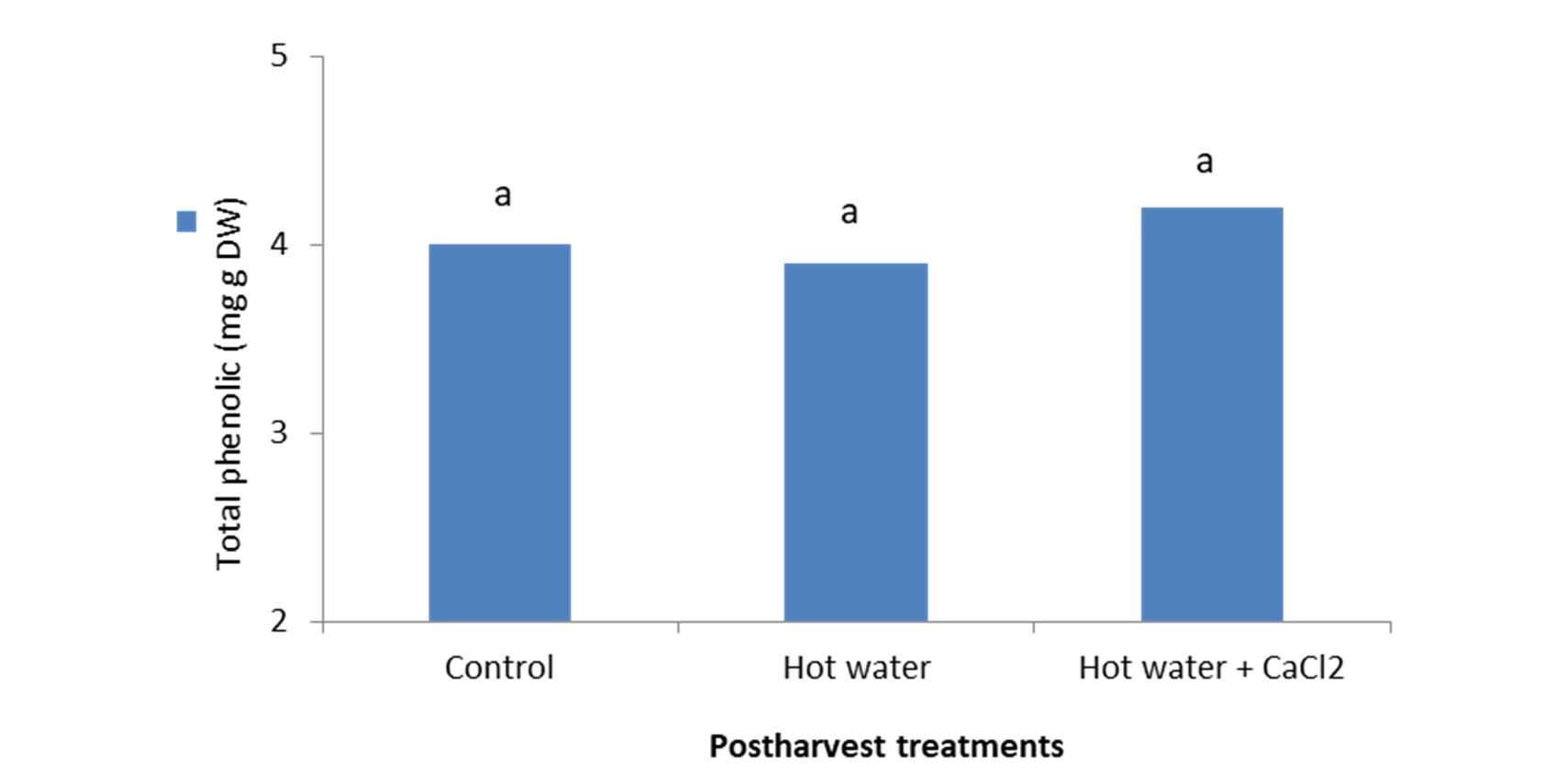
Figure 4 – Effect of hot water alone and hot water + 2% CaCl2 treatments on total phenolic compounds of fresh seedless barberry fruit stored at 2°C for 45 days
Calcium content
The treatment of the fruits with hot water + 2% CaCl2 significantly (p< 0.01) increased Ca content of the tissues as analysis of variance revealed. The results indicated that the highest Ca content (6.039 mg L-1) was related to the treatment with hot water + 2% CaCl2 and the lowest (2.457 mg L-1) was observed in control (Fig. 5).
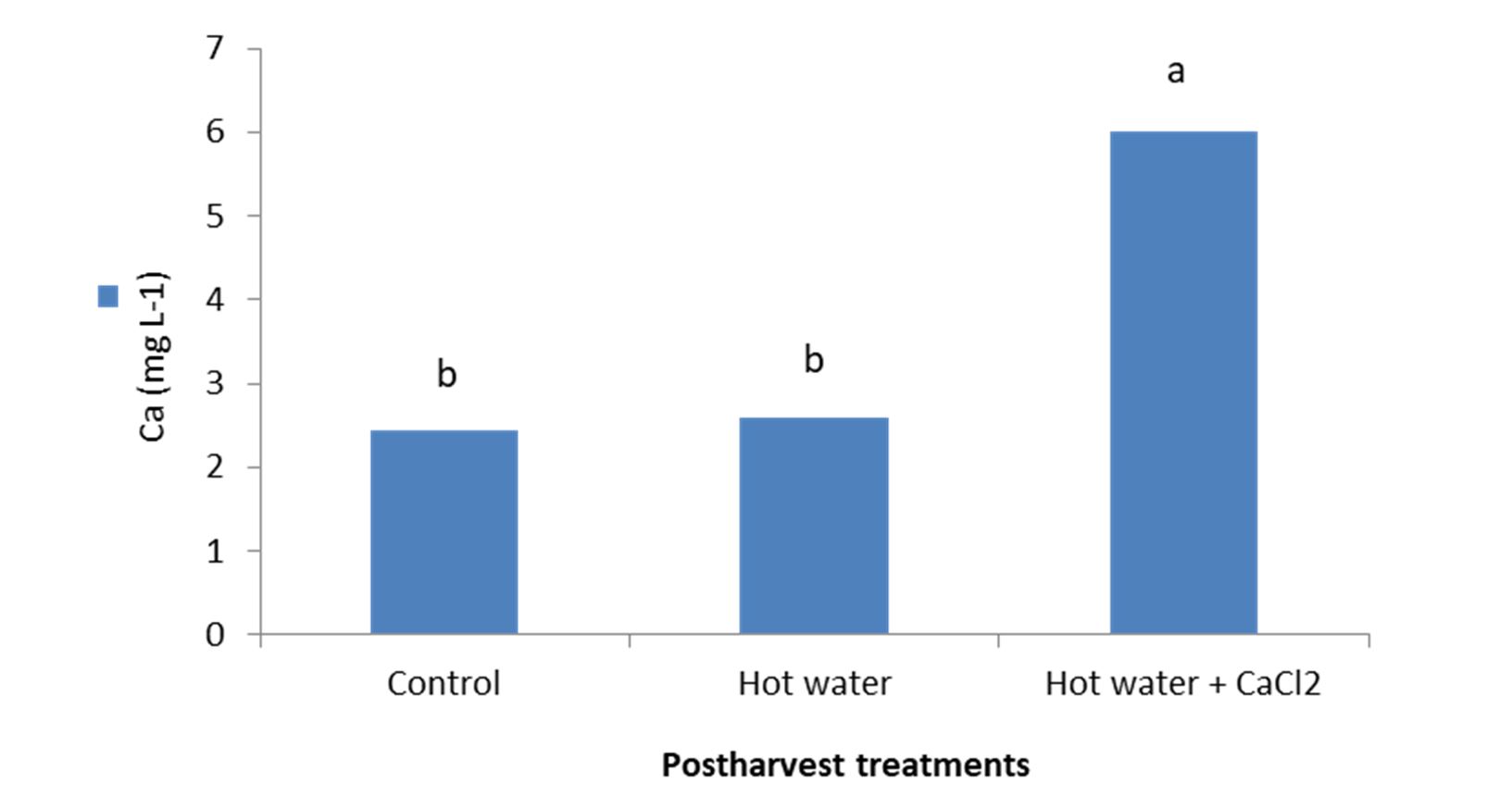
Figure 5 – Effect of hot water alone and hot water + 2% CaCl2 treatments on Ca content of fresh seedless barberry fruit stored at 2°C for 45 days
Ca ion is likely to enter into fruit tissue through the cracks in cuticle and epidermis (Glenn and Poovaiah, 1985). A significant increase was observed in Ca content of the fruits immersed in cold water containing Ca (Wang et al., 2014). Similarly, a study on the application of CaCl2 at four rates of 0.5, 1, 1.5, and 2% showed that the highest Ca content of the tissues after the storage time was obtained from the treated samples with 2% CaCl2 (Madani et al., 2014).
Likewise, the postharvest appli-cation of CaCl2 considerably en-hanced Ca content of the fruit tissue in raspberries and strawberries (Carlos, 2014; Turmanidze et al., 2017) and apple (Chardonnet, 2003; Torres et al., 2017) stored under refrigeration.
CONCLUSION
Owing to less attention to use postharvest technologies in barberry industry in Iran, the main part of harvested fruit are dried. However, demand for fresh barberry fruits raised and so there is a need to prepare fresh produce with safety for marketing. The results of this study revealed that postharvest application of hot water and calcium chloride treatments improved the appearance quality and nutritional values of fresh seedless barberry fruit, as well as extend the cold storage life, likely due to reduced pathogen contamination. However, further research is needed to recommend for commercial application.
REFERENCES
Aghdam, M.S., Dokhanieh, A.Y., Hassanpour, H. & Fard, J.R. (2013). Enhancement of antioxidant capacity of cornelian cherry (Cornus mas) fruit by postharvest calcium treatment. Sci.Hortic.,161: 160-164, https://doi.org/10.1016/j.scienta.2013.07.006
Al-Amier, H. & Craker, L.E. (2007). In-vitro selection for stress tolerant spearmint. Reprinted from: Issues in New Crops and New Uses, J. Janick and A. Whipkey (eds.). ASHS Press, Alexandria, VA, pp. 306-310.
Albertini, S., Reyes, A.E.L., Trigo, J.M., Sarriés, G.A. & Spoto, M.H.F. (2016). Effects of chemical treatments on fresh-cut papaya. Food Chem., 190: 1182-1189, https://doi.org/10.1016/j.foodchem.2015.06.038
Arora, A., Byrem, T.M., Nair, M.G. & Strasburg, G.M. (2000). Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch.Biochem.Biophys., 373(1): 102-109, https://doi.org/10.1006/ abbi.1999.1525
Barbagallo, R.N., Chisari, M. & Caputa, G. (2012). Effects of calcium citrate and ascorbate as inhibitors of browning and softening in minimally processed ‘Birgah’ eggplants. Postharvest Biol.Technol., 73: 107-114, https://doi.org/10.1016/ j.postharvbio.2012.06.006
Brambilla, A., Maffi, D. & Rizzolo, A. (2011). Study of the influence of berry-blanching on syneresis in blueberry purées. Procedia Food Sci., 1: 1502-1508, Procedia Food Scie., 1 (2011) 1502-1508, doi:10.1016/j.profoo.2011.09.222
Chardonnet, C.O., Charron, C.S., Sams, C.E. & Conway, W.S. (2003). Chemical changes in the cortical tissue and cell walls of calcium-infiltrated ‘Golden Delicious’ apples during storage. Postharvest Biol. Technol., 28(1): 97-111, https://doi. org/10.1016/S0925-5214(02)00139-4
Cybulska, J., Zdunek, A. & Konstankiewicz, K. (2011). Calcium effect on mechanical properties of model cell walls and apple tissue. J. Food Eng., 102(3): 217-223, https://doi.org/10.1016/ j.jfoodeng.2010.08.019
Escribano, S. & Mitcham E. J. (2014). Progress in heat treatments. Stewart Postharvest Review, 10(3): 1-6.
Fallik, E. (2004). Prestorage hot water treatments (immersion, rinsing, and brushing). Postharvest Biol.Technol., 32(2): 125-134, https://doi.org/10. 1016/j.postharvbio.2003.10.005
García, J.M., Herrera, S. & Morilla, A. (1996). Effects of postharvest dips in calcium chloride on strawberry. J.Agric.Food Chem., 44(1): 30-33, DOI: 10.1021/jf950334l
Giovanelli, G., Brambilla, A., Rizzolo, A. & Sinelli, N. (2012). Effects of blanching pre-treatment and sugar composition of the osmotic solution on physico-chemical, morphological and antioxidant characteristics of osmodehydrated blueberries (Vaccinium corymbosum L.). Food Res.Intern., 49(1): 263-271, https://doi.org/10.1016/j.foodres.2012.08.015
Glenn, G.M. & Poovaiah, B.W. (1985). Cuticular permeability to calcium compounds in ‘Golden Delicious’ apple fruit. J.Am.Soc.Hortic.Sci., USA., 110: 166-171.
Grace, S.C. & Logan, B.A. (2000). Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos. Trans.R.Soc.Lond.B.Biol.Sci., 355 (1402): 1499-1510, doi: 10.1098/ rstb.2000.0710
Guerreiro, A.C., Gago, C.M., Miguel, M.G. & Antunes, M.D. (2013). The effect of temperature and film covers on the storage ability of Arbutus unedo L. fresh fruit. Sci.Hortic., 159: 96-102, https://doi.org/10.1016/j.scienta.2013.04.030
Gulen, H., & Eris, A. (2004). Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci., 166(3): 739-744, https://doi.org/10.1016/ j.plantsci.2003.11.014
Hosseini, A. & Moradinezhad, F. (2018). Effect of short-term high CO2 treatment on quality and shelf life of button mushroom (Agaricus bisporus) at refrigerated storage. J.Hortic. Postharvest Res., 1(1): 37-48, DOI: 10.22077/jhpr.2018.1198. 1006
Irfan, P.K., Vanjakshi, V., Prakash, M.K., Ravi, R. & Kudachikar, V.B. (2013). Calcium chloride extends the keeping quality of fig fruit (Ficus carica L.) during storage and shelf-life. Postharvest Biol.Technol., 82: 70-75, https://doi.org/10.1016/ j.postharvbio.2013.02.008
Jacobo-Velázquez, D.A., Martínez-Hernández, G.B., del C. Rodríguez, S., Cao, C.M. & Cisneros-Zevallos, L. (2011). Plants as biofactories: physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J.Agric.Food Chem., 59(12): 6583-6593, DOI: 10.1021/jf2006529
Javanmardi, J. & Kubota, C. (2006). Variation of lycopene, antioxidant activity, total soluble solids and weight loss of tomato during postharvest storage. Postharvest Biol.Technol., 41(2): 151-155, https://doi.org/10.1016/j.postharvbio.2006.03.008
Kafi, M., Balandri, A., Rashed Mohasel, M., Karbasi, A., Marashi, H. & Maskouki, A. (2002). Barberry: production and process technology. Language and Literature Institute, pp. 45-60 (In Persian).
Karabulut, O.A., Smilanick, J.L., Crisosto, C.H. & Palou, L. (2010). Control of brown rot of stone fruits by brief heated water immersion treatments. Crop Prot., 29(8): 903-906, https://doi.org/10.1016/j.cropro. 2010.03.010
Kays, S.J., Paull, R.E., Zuidema, P.A., Leffelaar, P.A., Gerritsma, W., Mommer, L. & Magallanes, M.E. (2004). Postharvest biology. Agricultural Systems (RU). 2005, 84: 195-225.
Li, J.W., Fan, L.P., Ding, S.D. & Ding, X.L. (2007). Nutritional composition of five cultivars of Chinese jujube. Food Chem., 103(2): 454-460, https://doi.org/10.1016/j.foodchem.2006.08.016
Madani, B., Mohamed, M.T.M., Biggs, A.R., Kadir, J., Awang, Y., Tayebimeigooni, A. & Shojaei, T.R. (2014). Effect of pre-harvest calcium chloride applications on fruit calcium level and post-harvest anthracnose disease of papaya. Crop Prot., 55: 55-60, https://doi. org/10.1016/j.cropro.2013.10.009
Mahajan, P.V., Caleb, O.J., Singh, Z., Watkins, C.B. & Geyer, M. (2014). Postharvest treatments of fresh produce. Philos.Trans.A.Math.Phys. Eng.Sci., 372: 1-19, 20130309, https://doi.org/10.1098/rsta.2013.0309
Makkar, H. P., Blümmel, M., Borowy, N.K. & Becker, K. (1993). Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J.Sci. Food Agric., 61: 161-165, https://doi.org/10.1002/jsfa.2740610205
Martin-Diana, A.B., Rico, D., Frias, J.M., Barat, J.M., Henehan, G.T. M. & Barry-Ryan, C. (2007). Calcium for extending the shelf life of fresh whole and minimally processed fruits and vegetables: a review. Trends Food Sci.Technol., 18(4): 210-218, https://doi.org/10.1016/j.tifs.2006.11.027
Mattheis, J.P., Buchanan, D.A. & Fellman, J.K. (1997). Volatile constituents of Bing sweet cherry fruit following controlled atmosphere storage. J Agric.Food Chem., 45(1): 212-216, DOI: 10.1021/jf960234v
Moradinezhad, F. & Khayyat, M. (2014). Effects of intermittent warming and prestorage treatments (hot water, salicylic acid, calcium chloride) on postharvest life of pomegranate fruit cv.‘Shishe-Kab’ during long-term cold storage. Int.J.Hortic. Sci. Technol., 1(1): 43-51, DOI: 10.22059/ijhst.2014.50517
Moreira, M.D.R., Ponce, A.G., Del Valle, C.E. & Roura S.I. (2006). Ascorbic acid retention, microbial growth, and sensory acceptability of lettuce leave subjected to mild heat shocks. J. Food Sci., 71:188-192, https://doi. org/10.1111/j.1365-2621.2006.tb08924.x
Murata, M., Tanaka, E., Minoura, E. & Homma, S. (2004). Quality of cut lettuce treated by heat shock: prevention of enzymatic browning, repression of phenylalanine ammonia-lyase activity, and improvement on sensory evaluation during storage. Biosci.Biotechnol. Biochem., 68(3): 501-507, -https://doi.org/10.1271/bbb.68.501
Poovaiah, B.W. (1986). Role of calcium in prolonging the storage life of fruits and vegetables. Food Technol., 40(5): 86-89.
Rabiei, V. & Rahmani, S. (2014). Influence of salicylic acid, calcium chloride and hot water treatment on quantitative, qualitative parameters and storage life of pomegranate cv. Meykhosh. Journal of Horticulture Science, 28(1): 107-115 (In Persian with English Abstract).
Ramezanian, A., Rahemi, M. & Vazifehshenas, M.R. (2009). Effects of foliar application of calcium chloride and urea on quantitative and qualitative characteristics of pomegranate fruits. Sci.Hortic., 121(2): 171-175, https://doi.org/10.1016/j.scienta.2009.01.039
Rapisarda, P., Fanella, F. & Maccarone, E. (2000). Reliability of analytical methods for determining anthocy-anins in blood orange juices. J.Agric.Food Chem., 48(6): 2249-2252, DOI: 10.1021/jf991157h
Rein, M. (2005). Copigmentation reactions and color stability of berry anthocyanins. Doctoral dissertation, University of Helsinki, Helsinki, FI, 87 p.
Rossi, M., Giussani, E., Morelli, R., Scalzo, R.L., Nani, R.C. & Torreggiani, D. (2003). Effect of fruit blanching on phenolics and radical scavenging activity of highbush blueberry juice. FoodRes. Int., 36(9-10): 999-1005, https://doi. org/10.1016/j.foodres.2003.07.002
Sablani, S.S., Andrews, P.K., Davies, N.M., Walters, T., Saez, H., Syamaladevi, R.M. & Mohekar, P.R. (2010). Effect of thermal treatments on phytochemicals in conventionally and organically grown berries. J Sci.Food Agric., 90(5): 769-778, https://doi.org/10.1002/jsfa. 3882
Sayari, M., Babalar, M., Kalantari, S., Alizadeh, H. & Asgari, M. (2009). Effects of salicylic acid on chilling resistance and phenylalanine ammonia lyase activity in ‘Malase Saveh’ pomegranate (Punica granatum) during cold storage. Iranian Journal of Horticultural Science, 40(3): 21-38 (In Persian with English Abstract).
Siddiq, M., Roidoung, S., Sogi, D.S. & Dolan, K.D. (2013). Total phenolics, antioxidant properties and quality of fresh-cut onions (Allium cepa L.) treated with mild-heat. Food Chem., 136(2): 803-806, https://doi.org/ 10.1016/j.foodchem.2012.09.02
Skrede, G., Wrolstad, R.E. & Durst, R.W. (2000). Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.). J. Food Sci., 65(2): 357-364, https://doi.org/10.1111/j.1365-2621. 2000.tb16007.x
Torres, E., Recasens, I., Lordan, J. & Alegre, S. (2017). Combination of strategies to supply calcium and reduce bitter pit in ‘Golden Delicious’ apples. Sci.Hortic., 217: 179-188, https://doi.org/10.1016/j.scienta.2017.01.028
Turmanidze, T., Gulua, L., Jgenti, M. & Wicker, L. (2017). Potential antioxidant retention and quality maintenance in raspberries and strawberries treated with calcium chloride and stored under refrigeration. Braz.J. Food Technol., 20, http://dx.doi.org/10.1590/1981-6723.8916
Wang, Y., Xie, X. & Long, L.E. (2014). The effect of postharvest calcium application in hydro-cooling water on tissue calcium content, biochemical changes, and quality attributes of sweet cherry fruit. Food Chem., 160, 22-30, https://doi.org/10.1016/ j.foodchem.2014.03.073
Zargari, A. (1993). Medicinal herbs (Vol. 4th). Tehran, Iran: Tehran University Press (In Persian).



