Erawan Baothong, Pisit Chareonsudjai
ABSTRACT. The research aimed to extract high-quality pectin from water hyacinth for less soluble hydrogel production. The product adds value to water hyacinth waste and helps solve environmental problems. The high degree of esterification pectin can be prepared as a hydrogel, which can be used in various pollution treatment applications. The quality of pectin depended on raw materials and extraction conditions. The optimum condition was initially predicted using the response surface method (RSM). Three extraction variables were studied, including pH 1.0-4.0, temperature 50-110ºC, and extraction time 30-240 min. A total of seventeen runs including five replicate runs were studied. Functional groups of pectin were studied using Fourier Transform Infrared spectrophotometry. The degree of esterification and emulsifying properties were determined for assessing the quality of extracted pectin. The result revealed that the extraction conditions strongly affected both the yield and the quality. The condition of pH 2.5, 110ºC, and 30 minutes had the highest degree of esterification of 94.13 % but low yield (1.42%). On the other hand, under the conditions of pH 1.0, 110ºC, and 135 min extract time had the highest yield of 3.85% (about 76.6% of pectin content) however the degree of esterification is low at 43.25 %. Two mathematical models were proposed for yield and number of ester groups. The result will be used for the selection of high-quality pectin to produce insoluble hydrogel for pollution treatment in the future.
Keywords: Eichhornia crassipes; high-quality pectin; optimum condition; water hyacinth.
Cite
ALSE and ACS Style
Baothong, E.; Chareonsudjai, P. Optimal conditions for extracting high-quality pectin from water hyacinth (Eichhornia crassipes) for less soluble hydrogel production. Journal of Applied Life Sciences and Environment 2024, 57, 265-283.
https://doi.org/10.46909/alse-572136
AMA Style
Baothong E, Chareonsudjai P. Optimal conditions for extracting high-quality pectin from water hyacinth (Eichhornia crassipes) for less soluble hydrogel production. Journal of Applied Life Sciences and Environment. 2024; 57 (2): 57, 265-283.
https://doi.org/10.46909/alse-572136
Chicago/Turabian Style
Baothong, Erawan, and Pisit Chareonsudjai. 2024. “Optimal conditions for extracting high-quality pectin from water hyacinth (Eichhornia crassipes) for less soluble hydrogel production” Journal of Applied Life Sciences and Environment 57, no. 2: 57, 265-283.
https://doi.org/10.46909/alse-572136
View full article (HTML)
Optimal Conditions for Extracting High-Quality Pectin From Water Hyacinth (Eichhornia Crassipes) for Less Soluble Hydrogel Production
Erawan BAOTHONG and Pisit CHAREONSUDJAI*
Department of Environmental Science, Faculty of Science, Khon Kaen University, 40002, Thailand; email: zempujin@gmail.com
*Correspondence: pisit@kku.ac.th
Received: Feb. 06, 2024. Revised: Mar. 10, 2024. Accepted: Mar. 24, 2024. Published online: Apr. 24, 2024
ABSTRACT. The research aimed to extract high-quality pectin from water hyacinth for less soluble hydrogel production. The product adds value to water hyacinth waste and helps solve environmental problems. The high degree of esterification pectin can be prepared as a hydrogel, which can be used in various pollution treatment applications. The quality of pectin depended on raw materials and extraction conditions. The optimum condition was initially predicted using the response surface method (RSM). Three extraction variables were studied, including pH 1.0-4.0, temperature 50-110ºC, and extraction time 30-240 min. A total of seventeen runs including five replicate runs were studied. Functional groups of pectin were studied using Fourier Transform Infrared spectrophotometry. The degree of esterification and emulsifying properties were determined for assessing the quality of extracted pectin. The result revealed that the extraction conditions strongly affected both the yield and the quality. The condition of pH 2.5, 110ºC, and 30 minutes had the highest degree of esterification of 94.13 % but low yield (1.42%). On the other hand, under the conditions of pH 1.0, 110ºC, and 135 min extract time had the highest yield of 3.85% (about 76.6% of pectin content) however the degree of esterification is low at 43.25 %. Two mathematical models were proposed for yield and number of ester groups. The result will be used for the selection of high-quality pectin to produce insoluble hydrogel for pollution treatment in the future.
Keywords: Eichhornia crassipes; high-quality pectin; optimum condition; water hyacinth.
INTRODUCTION
Water hyacinth (Eichhornia crassipes) is a multi-season water plant in the Pontederiaceae family. It can survive under many water quality conditions, quickly breed, and distribute to many water bodies worldwide, causing serious problems, particularly blocking water transportation (Carlini et al., 2018). Water hyacinth was currently managed by mechanical harvesting, composting, and handicrafts (Weiping et al., 2018) or left on the river bank, which was not efficient enough due to the large volume and no value-added product to generate business investment. Therefore, finding a way to get rid of water hyacinth and convert it into a high-value product was necessary for incentive management. Its cell walls generally contain many molecularly linked polysaccharides, including cellulose, hemicellulose, and pectin, which can be used in many industries (Audenhove et al., 2021). Optimum extraction conditions however are necessary for these products.
Response Surface Methodology (RSM) is a process dealing with numerous parameters and possible interactions resulting in the desired response (Hundie, 2020). Optimization extraction process when numerous parameters and possible interactions may impact the desired response, especially, to study optimum conditions for extracting pectin from raw materials (Hundie, 2020). Optimizing extraction processes for many raw materials directed by RSM are widely reported, such as the optimization variables of durian rind and coconut husk for pectin extraction (Baothong, 2018), citrus fruit peel (Shukla et al., 2014), and sunflower heads (Ma et al., 2020). However, RSM-based models are only for a restricted range of input parameters and thus, restrict the use for non-linear behavior. It should be noted that in these extraction processes, the output usually is influenced by many input variables (Peng et al., 2020). Thus, this model is suitable for examining the response to non-linear variables (Pasandide et al., 2017).
The degree of esterification (%DE) was used to consider pectin quality (Robledo and Vázquez, 2023). In the past, pectin was found in water hyacinth since 1989 about 5% of pectin content (Naohara and Ishii, 1989). However, an appropriate extraction method was not reported. Recently, the %DE value of pectin extracted from water hyacinth of 62.26% ± 1.32 was reported under one condition (Jariyapamornkoon et al., 2023). However, it is not high enough for environmental applications. Therefore, it is necessary to find other optimum conditions for extracting pectin from water hyacinth. Generally, hot mineral acid conditions were used to extract pectin because of its high extraction yield and low cost (Audenhove et al., 2021). The most important factors for extracting pectin from agricultural plant waste were pH, temperature, and extraction time. Generally, higher extraction yields are found under harsher conditions such as lower pH, longer extraction time, and higher temperatures (Audenhove et al., 2021) because the links in the side chain of neutral sugar are more acidic than those of the pectin backbone. The high-quality pectin is considered from the number of carboxylic groups that are esterified with alcohol. If the percentage of esterified carboxyl groups is more than 50%, the pectin is referred to as a high degree of esterification pectin (HEP). Whereas the percentage is less than 50%, this pectin is called a low-degree esterification pectin (LEP) (Narasimman and Sethuraman, 2016). Pectin is a biopolymer that consists of three main domains, namely homogalacturonan and rhamnogalacturonan I, II. The homogalacturonan is a linear polymer consisting only of α-1,4-linked galacturonic acid units which can be methyl-esterified on C-6 and O-acetylated on O-2 or O-3. The percentage of methyl-esterified galacturonic acid unit is expressed as %DE.
There is much research using pectin for environmental protection, such as using pectin hydrogel to absorb metals and heavy metals in wastewater (Wang et al., 2019). The commonly used pectin for adsorption is the LEP type because it has a higher adsorption capacity than the HEP type (Sabando et al., 2023). Because the adsorption of heavy metals by the pectin hydrogel occurs in the carboxyl group (Said et al., 2023). Therefore, if the pectin hydrogel has a high ester group, it will have a lower active site for heavy metal adsorption (Said et al., 2023).
However, it was found that LEP pectin hydrogel has a higher water solubility than HEP because the carboxyl groups on the surface of the pectin polymer matrix could react with water molecules in the dispersion medium and dissolve (Sabando et al., 2023). Therefore, fewer free carboxyl groups on the surface of the pectin hydrogel, and higher water solubility appeared (Said et al., 2023), resulting in the inability to use it in wastewater. Therefore, HEP pectin hydrogel with less solubility will be used as an alternative in heavy metal adsorption. The HEP waste after several times use and removal of the metal is biodegradable and does not cause environmental problems. For that reason, the objective of this research is to study the new raw material (water hyacinth) and suitable extraction methods to obtain the higher esterification pectin with high ester groups to produce less soluble hydrogel. Multiple linear regression statistics will be applied for the prediction of extraction yield and quality of pectin, which will be handy for future use.
MATERIALS AND METHODS
A. Water hyacinth preparation
Water hyacinth was randomly collected from the drainage canal of Udon Thani municipal, Udon Thani province, Thailand for 5 kg in September 2017. A mature plant with a stem longer than 30 centimeters was collected. Roots were removed and then the stem and leaves were coarsely chopped by hand to the size of about 5 centimeters and blended without adding water in a blender (Phillip model HR2115) for 3 minutes to fine particles. The mixture was preserved at 4ºC in a refrigerator for less than three days for pectin extraction.
B. Pectin extraction
The 100 g dried weight equivalent samples of water hyacinth were extracted according to a 3D response surface plot by Box-Behnken design (BBD). Three input parameters were studied including pH, water temperature, and extraction time. Seventeen runs were suggested by the program as shown in Table 1. Runs #2, #16 and #7, #9, #11 were replicates of some random conditions. The pH of the solution was adjusted using conc. HCl (AR grade, RCI Labscan), the temperature was controlled by a thermostat (EGO company model 55.13262.130, Thailand), and the extraction time was 30-240 minutes.
Table 1
RSM suggesting conditions for pectin extraction
|
Variables |
Runs |
||
|
pH |
Temperature (ºC) |
Time (min) |
|
|
1.0 |
50 |
135 |
1 |
|
1.0 |
80 |
30 |
3 |
|
1.0 |
80 |
240 |
15 |
|
1.0 |
110 |
135 |
12 |
|
2.5 |
50 |
30 |
4 |
|
2.5 |
50 |
240 |
8 |
|
2.5 |
80 |
135 |
7 |
|
2.5 |
80 |
135 |
9 |
|
2.5 |
80 |
135 |
11 |
|
2.5 |
110 |
30 |
13 |
|
25 |
110 |
135 |
17 |
|
2.5 |
110 |
240 |
6 |
|
4.0 |
50 |
135 |
2 |
|
4.0 |
50 |
135 |
16 |
|
4.0 |
80 |
30 |
14 |
|
4.0 |
80 |
240 |
10 |
|
4.0 |
110 |
135 |
5 |
After extraction, the samples were filtered through a cheesecloth; 95% ethanol was added at the ratio of 1:1 (v/v) and incubated at 4ºC for 12 hours. The filtrates were then centrifuged at 10,000 rpm for 15 minutes using HAEMATOKRIT Premiere model XC-3012. The precipitate was washed with 70% ethanol and the precipitate was collected. The pectin was dried in a vacuum oven (France Etuves model XLF020) at 60ºC under vacuum overnight. The pectin precipitate was grounded and sieved using standard sieve no.60 given the diameter particle lesser than 250 µm for further experiments. The yield of pectin was calculated by Equation 1 as follows (Wai et al., 2010):
![]()
C. Characterization of the pectin
The pectin was analyzed by Fourier transform infrared (FTIR) spectrophotometry in the wavenumber range of 4000-800 cm–1 (Perkin Elmer, Spectrum Two, USA) using a KBr pellet method. Standard pectin AR grade with known DE values of 63.0 – 66.0 % was obtained from LOBA Chemie (Mumbai, India).
D. Quality of pectin
The quality of pectin was assessed using two methods: 1) the emulsifying properties and 2) the degree of esterification.
1) The Emulsifying property was evaluated according to the method described by Dalev and Simeonova (1995). Emulsifying activity (EA) was calculated by Equation 2:
![]()
where Wv is the whole volume of the solution and ELV is the emulsified layer volume
Emulsion stabilities after 1 and 30 days of storage at 4 and 23ºC were calculated using the Equation 3:
![]()
where VEr and VEi are the emulsified layer volumes after centrifuging and the initial emulsified layer volumes, respectively.
2) The degree of esterification (%DE) of pectin extracted from water hyacinth. The %DE of pectin was analyzed by the titrimetric method (USP NF21, 2023). The %DE was calculated by Equation 4 as previously described by Santos et al. (2013):
![]()
where V1 is the volume of the initial titer and V2 is the volume of the final titer
E. Statistical analysis
The data were analyzed statistically using Design-Expert® software, version 13, Stat-Ease, Inc., Minneapolis, MN, USA, www.statease.com. Multiple linear regression (MLR) was used to estimate the relationship between the pectin extraction yield, pectin quality, and the extraction conditions. ANOVA was used to analyze the variables of the extraction process.
RESULTS
The results and discussions were separated into three parts including: A) The relationship between pectin yield and the extraction variables, B) The characterization of the pectin and C) The relationship between pectin quality and the extraction variables.
The details of each step are given below.
A. The relationship between pectin yield and the extraction variables
The pectin extraction yields from water hyacinth are shown in Table 2. The control application runs showed similar yield and %DE within the groups (#2, #16) and (#7, #9 and #11). The highest yield was 3.85 % in run #12 (pH 1.0, temperature 110ºC, and extraction time 135 min). The extraction yield of pectin was significantly related to the pH. It was found that lower pH resulted in a trend of higher percentage yield (R2=0.4558) (Figure 1). With increasing extraction time, the trend of percent yield slightly increased (R2=0.098) at the range of 1.5-2.0% yield and the temperature did not affect the yield. In addition, the hydrogen ions from hydrochloric acid activated the hydrolysis of pectin from proto-pectin and improved the efficacy to precipitate pectin due to their higher relevance for cations which stabilizes the pectin molecule (Sandarani, 2017).
This result clearly shows that the extraction yield is increased at lower pH as the concentration of hydrogen ions penetrates the polymer structure resulting in better separation of pectin (Yapo, 2009). According to the results of this study, it was found that the pectin extraction efficiency from water hyacinth was as high as 76.6% compared with the 5% content by Naohara and Ishii (1989).
The results from Table 2 were statistically analyzed by multiple factor linear regression and found that the equations with uncoded factors for extract yield prediction were given in Equation (5) as:

where X1, X2, and X3 are the pH, temperature and extraction time, respectively.
The R-square of yield Equation (5) was 0.86. The total extraction yield reflects the extraction efficiency of pectin (Dang et al., 2014; Moonsoor et al., 2001; Singthong et al., 2004). The highest pectin yield was extracted at pH 1.0, 110ºC for 135 min (run #12) at 3.85 % w/w. When substituting the values from run 12 to the Equation (5), the result was 3.33% which is close to the experiment yield of 3.85%. This revealed that Equation (5) could predict the pectin yield.
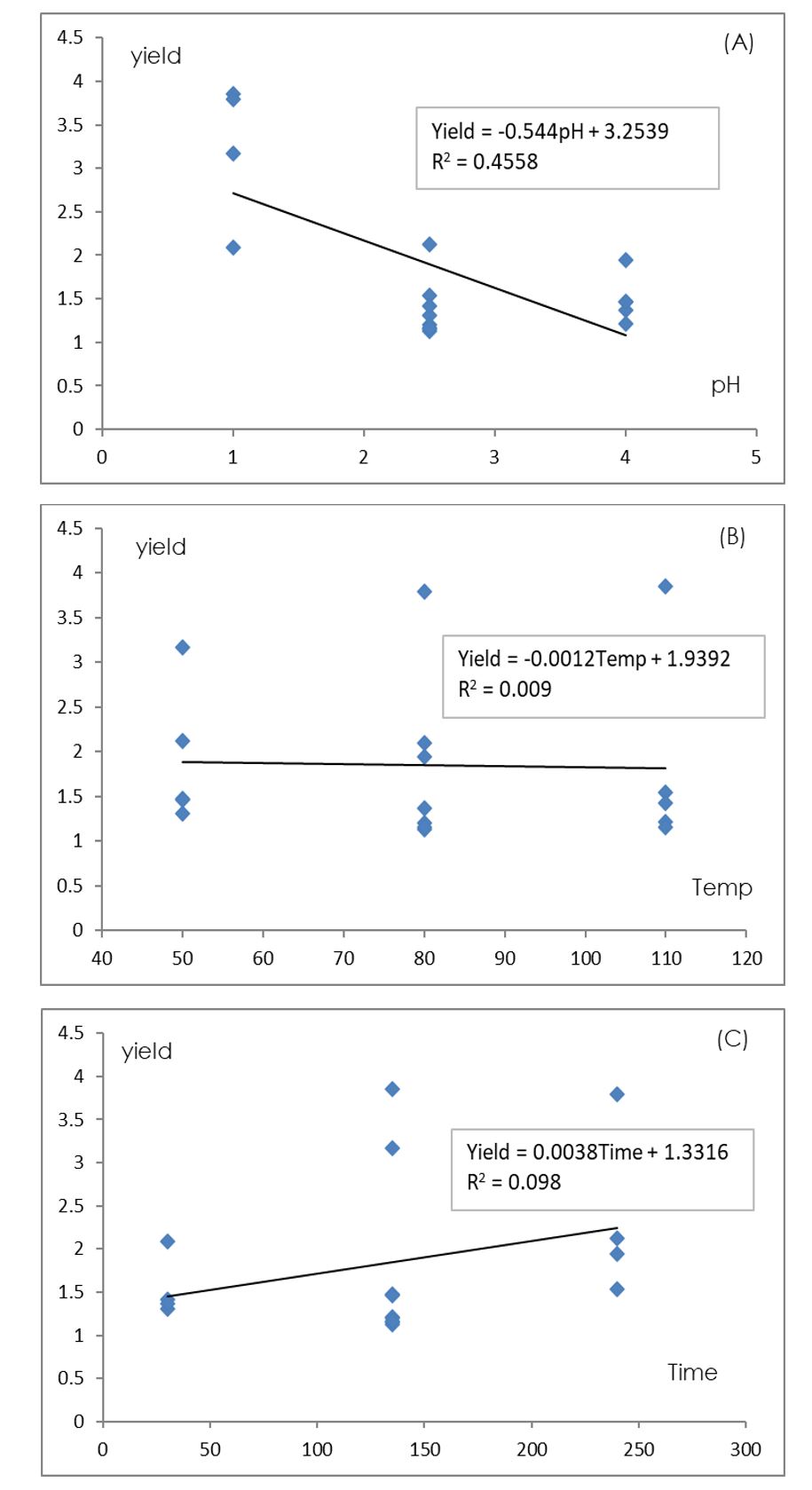
Figure 1 – Relationship between extraction yield of pectin extracted from water hyacinth and (A) pH value, (B) temperature and (C) extraction time
Table 2
Yield and DE of each condition of pectin extraction
|
Runs |
Variables |
Response |
|||
|
pH |
Temperature (ºC) |
Time (min) |
Yield (%) |
DE (%) |
|
|
1 |
1.0 |
50 |
135 |
3.17 |
0 |
|
3 |
1.0 |
80 |
30 |
2.09 |
80.51 |
|
15 |
1.0 |
80 |
240 |
3.79 |
0 |
|
12 |
1.0 |
110 |
135 |
3.85 |
43.25 |
|
4 |
2.5 |
50 |
30 |
1.31 |
54.44 |
|
8 |
2.5 |
50 |
240 |
2.12 |
63.44 |
|
7 |
2.5 |
80 |
135 |
1.13 |
63.22 |
|
9 |
2.5 |
80 |
135 |
1.15 |
66.10 |
|
11 |
2.5 |
80 |
135 |
1.20 |
66.90 |
|
13 |
2.5 |
110 |
30 |
1.42 |
94.13 |
|
17 |
25 |
110 |
135 |
1.16 |
53.68 |
|
6 |
2.5 |
110 |
240 |
1.54 |
0 |
|
2 |
4.0 |
50 |
135 |
1.47 |
38.48 |
|
16 |
4.0 |
50 |
135 |
1.46 |
38.66 |
|
14 |
4.0 |
80 |
30 |
1.37 |
67.15 |
|
10 |
4.0 |
80 |
240 |
1.94 |
60.82 |
|
5 |
4.0 |
110 |
135 |
1.20 |
53.44 |
Considering the appropriate model for extraction yield, both quadratic and cubic models were significant (p<0.5) in response to the extraction. The quadratic model was suggested although the cubic model was aliased (Table 3). The F-value and p-value of the extraction model were 13.59 and 0.0012, respectively, implying that the model was significant. The adequate precision ratio was 11.418, moreover, indicates an adequate signal. This model can be used to navigate the design space of extraction yield.
The influence of process variables over the extraction yield was graphically plotted using a 3D response surface plot. It was generated by maintaining two factors that were varied in their ranges (Masmoudi et al., 2008; Thirugnanasamdham et al., 2014) and the result of extraction yield is depicted in Figure 2.
The effect of extraction variables on the pectin extraction yield was graphically plotted using 3D response surface plots displayed in contour lines and color bands as shown in Figure 2. The plots were generated by maintaining two factors (pH and time in this case), that were varied in their range (Masmoudi et al., 2008; Thirugnanasamdham et al., 2014). From Figure 2, the sequence of yield from low to high is blue, green, yellow, and red, respectively. The extraction yield therefore was greatly influenced by pH and extraction time. The highest yield from Figure 2 (the reddest) was at pH 1.0 and 240 min time in run #15. However, the experimental yield from run #15 was 3.79% compared to run #12 of 3.85% yield. This result may be due to the combination of concurrent phenomena occurring during pectin extraction with a long time and low temperature because of the release of sugar as a product of pectin hydrolysis and degradation by heat (Oliveira et al., 2016). This could be explained by the degrading of the pectin polymer which affects the pectin yield decrease (Levigne et al., 2002). These results demonstrated that increasing extraction time and suitable temperature increased the extraction yield. The optimum conditions suggested by the software are shown in Figure 3. This optimum condition of pH 1.00533, temperature 88.3172, and extraction time 237.853, resulted in the highest yield of pectin from water hyacinth.
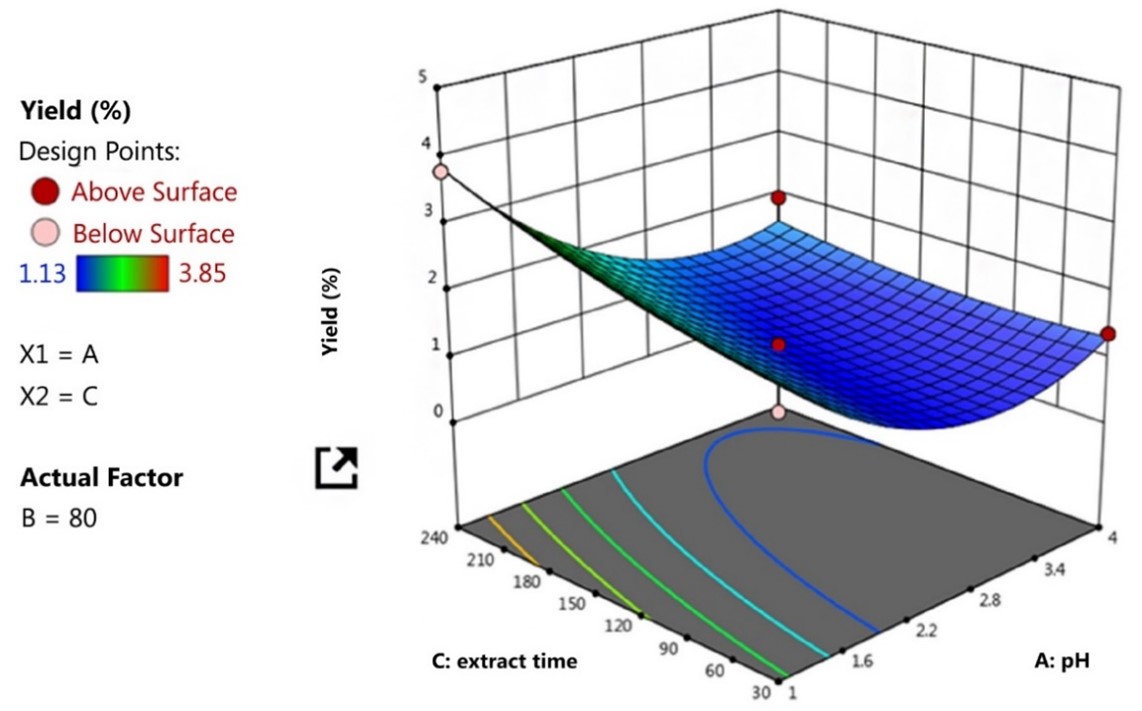
Figure 2 – Three-dimensional (3D) surface plots for a yield of pectin from water hyacinth. The percentage yield of pectin in a sequence from low to high was blue, green, yellow and red, respectively. (The red dot in the middle and every corner of the diagram was the design point appointed by the program to demonstrate that the design of the experimental conditions used in this study obtained from maximum values, median and minimum values of the factors to be studied)
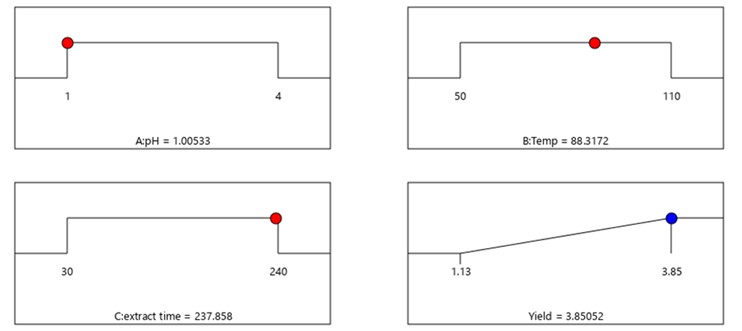
Figure 3 – The highest extracted pectin yield under an optimum condition of pH, temperature and time
Table 3
Sequential model sum of squares and ANOVA for response surface linear model of extraction yield
|
Source |
Sum of Squares |
df |
Mean Square |
F–value |
p–value |
|
|
Mean |
56.93 |
1 |
56.93 |
– |
– |
|
|
Linear |
7.25 |
3 |
2.42 |
5.16 |
0.0145 |
|
|
2FI |
0.6592 |
3 |
0.2197 |
0.4044 |
0.7531 |
|
|
Quadratic |
4.71 |
3 |
1.57 |
15.23 |
0.0019 |
suggested |
|
Cubic |
0.7151 |
3 |
0.2384 |
137.78 |
0.0002 |
aliased |
|
Residual |
0.0069 |
4 |
0.0017 |
– |
– |
|
|
Total |
70.27 |
17 |
4.13 |
– |
– |
|
|
Model |
12.62 |
9 |
1.40 |
13.59 |
0.0012 |
significant |
|
A-pH |
5.97 |
1 |
5.97 |
57.87 |
0.0001 |
|
|
B-Temp |
0.0003 |
1 |
0.0003 |
0.0030 |
0.9576 |
|
|
C- time |
1.28 |
1 |
1.28 |
12.41 |
0.0097 |
|
|
AB |
0.2209 |
1 |
0.2209 |
2.14 |
0.1867 |
|
|
AC |
0.3192 |
1 |
0.3192 |
3.10 |
0.1219 |
|
|
BC |
0.1190 |
1 |
0.1190 |
1.15 |
0.3183 |
|
|
A² |
4.04 |
1 |
4.04 |
39.17 |
0.0004 |
|
|
B² |
0.3289 |
1 |
0.3289 |
3.19 |
0.1173 |
|
|
C² |
0.0973 |
1 |
0.0973 |
0.9432 |
0.3638 |
|
|
Residual |
0.7220 |
7 |
0.1031 |
– |
– |
|
|
Lack of Fit |
0.7151 |
3 |
0.2384 |
137.78 |
0.0002 |
significant |
|
Pure Error |
0.0069 |
4 |
0.0017 |
– |
– |
|
|
Cor Total |
13.34 |
16 |
– |
– |
– |
|
|
Std. Dev. |
|
|
0.3212 |
|
|
|
|
Mean |
|
|
1.83 |
|
|
|
|
C.V. % |
|
|
17.55 |
|
|
|
|
R² |
|
|
0.9459 |
|
|
|
|
Adjusted R² |
|
|
0.8763 |
|
|
|
|
Predicted R² |
|
|
0.8416 |
|
|
|
|
Adeq Precision |
|
|
11.4182 |
|
|
|
B. Characterization of the pectin
Functional groups were analyzed by Fourier Transform Infrared (FTIR) spectrophotometry and the results of the standard and run #13 are shown in Figure 4 (A) and (B), respectively. Its spectra of pectin from water hyacinth were generally similar to the standard pectin in Figure 4 (A).
The spectra were determined to affirm the functional groups of extracted pectin. The %DE was estimated by the characteristic peaks of ester groups in the spectra (Begum et al., 2014; Kyomugasho et al., 2015). Wavenumber range of 950 and 1200 cm–1 as the “fingerprint” region of carbohydrates pectin groups (ether and cyclic C-C bond) (Masmoudi et al., 2008; Santos et al., 2013).
Similar spectra of the standard pectin and the water hyacinth extract in the “fingerprint” region implied that the extracts were pectin. Some carbonyl peaks at 1460 – 1592 cm–1 and 1683 – 1702 cm–1 were related to the free and esterified carboxyl groups, respectively (Thirugnanasambandham et al., 2014). The increase in the intensities and peak area of the esterified carboxyl groups will also increase the %DE (Oliveira et al., 2016). The peak at 1700 cm–1 was ascribed to the C=O stretching of methyl esterified uronic carboxyl group.
This free polysaccharide represents this shift. Determination of the %DE value can be done using the peak area at 1702 cm–1 (free carboxyl group) and 1460 cm–1 (esterified group) (Begum et al., 2014).
The peak at 2841 cm–1 showed the methoxyl group and was ascribed to the quality of pectin.
C. Relationship of pectin quality and the extraction variables
The quality of extracted pectin from water hyacinth is shown in Table 2.
The DE was plotted against pH, temperature, and time in Figure 5. It was found that increasing the extraction time resulted in a trend of decreasing DE (R2=0.3077).
On the contrary, increasing pH and temperature had the trend of slightly increasing DE (R2=0.0676 and 0.0204, respectively). The %DE of pectin was investigated according to BBD (17 runs) and its results are shown in Table 2. The highest DE was 94.13 % in run #13 from the extraction condition of pH 2.5, temperature 110ºC, and extraction time of 30 min.
In addition, some conditions, such as, run #1, #6, and #15 with a long extraction time showed different characteristic peaks other than the standard pectin assuming that they were not pectin (DE=0).
The degree of esterification was analyzed by multiple factor linear regression and found that the equations with uncoded factors for DE prediction were given as Equation (6) below:

where X1, X2, and X3 are the pH of the solution, temperature, and extraction time, respectively.
The R-square of Equation (6) was 0.97. The DE reflects the extraction quality of pectin. When substituting the values from run #13 in the quality Equation (6), the calculated DE was 99.06% and is comparable to 94.13% from the actual value. If high methoxyl pectin (HMP) has a degree of esterification values higher than 50%, then many conditions in this study could produce HMP for example run #13, #3, #8 etc.
The 2FI and quadratic models were the appropriate model for the quality of pectin from water hyacinth. The cubic model was aliased, which implies that it cannot be selected (Table 4).
The 2FI and quadratic model were significant with p-values of 0.0266 and 0.0256, respectively. The adequate precision ratio was 12.3274 indicating an adequate signal and was possible to navigate the design space of pectin quality.
The effect of extraction variables on the %DE is depicted in Figure 6. It demonstrates the effect of extraction time and temperature on the %DE.
The highest DE value of 94.13% was found when the pH of 3.2 and the extraction time was at 135 min and 80ºC. Indeed, similar studies have found behaviors for the DE value as a function of extraction conditions (Yapo et al., 2007).
Other research (Baothong, 2018; Oliveira et al., 2016) showed the extraction of pectin from each material using high pH values produced pectin with higher DE values. This can be described as the higher de-esterification of the polygalactoronic chain at very low pH conditions.
The results revealed that extracted pectin from water hyacinth with high %DE values where extraction time not longer than 30 minutes in runs #3 and #13, which the %DE values higher than 80%. In run #14, even though the pH value was high (4.0) and the extraction time was low, the %DE value was still considered high (67.15%). Although only limit conditions were tested, the optimum extraction conditions at a short time was likely to respond to a high %DE value.
Figure 7 shows the optimum condition of each parameter calculated by the RSM software to achieve a high %DE. The optimum condition was 2.59, 99.80, and 42.67 for pH, temperature, and extraction time, respectively. The %DE at the optimum condition was 96.87%.
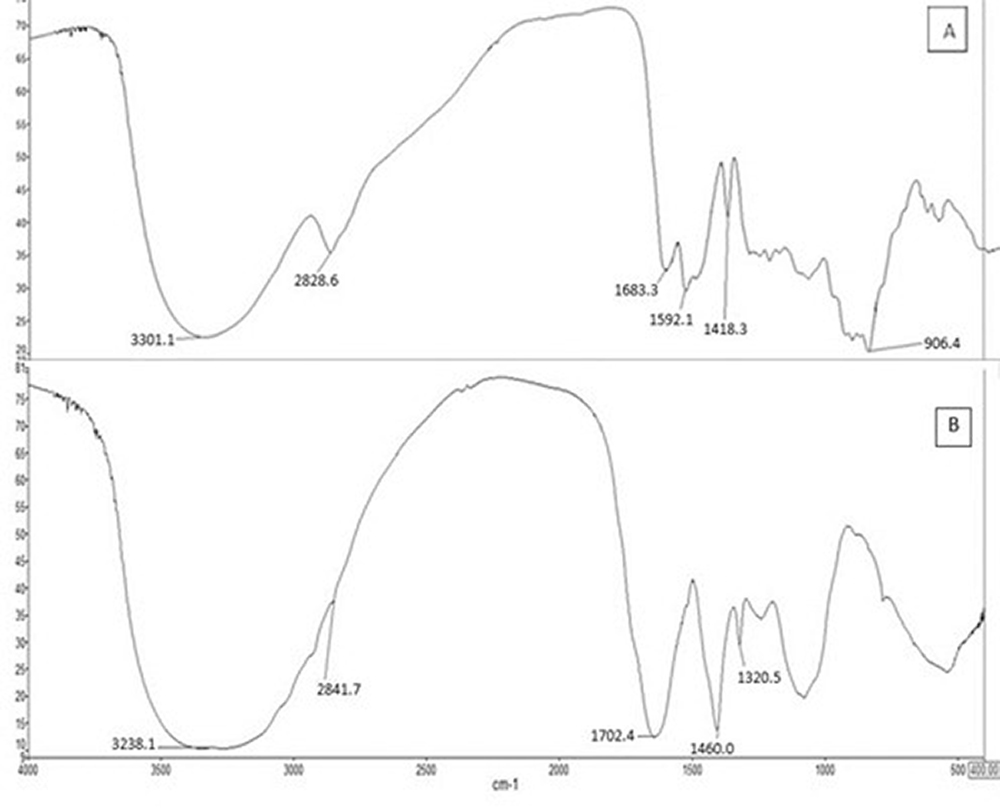
Figure 4 – FTIR spectra of water hyacinth pectin obtained at (A) standard pectin, (B) run #13 (The highest quality pectin)
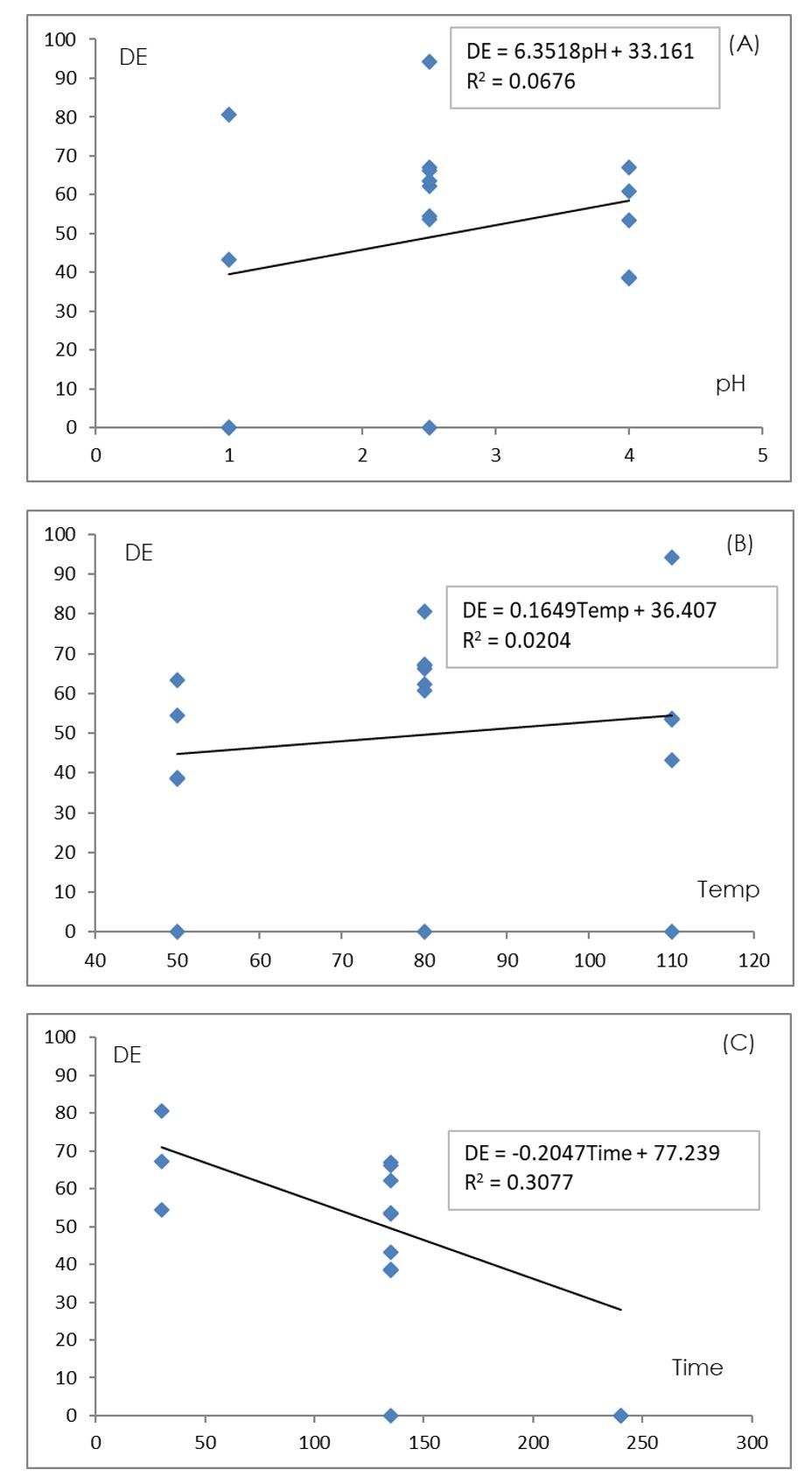
Figure 5 – Relationship between DE of pectin extracted from water hyacinth and (A) pH, (B) temperature and (C) extraction time
Table 4
Sequential model sum of squares and ANOVA for response surface linear model of %DE
|
Source |
Sum of Squares |
df |
Mean Square |
F–value |
p–value |
|
|
Mean |
45474.29 |
1 |
45474.29 |
– |
– |
|
|
Linear |
5000.27 |
3 |
1666.76 |
3.00 |
0.0694 |
|
|
2FI |
4234.70 |
3 |
1411.57 |
4.72 |
0.0266 |
suggested |
|
Quadratic |
2137.28 |
3 |
712.43 |
5.83 |
0.0256 |
suggested |
|
Cubic |
840.23 |
3 |
280.08 |
73.06 |
0.0006 |
aliased |
|
Residual |
15.33 |
4 |
3.83 |
– |
– |
|
|
Total |
57702.10 |
17 |
3394.24 |
– |
– |
|
|
Model |
11372.25 |
9 |
1263.58 |
10.34 |
0.0028 |
significant |
|
A-pH |
1155.12 |
1 |
1155.12 |
9.45 |
0.0180 |
|
|
B-Temp |
148.44 |
1 |
148.44 |
1.21 |
0.3069 |
|
|
C- time |
3696.71 |
1 |
3696.71 |
30.25 |
0.0009 |
|
|
AB |
200.08 |
1 |
200.08 |
1.64 |
0.2415 |
|
|
AC |
1375.67 |
1 |
1375.67 |
11.26 |
0.0122 |
|
|
BC |
2658.95 |
1 |
2658.95 |
21.75 |
0.0023 |
|
|
A² |
1064.86 |
1 |
1064.86 |
8.71 |
0.0214 |
|
|
B² |
949.96 |
1 |
949.96 |
7.77 |
0.0270 |
|
|
C² |
46.05 |
1 |
46.05 |
0.3767 |
0.5588 |
|
|
Residual |
855.56 |
7 |
122.22 |
|
|
|
|
Lack of Fit |
840.23 |
3 |
280.08 |
73.06 |
0.0006 |
significant |
|
Pure Error |
15.33 |
4 |
3.83 |
|
|
|
|
Cor Total |
12227.81 |
16 |
|
|
|
|
|
Std. Dev. |
|
|
11.06 |
|
|
|
|
Mean |
|
|
51.72 |
|
|
|
|
C.V. % |
|
|
21.38 |
|
|
|
|
R² |
|
|
0.9300 |
|
|
|
|
Adjusted R² |
|
|
0.8401 |
|
|
|
|
Predicted R² |
|
|
0.8416 |
|
|
|
|
Adeq Precision |
|
|
12.3274 |
|
|
|
The emulsifying properties of the highest %DE pectin from Run #13, which was extracted under optimal extraction conditions were studied. The emulsifying activity (EA) and emulsion stability (ES) of the highest %DE pectin are shown in Table 5. This research shows that the emulsion stability had a high level under the two different tested storage temperatures, emulsion stability levels at 4ºC after 1 day and 30 days, were 92.3% and 87.4%, respectively. But emulsion stability levels at 23ºC after 1 day and 30 days, decreased to 79.6% and 51.2%, respectively. Therefore, the factors that greatly affect the emulsifying activity of pectin extracted from water hyacinth were temperature and time (Bichara et al., 2016; Hosseini et al., 2016).
DISCUSSION
The response of pectin extraction in this case is a non-linear combination condition of three parameters (pH, temperature, and extraction time). Using the response-surface model was suitable and could reduce the number of wet experiments (Pasandide et al., 2017; Peng et al., 2020).
Percentage pectin yields extracted from raw materials are significantly varied in the range of 1.42-71.5% as shown in Table 6. Although pectin could be extracted from water hyacinth, the extraction yield was comparatively lower than other materials. This reflects the fact that the cell walls of water hyacinths contain very low (about 5%) pectin (Naohara and Ishii, 1989). The quality of the pectin extracted from water hyacinth on the contrary had the highest %DE of 94.13%. This number was higher than other research, which extracted pectin from water hyacinth with a %DE value of 62.26% in conditions with a pH of 2.0, temperature of 80ºC, and extraction time of 60 minutes. However, the extraction time was too long. The ester group in the pectin chain is converted to a carboxylic group. The -CH3 of the ester group will be replaced by a hydrogen atom and the %DE value will be lower (Ji-u and Neamsorn, 2022).
High-quality pectin can be used in many industries. The price of high-quality pectin was $99.17/Kg for Laboratory and pharmaceutical grades (Happi et al., 2008). Furthermore, a high degree of esterification provided the active sites for cross-linked to other cations such as calcium and boron to form stable (hard to be dissolved) hydrogel which can be used as a base for heavy metal adsorption. Thus, the optimum condition for high %DE pectin in this study can improve an adsorbent that is less soluble and can be used for adsorption for wastewater treatment repeatedly and efficiently. Therefore, it is another alternative way to manage water hyacinth problems in water sources.
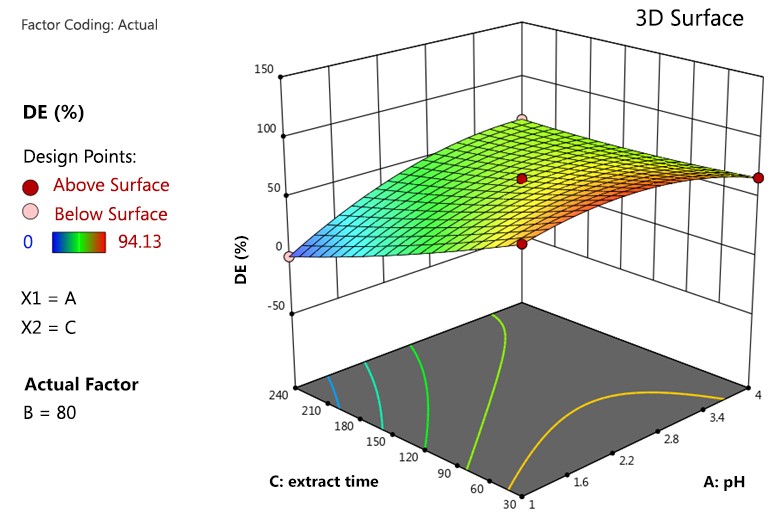
Figure 6 – Three-dimensional (3D) surface plots for the %DE from water hyacinth The %DE in a sequence from low to high was blue, green, yellow and red, respectively
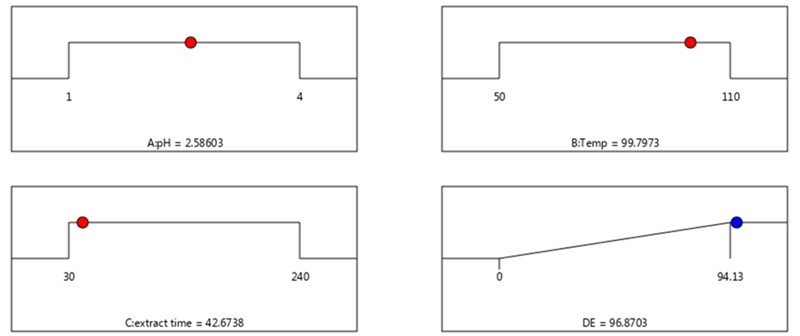
Figure 7 – The highest %DE under an optimum condition of pH, temperature and time
Table 5
Emulsifying activity and emulsion stability (%v/v) of pectin solutions (0.5%w/w)
|
Storage time (day) |
Emulsion activity (%) |
Emulsion stability (%) |
|||
|
1 |
30 |
||||
|
Temperature (ºC) |
|
4 |
23 |
4 |
23 |
|
Pectin Run #13* |
42 |
92.3 |
79.6 |
87.4 |
51.2 |
* Pectin with the highest %DE (at the temperature 110ºC, extraction time 30 min and pH 2.5)
Table 6
Yield and DE of pectin extracted from each material
|
Material |
%DE |
%Yield |
References |
|
Water hyacinth Water hyacinth |
94.13 62.26 |
1.42 5.42 |
This study Jariyapamornkoon et al. (2023) |
|
Broccoli |
76.50 |
71.50 |
Christiaens et al. (2011) |
|
Durian rind mixed with coconut coir |
65.80 |
9.10 |
Baothong (2018) |
|
Dragon fruit |
– |
7.50 |
Thirugnanasambandham et al. (2014) |
|
Pomegranate peel |
47.43 |
25.96 |
Pereira et al. (2016) |
|
Lemon peel |
– |
11.21 |
Ciriminna et al. (2016) |
CONCLUSIONS
The pectin extracted from water hyacinth was classified as high quality because of the high %DE. Extraction conditions affected both the extraction yield and quality of pectin. For future use, multiple linear regression models for the extraction yield and quality of pectin were proposed. The extraction yield was affected by pH and extraction time. The degree of esterification is affected by extraction time and pH. The optimum condition given high-quality pectin from water hyacinth of 94.13% DE was pH 2.5, 110ºC, and an extraction time of 30 min. The high-quality pectin can be applied in many industries including repeatable desorption hydrogel granules for wastewater treatment.
Author Contributions: Conceptualization, methodology, supervision, review: PC; Planning, writing, data curation, investigation: EB. All authors declare that they have read and approved the publication of the manuscript in this present form.
Acknowledgments: This research is supported by the Office of the Higher Education Commission, Ministry of Education, Thailand. Thanks to Stat–Ease, Inc. for Design-Expert® software, version 13. We would like to thank Mr. Markus Davis who is an English language teacher at Xi’an Jiaotong–Liverpool University, Suzhou, China for correcting the English in this manuscript.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
Audenhove, J.V.; Bernaerts, T.; De Sme, V.; Delbaere, S.; Van Loey, A.M.; Hendrickx, M.E. The Structure and Composition of Extracted Pectin and Residual Cell Wall Material from Processing Tomato: The Role of a Stepwise Approach versus High-Pressure Homogenization-Facilitated Acid Extraction. Foods. 2021, 10, 1-23. https://doi.org/10.3390/foods10051064
Baothong, E. Utilization of Durian Rind and Coconut Husk for Pectin Extraction. Journal of Research Unit on Science, Technology and Environment for Learning. 2018, 9, 200-210.
Begum, R.; Aziz, M.G.; Uddin, M.B.; Yusof, Y.A. Characterization of Jackfruit (Artocarpus heterophyllous) waste pectin as influenced by various extraction conditions. Agriculture and Agricultural Science Procedia. 2014, 2, 244-251. https://doi.org/10.1016/j.aaspro.2014.11.035
Bichara, L.C.; Alvarez, P.E.; Bimb, M.V.F.; Vaca, H.; Gervasi, C.; Brandan, S.A. Structural and Spectroscopic study of pectin isolated from citrus peel by using FTIR and FT-Raman spectra and DFT calculations. Infrared Physics & Technology. 2016, 76, 315-327. https://doi.org/10.1016/j.infrared.2016.03.009
Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its Physicochemical properties. Carbohydrate Polymer. 2016, 140, 59-65. https://doi.org/10.1016/j.carbpol.2015.12.051
Carlini, M.; Castellucci, S.; Mennuni, A. Water hyacinth biomass: chemical and thermal pre-run for energetic utilization in the anaerobic digestion process. Energy Procedia. 2018, 148, 431-438. https://doi.org/10.1016/j.egypro.2018.08.106
Christiaens, S.V.; Buggenhout, K.; Houben, I.; Fraeye, A.M.; Loey, V.; Hendrickx, M.E. Towards a better understanding of the pectin structure function relationship in broccoli during processing. Part I macroscopic and molecular analyses. Food Research International. 2011, 44, 1604-1612. https://doi.org/10.1016/j.foodres.2011.04.029
Ciriminna, R.; Fidalgo, A.; Delisi, R.; Ilharco, L.; Pagliaro, M. Pectin production and global market. Agro Food Industry Hi Tech. 2016, 27, 17-20.
Dalev, P.G.; Simeonova, L.S. Emulsifying properties of protein–pectin complexes and their use in oil-containing foodstuffs. Journal of the Science of Food and Agriculture. 1995, 68, 203-206. https://doi.org/10.1002/jsfa.2740680211
Dang, B.K.; Do, T.T.; Pham, M.T.; Dang, T.B.O.; Le, T.H.V.; Phan, D.Q.; Pham, D.L. Optimization the pectin extraction process from amberalla peel by the combined oxalic acid and microwave and comparison of characteristics with the pectins obtain to traditional extraction method. Annals Food Science and Technology. 2014, 15, 231-239.
Happi, T.E.; T.; Ronkart, S.N.; Christelle, R.; Bernard, W.; Michel, P. Characterization of pectins extracted from banana peels (Musa AAA) under different conditions using an experimental design. Food Chemistry. 2008, 108, 463-471. https://doi.org/10.1016/j.foodchem.2007.10.078
Hundie, K.B. Ultrasound-Assisted Optimization of Pectin Extraction from Orange Peel Using Response Surface Methodology (RSM) and Artificial Neural Network (ANN). International Journal of Applied Science and Engineering. 2020, 8. https//:doi.org/ 10.30954/2322-0465.2.2020.1
Jariyapamornkoon, N.; Phongthajitr, C.; Sritharet, N.; Sutthitham, W. Preservation of chicken egg quality using pectin derived from water hyacinth. Applied Food Research. 2023, 3. https://doi.org/10.1016/j.afres.2023.100355.
Ji–u, P.; Neamsorn, N. Effect of Extraction Methods on Physical and Chemical Properties of Pectin from Overripe ’Namwa’ Banana. Journal of Food Technology, Siam University. 2022, 17, 26-36.
Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit-and vegetable-based matrices. Food Chemistry. 2015, 176, 82-90. https://doi.org/10.1016/j.foodchem.2014.12.033
Levigne, S.; Ralet, M.C.; Thibault, J.F. Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohydrate Polymers. 2002, 49, 145-153. https://doi.org/10.1016/S0144-8617(01)00314-9
Ma, X.; Jing, J.; Wang, J.; Xu, J.; Hu, Z. Extraction of Low Methoxyl Pectin from Fresh Sunflower Heads by Subcritical Water Extraction. ACS Omega. 2020, 5, 15095-15104. https://doi.org/10.1021/acsomega.0c00928
Masmoudi, M.; Besbes, S.; Chaabouni, M.; Robert, C.; Paquot, M.; Blecker, C.; Attia, H. Optimization of pectin extraction from lemon by product with acidified date juice using response surface methodology. Carbohydrate Polymer. 2008, 74, 185-192. https://doi.org/10.1016/j.carbpol.2008.02.003
Moonsoor, M.A.; Kalapathy, U.; Proctor, A. Determination of polygalacturonic acid content in pectin extracts by diffuse reflectance Fourier transform infrared spectroscopy. Food Chemistry. 2001, 74, 233-238. https://doi.org/10.1016/S0308-8146(01)00100-5
Naohara, J.; Ishii, T. Contents of Pectic Substances in Water Hyacinth. Nippon Shokuhin Kogyo Gakkaishi. 1989, 36, 583-589.
Narasimman, P.; Sethuraman, P. An overview on the fundamentals of pectin. International Journal of Advanced Research. 2016, 4, 1855-1860. https://doi.org/10.21474/IJAR01/2593
Oliveira, T.I.S.; Rosa, M.F.; Canalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azerefo, H.M.C. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chemistry. 2016, 198, 113-118. https://doi.org/10.1016/j.foodchem.2015.08.080
Pasandide, B.; Khodaiyan, F.; Mousavi, Z.E.; Hosseini, S.S. Optimization of aqueous pectin extraction from Citrus medica peel. Carbohydrate Polymers. 2017, 178, 27-33. http://dx.doi.org/10.1016/j.carbpol.2017.08.098
Peng, X.; Yang, G.; Shi, Y.; Zhou, Y.; Zhang, M.; Li, S. BoxBehnken design based statistical modeling for the extraction and physico chemical properties of pectin from sunflower heads and the comparison with commercial low-methoxyl pectin. Scientific Reports. 2020, 10, 1-10. https://doi.org/10.1038/s41598-020-60339-1
Pereira, P.H.; Oliveira, T.I.; Rosa, M., Cavalcante, F.L.; Moates, G.K.; Wellner, N.; Waldron, K.W.; Azeredo, H.M.C. Pectin extraction from pomegranate peels with citric acid. International Journal of Biological Macromolecules. 2016, 88, 373-379. https://doi.org/10.1016/j.ijbiomac.2016.03.074
Robledo, V.R.; Vázquez, L.I.C. Pectin – Extraction, Purification, Characterization and Applications. https://www.intechopen.com/chapters/66458 (accessed on 25 December 2023).
Sabando, J.M.; Coin, F.; Melillo, J.H.; Goyanes, S.; Cerveny, S.A. Review of Pectin-Based Material for Applications in Water Treatment. Materials. 2023, 16, 2207. https://doi.org/10.3390/ma16062207
Said, N.S.; Olawuyi, I.F.; Lee, W.Y. Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications. Gels. 2023, 9, 732. https://doi.org/10.3390/gels9090732
Sandarani, M. A Review: Different Extraction Techniques of Pectin. Journal of Pharmacognosy & Natural Products. 2017, 3. https://doi.org/10.4172/2472-0992.1000143
Santos, J.D.G.; Espeleta, A.F.; Branco, A.; Assis, S.A. Aqueous Extraction of Pectin from Sisal Waste. Carbohydrate Polymer. 2013, 92, 1997-2001. https://doi.org/10.1016/j.carbpol.2012.11.089
Shukla, R.N.; Bala, K.L.; Kumar, A.A.; Mishra, A.; Yadav, K.C. Extraction of Pectin from Citrus Fruit Peel and Its Utilization in Preparation of Jelly. International Journal of Engineering Research & Technology. 2014, 3, 1925-1932.
Singthong, J.; Cui, S.W.; Ningsanond, S.; Goff, H.D. Structural characterization degree of esterification and some gelling properties of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohydrate Polymers. 2004, 58, 391-400. https://doi.org/10.1016/j.carbpol.2004.07.018
Thirugnanasambandham, K.; Sivakumar, V.; Maran, J.P. Process optimization and analysis of microwave assisted extraction of pectin from dragon fruit peel. Carbohydrate Polymer. 2014, 112, 622-626. https://doi.org/10.1016/j.carbpol.2014.06.044
USP NF 21. The United States Pharmacopeia–The National Formulary. Rockvile, Maryland, USA, 2003, pp. 1401-1402.
Wai, W.W.; Alkarkhi, A.F.M.; Easa, A.M. Effect of extraction conditions on yield and degree of esterification of durian rind Pectin: An experimental design. Food and Bioproducst Processing. 2010, 88, 209-214. https://doi.org/10.1016/j.fbp.2010.01.010
Wang, R.; Liang, R.; Dai, T.; Chen, J.; Shuai, X.; Liu, W. Pectin-based adsorbents for heavy metal ions: A review. Trends in Food Science & Technology. 2019, 91, 319-329. https://doi.org/10.1016/j.tifs.2019.07.033
Weiping, S.; Qingping, S.; Meisheng, X.; Zhengshun, W.; Zhitong, Y. The Resource Utilization of Water Hyacinth (Eichhornia crassipes [Mart.] Solms) and Its Challenges. Resources. 2018, 7, 1-9. https://doi.org/10.3390/resources7030046
Yapo, B.M. Pectin quantity, composition and physicochemical behavior as influenced by the purification process. Food Research International. 2009, 42, 1197-1202. https://doi.org/10.1016/j.foodres.2009.06.002
Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chemistry. 2007, 100, 1356-1364. https://doi.org/10.1016/j.foodchem.2005.12.012
Academic Editor: Dr. Iuliana Motrescu
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.



