A. Kheloufi, L.M. Mansouri, B. Bouafia, Y. Khamari, H. Kheloufi, Y. Bouguern
ABSTRACT. Astragalus armatus Willd. subsp. armatus is an endemic shrub of the Northern Africa. Its cultivation and domestication are very limited because of difficulty with seed germination and establishment. In this study, we investiga-ted some plant morphological characteris-tics in real time and in situ (leaves, fruit and seeds) of different ecotypes of A. armatus, collected from two sites in Algeria (Arid Steppe of Aïn Naga and Condorcet Mountain), which elevation and climate data are very different. Moreover, the role played by the seed coat in seed dormancy of these two different populations was tested by the effects of the pretreatment and its duration on the performance of seed germination, by considering the final germination percentage (FGP) and the mean germina-tion time (MGT). These parameters are estimated for 10 days in Petri dishes and stored in darkness at (25°C). Pre-sowing treatments included immersion in concentrated sulphuric acid for 30, 60 and 90 min, and immersion in hot water (100°C) for 10 min. Statistical analysis showed that the treatment and the eco-types effects on both FGP and MGT were highly significant (p< 0.0001). Untreated seeds of both ecotypes of A. armatus failed to germinate (except for a few of Condorcet Mountain ecotypes). For both populations, the most effective treatment was immersion in sulphuric acid for 60 min for the ecotype of Arid Steppe of Aïn Naga, and only 30 min for Condorcet Mountain. An excellent germinative strength is characterized by a higher FGP and a reduced MGT. The morphological characteristic and seed germination could be attributed to intraspecific variations resulting from the natural selection of the same species.
Keywords: Astragalus armatus; desert shrub; ecotype; germination; scarification.
View full article (HTML)
Morphological characteristics and seed germination improvement of two ecotypes of Astragalus Armatus Willd. subsp. Armatus in Algeria
A. Kheloufi1*, L.M. Mansouri1, B. Bouafia1, Y. Khamari1, H. Kheloufi1, Y. Bouguern1
1Department of Ecology and Environment, Faculty of Natural and Life Sciences, University of Batna 2, Batna, Algeria
*E-mail: abdenour.kheloufi@yahoo.fr
Received: July 24, 2018. Revised: Oct. 21, 2018. Accepted: Nov. 19, 2018. Published online: Oct. 3, 2019
ABSTRACT. Astragalus armatus Willd. subsp. armatus is an endemic shrub of the Northern Africa. Its cultivation and domestication are very limited because of difficulty with seed germination and establishment. In this study, we investiga-ted some plant morphological characteris-tics in real time and in situ (leaves, fruit and seeds) of different ecotypes of A. armatus, collected from two sites in Algeria (Arid Steppe of Aïn Naga and Condorcet Mountain), which elevation and climate data are very different. Moreover, the role played by the seed coat in seed dormancy of these two different populations was tested by the effects of the pretreatment and its duration on the performance of seed germination, by considering the final germination percentage (FGP) and the mean germina-tion time (MGT). These parameters are estimated for 10 days in Petri dishes and stored in darkness at (25°C). Pre-sowing treatments included immersion in concentrated sulphuric acid for 30, 60 and 90 min, and immersion in hot water (100°C) for 10 min. Statistical analysis showed that the treatment and the eco-types effects on both FGP and MGT were highly significant (p< 0.0001). Untreated seeds of both ecotypes of A. armatus failed to germinate (except for a few of Condorcet Mountain ecotypes). For both populations, the most effective treatment was immersion in sulphuric acid for 60 min for the ecotype of Arid Steppe of Aïn Naga, and only 30 min for Condorcet Mountain. An excellent germinative strength is characterized by a higher FGP and a reduced MGT. The morphological characteristic and seed germination could be attributed to intraspecific variations resulting from the natural selection of the same species.
Keywords: Astragalus armatus; desert shrub; ecotype; germination; scarification.
INTRODUCTION
As annual or perennial herbs, subshrubs, or shrubs, the plants of Astragalus (L.) (Fabaceae) are widely distributed throughout the temperate and arid regions (Zakhia et al., 2004). So far, the genus has been estimated to contain 2000-3000 species and more than 250 taxonomic sections in the world (Li et al., 2014). Several Astragalus species were studied for their antiviral, cardiotonic, antioxi-dant, cytotoxic, anticancer, immuno-stimulant, anti-inflammatory and anal-gesic activities (Labed et al., 2016). Astragalus armatus Willd. subsp. armatus is a thorny shrub locally known as “ketad”. It is an arid and semi-arid flowering plant that consti-tutes a significant element of the North African vegetation (Labed et al., 2016). This shrub is an endemic species to Algeria and is mainly found in North Sahara (Ozenda, 1991) and is adapted to severe climatic conditions of edaphic poverty (Rodelas and González-López, 2013).
It has been reported that harvesting most of commercial medicinal plants that are obtained by destructive harvest techniques of these species occurs essentially during the flowering period, before seed set, thereby lowering regeneration and causing the gradual degradation of wild populations (Saharkhiz et al., 2015). One of the most appropriate actions for safeguarding overexploited species would be to improve propagation techniques and to encourage cultivation. This strategy has been widely adopted in Europe, China, and India for many medicinal plants (Lubbe and Verpoorte, 2011).
There are two types of frequently used pretreatments, mechanical nature (break) and wet nature (immersion in a corrosive solution). The soaking into hot water or acids presents the advantage to treat an important quantity of seeds at the same time. However, the duration of the immersion must be determined to conclude the best time required to raise the coat dormancy. The present study aimed at identifying easily applied pretreatments (time of soaking into concentrated sulphuric acid and soaking in boiled water) that can be used to treat massive quantities of seeds to assure fast, homogeneous and synchronized seed germination of two ecotypes of Astragalus armatus subsp. armatus collected in Algeria (Biskra and Batna). A secondary aim of this study is to investigate and measure some morphological charac-terristics of fruit, seeds and leaves for each ecotype. This comparison pro-vides a test of the hypothesis that species in mountains region should be controlled by altitudinal barriers than lowland species.
MATERIALS AND METHODS
Collection and origin of seeds
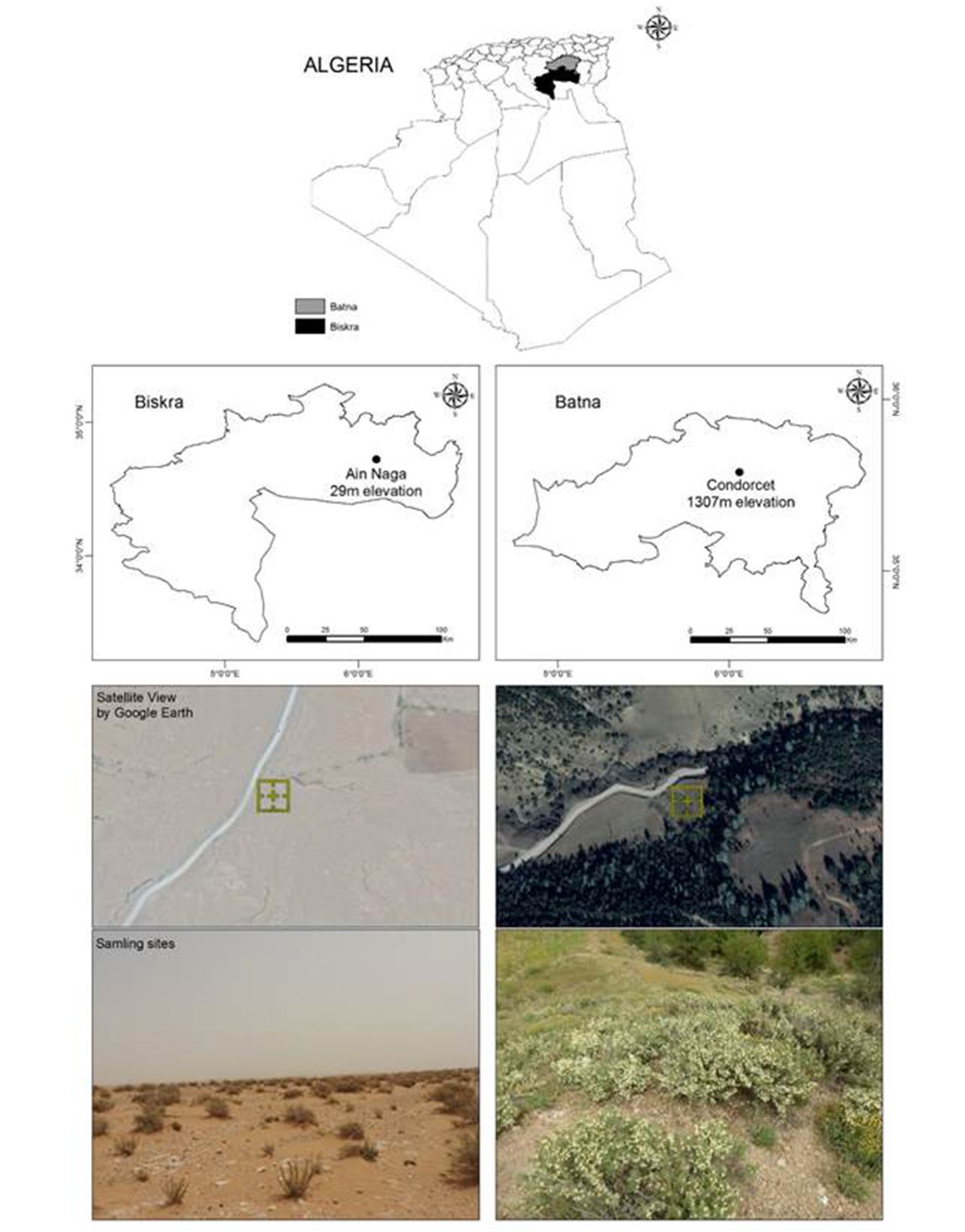
Seeds of A. armatus were collected from two wild populations at Batna (Mountain of Condorcet), and Biskra (Arid Steppe of Aïn Naga), located in Algeria (Fig. 1).
The geographic characteristics of the collection sites of the different ecotypes are presented in Table 1. Georeferenced maps were realized by GIS software (ArcGIS 10.3). Geographical positions were recorded using a Magellan eXplorist 200 GPS Receiver.
In this study, we have retained the average annual temperature, average annual maximum temperature, average annual minimum temperature, total annual precipitation and total rainy days during the year, of 38 year-period (1980-2018).
Climatic data was provided by the World Climate Data: Tutiempo available on the website: www.tutiempo.net.
Morphological characteristics
Leaves and mature fruits were collected directly from a total of 10 shrubs per ecotypes of A. armatus and stored in paper bags (Fig. 2). After collection, they were transported to the Laboratory of Ecology and Environment, University of Batna 2 (Algeria), where they were selected properly, described and photographed. In the laboratory, 50 leaves, 50 fruits and 50 seeds were analyzed.
The fruits and the seeds were analyzed in relation size (length, width) using a caliper. The leaves were observed for number of leaflets. After processing, seeds were reserved for germination test (Fig. 3).
Improvement of seed germination
The seed sample was obtained by mixing the seeds. Seeds of every population underwent several pretreat-ments: a chemical treatment, which consisted of immersion in 98% sulphuric acid for 30, 60 or 90 min, followed by washing in distilled water; or hot water treatment, soaking seeds in hot water (100°C) for 10 min, followed by washing in fresh distilled water. For control, seeds were not treated. It was conducted to be able to compare the effect of no pretreatment on germination.
The sowing (four replicates of 10 seeds × 5 treatments × 2 ecotypes) was realized in Petri dishes of 10 cm diameter, papered with two layers of Whatman filter paper and soaked with 20 ml of distilled water and then placed in a culture chamber in the obscurity at the laboratory temperature 25 (±2°C), during 10 days of incubation.
The Petri dishes were arranged every two days, according to a randomized design to eliminate any effect of the position in the seed culture room (Kheloufi et al., 2017). The counts of germinated seeds were made every day until the 10th day of incubation and were expressed in percentage (The criterion of germination was 2 mm radicle protru-sion).
In the germination tests, final germination percentage (FGP) and mean germination time (MGT) for each ecotypes and pretreatment were calculated by using the following procedures and formulas:

where, FGP is final germination percentage, ni is the number of germinated seeds at final day of test, and N is the total number of incubated seeds per test (Côme, 1970).
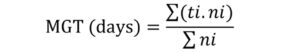
where, MGT is mean germination time, ti is the number of days from beginning of the test, ni is the number of germinated seeds recorded at time t(i), and Σni is the total number of germinated seeds (Orchard, 1977).
Table 1
Geographical coordinates and climate of two populations of A. armatus in Algeria
|
Climatic data (1980-2018) |
Batna (Mountain of Condorcet) |
Biskra (Arid Steppe of Aïn Naga) |
|
Elevation |
1307 m |
29 m |
|
Latitude |
35°34’49.99″ N |
34°44’20.86″ N |
|
Longitude |
6° 4’11.31″ E |
6° 7’5.89″ E |
|
Average annual temperature (°C) |
15,6 |
22.7 |
|
Average annual maximum temperature (°C) |
22.8 |
28.4 |
|
Average annual minimum temperature (°C) |
8.3 |
16.9 |
|
Total annual precipitation (mm) |
323.8 |
160.6 |
|
Total rainy days during the year |
73 |
30 |

Figure 2 – Two ecotypes of Astragalus armatus Willd. subsp. armatus (A) ecotype of Aïn Naga Steppes; (B) ecotype of Condorcet Mountain
Statistical analysis
The morphological characteristics and the effects of pretreatments on both variables were tested by analysis of variance (ANOVA). Multiple compare-sons of means were performed with Duncan’s test (α ≤ 0.05).
All statistical methods were performed using SAS Version 9.0 (Statistical Analysis System) (2002) software.
RESULTS AND DISCUSSION
Morphological description
The distribution of plant species among habitats is determined by a wide range of climatic and edaphic factors.
Habitat heterogeneity combined with natural selection often lead to multiple, genetically distinct ecotypes within a single species (Hufford and Mazer, 2003). Thus, ecotypes are populations of a particular species that are evolutionally adapted to specific environmental conditions. These eco-types, or populations that occurring in distinct habitats, vary from one to another in morphological traits such as shape, size, or leaf color (Kjemtrup et al., 2003), as well as traits related to seed germination (Hufford and Mazer, 2003).

Figure 3 – Stems of 10 cm long of A. armatus (A); seeds and fruits morphometrics of A. armatus (B); seed germination in Petri dish of A. armatus (C)
ANOVA showed that ecotype had a significant effect on the traits: leaflets number per leaf (p = 0,0206), fruit length and width (p< 0,0001), and seed width (p< 0,0001), whereas seed length was not significant at 5% probability level, as illustrated in Table 2. Effectively, seed length of both populations of A. armatus seems similar.
The greatest difference was observed in the case of 1000-seed weight. The Condorcet Mountain ecotype showed the highest weight (10,81 g), followed by the Arid Steppe of Aïn Naga (8,18 g) (Table 2). Relationships between seed mass and germination are less well understood than relationships between seed mass and other life-history stages.
Differences in seed mass among species are due in part to different levels of starch and endosperm nutrients, and may influence germination percentage (Kidson and Westoby, 2000).
Table 2
Variance analysis and morphological characteristics of leaves, fruit and seeds of A. astragalus ecotypes (Aïn Naga Steppe and Condorcet Mountain)
|
Ecotypes |
1000-seed weight (g) |
Leaflets per leaf |
Fruit size |
Seed size |
||
|
Length (mm) |
Width (mm) |
Length (mm) |
Width (mm) |
|||
|
Arid Steppe of Aïn Naga |
8,18 |
7,06 ± 1,44B |
14,9 ± 0,92A |
6,14 ± 0,60B |
3,35 ± 0,38B |
2,1 ± 0,24B |
|
Condorcet Mountain |
10,81 |
7,78 ± 1,60A |
12,3 ± 1,28B |
11,1 ± 1,23A |
3,56 ± 0,38A |
2,5 ± 0,24A |
|
F of Fisher |
— |
5,54 |
136,05 |
649,28 |
0,0055 |
56,26 |
|
P |
— |
0,0206 |
< 0,0001 |
< 0,0001 |
8,07 |
< 0,0001 |
For each ecotypes species, the same alphabet along the column indicates no significance difference (Duncan Multiple Range Test) (n=50).
As shown in Table 2, ecotype had a significant effect on the fruit length. The highest length of was found on plants from the Arid Steppe of Aïn Naga (14,9 ± 0,92 mm), followed by Condorcet Mountain with (12,3 ± 1,28 mm), respectively. However, fruit width of Condorcet Mountain population was larger with (11,1 ± 1,23 mm).
Ecotype had no significant effect on seed length. However, the high seed width of (2,5 ± 0,24 mm) was observed for Condorcet Mountain population.
Seed germination improvement
According to Kheloufi et al. (2017), the intensity of dormancy for the same species may vary according to genotype and environment in which seeds are produced.
The efficiency of scarification with sulphuric acid to overcome seed coat impermeability and increase seed germination has been reported for different species. However, the efficiency of this treatment varies with the acid concentration, plant species and treatment duration (Kheloufi, 2017).
The final phase of seed development involves the loss of water, cessation of reserve synthesis where after the seed enters a metabolically inactive state. Seed dormancy has been defined as a temporary failure of a viable seed to germinate in conditions that favour germination (Bewley, 1997). These conditions are a complex combination of water, light, temperature, gasses, mechanical restrictions, seed coats, and hormone structures. Dormancy in nature serves to protect the seed from conditions, which are temporarily suitable for germination, but which quickly revert to conditions too harsh for survival of the tender young seedling (Koornneef et al., 2002).
Thus, a seed coat relatively impermeable to moisture prevents germination during isolated showers in the middle of a long dry season, while permitting it during a sustained rainy season (Vázquez-Yanes and Orozco-Segovia, 1993). The seed coat has been shown to be a multifunc-tional organ which supplies nutrients to the embryo sac throughout development (van Dongen et al., 2003) and is functional during drying of the seed (Howe and Smallwood, 1982). The structural and chemical properties of the seed coat impose impermeability (Rolston, 1978), regu-late water entry once dormancy has been broken (Serrato-Valenti et al., 1993), provide a barrier against fungi (Mohamed-Yasseen et al., 1994), and reduce leakage from the embryo during imbibition (Simon and Harun, 1972). So, understanding this type of dormancy and identifying natural ways of breaking it becomes economically important.
Statistical analysis showed that the treatment and the ecotypes effects on both FGP and MGT were highly significant (p< 0.0001) (Table 3). Untreated seeds of both ecotypes of A. armatus failed to germinate (except for a few of Condorcet Mountain ecotypes). Mean germination time varied significantly across different provenances and due to pretreatments. Reduced mean germination time in acid treated seeds implies that the period of dormancy in seeds was reduced due to pretreatment of seeds in sulphuric acid. It is reported that acid treatment is an efficient method of enhancing seed germination of species with hard impermeable seed coat (Sy et al., 2001; Pérez-García and González-Benito, 2006; Hu et al., 2009). It stimulates fast and uniform germination of seeds.
Table 3
Variance analysis for the traits investigated of two Astragalus armatus ecotypes seeds in response to different pre-sowing at different durations for 10 days-period
|
Parameters |
Sources of variation |
Df |
F of Fisher |
P |
|
FGP |
TRT |
4 |
68,25 |
< 0,0001 |
|
Sp |
1 |
17,36 |
0,0002 |
|
|
TRT × Sp |
4 |
20,57 |
< 0,0001 |
|
|
MGT |
TRT |
4 |
20,85 |
< 0,0001 |
|
Sp |
1 |
45,78 |
< 0,0001 |
|
|
TRT × Sp |
2 |
0,00 |
1,0000 |
For both populations, the most effective treatment was immersion in sulphuric acid for 60 min for the ecotype of Arid Steppe of Aïn Naga, and only 30 min for Condorcet mountain. Increasing the duration of soaking in sulphuric acid from 30 to 60 min was favourable for lowland seeds, improving FGP from 90 to 100 (Table 4). However, extension of the soaking period to 90 min was unfavourable, reducing the FGP by 50%. Seeds soaked in boiled water had poor germination for Condorcet Mountain ecotypes and no germina-tion for the ecotype of Arid Steppe of Aïn Naga (Table 4). According to Trivedi and Joshi (2014), soaking in water, whatever its duration or tempe-rature, does not enhance germination for species with hard seed coats. According to de Paula et al. (2012), the required soaking time in hot water at 100°C also depends on the thickness
and hardness of the coat. The highest germination was obtained following a pretreatment of 30 min in sulphuric acid for the mountain seeds with 95% final germination, while 90 min soaking inhibited all germination process.
According to Nonogaki et al. (2010), the evaluation of germination capacity depends not only on the final germination percentage, but also the speed of germination. The correlation of these two factors is often used to determine the success of a pretreat-ment on overcoming dormancy.
The optimal duration of soaking is proportional to the coat rigidity. In this study, the seeds of Condorcet Mountain seem very sensitive to increase in the duration of soaking in the acid and under the boiling water treatment. This is in agreement with the works of Kheloufi (2017), which revealed that prolonged soaking in acid could damage the embryo and reduce germination.
Table 4
Final germination percentage (FGP) and mean germination time (MGT) for A. astragalus ecotypes species (Steppe of Aïn Naga and Condorcet Mountain) exposed to different pre-sowing treatments
|
Ecotypes |
TRT |
FGP |
MGT |
|
Arid Steppe of Aïn Naga |
Untreated |
0,00 ± 0,00D |
— |
|
BW |
0,00 ± 0,00D |
— |
|
|
30 min SA |
90.0 ± 11,54B |
2,58 ± 0,20A |
|
|
60 min SA |
100 ± 0,00A |
2,90 ± 0,57A |
|
|
90 min SA |
50.0 ± 0,00C |
1,20 ± 0,28B |
|
|
Condorcet Mountain |
Untreated |
15.0 ± 10,00C |
3,00 ± 0,00B |
|
BW |
15.0 ± 10,0C |
4,67 ± 1,15A |
|
|
30 min SA |
95.0 ± 10,0A |
2,94 ± 0,12B |
|
|
60 min SA |
60.0 ± 23,1B |
3,69 ± 0,37AB |
|
|
90 min SA |
0.00 ± 0,00D |
3,00 ± 0,00B |
For each ecotypes species, the same alphabet along the column indicates no significance difference (Duncan Multiple Range Test) (n=50).
To conclude, for several forest species, a special seed pretreatment is needed to get satisfactory germina-tion. The pretreatments do not allow the seeds to germinate, but improve the germination ability subsequently, when all favourable conditions are united. According to our results, ger-mination of a seed depends on the po-tential of embryo growth or potentials of growth preventor. These potentials depend particularly on seed structure that surrounded the embryo (endo-sperm, pericarp, glumes) (Schopfer and Plachy, 1985; Germanà et al., 2014). Other factors, like hormones and environmental factors, also affect embryo growth (Shu et al., 2016). This kind of dormancy happens when factors like water and gas are not permitted to enter the seed, so imbibition is not occurred and, consequently, resulting in decreasing seed germination (Bewley, 1997).
CONCLUSION
According to the obtained results, scarification treatments with sulphuric acid (98%) were effective, which caused dormancy breaking and seed germination induction of both populations of A. armatus.
This suggest that, considering only germination, it could be relatively easy for high altitude populations of the species studied to colonize open sites at lower eleva-tions. However, lowland populations of the same species may either require specific genetically based adaptations to low temperatures, or acclimation to low temperatures during seed deve-lopment, before their seeds can germinate on high-elevation sites.
This study is significant for land managers and conservation agencies with an interest in optimizing the germination of arid-zone seeds for rehabilitation of this threatened species.
REFERENCES
Bewley, J.D. (1997). Seed germination and dormancy. Plant Cell., 9 (7): 1055-1066, DOI: 10.1105/tpc.9.7. 1055
Côme, D. (1970). Obstacles to germi-nation. Masson et Cie. (Eds,), Paris, 162 p.
de Paula, A.S., Delgado, C.M.L., Paulilo, M.T.S. & Santos, M. (2012). Breaking physical dormancy of Cassia leptophylla and Senna macranthera (Fabaceae: Caesalpini-oideae) seeds: water absorption and alternating temperatures. Seed Sci.Res., 22(4): 259-267, DOI: 10.1017/S096025851 200013X
Germanà, M.A., Chiancone, B., Hammami, S.B.M. & Rapoport, H.F. (2014). Olive embryo in vitro germination potential: role of explant configuration and embryo structure among cultivars. Plant Cell.Tiss. Organ Cult., 118 (3): 409-417, DOI: 10.1007/s11240-014-0493-5
Howe, H.F. & Smallwood, J. (1982). Ecology of seed dispersal. Ann.Rev.Ecol.Syst.,13(1): 201-228, DOI: 10.1146/annurev.es.13.110182. 001221
Hu X.W., Wang Y.R., Wu Y.P., Baskin C.C. (2009). Role of the lens in controlling water uptake in seeds of two Fabaceae (Papilionoideae) species treated with sulphuric acid and hot water. Seed Sci.Res., 19 (2): 73-80, DOI: 10.1017/S096025 8509301099
Hufford, K.M. & Mazer, S.J. (2003). Plant ecotypes: genetic differentiation in the age of ecological restoration. Trends Ecol.Evo., 18(3): 147-155, DOI: 10.1016/S0169-5347(03)000 02-8
Kheloufi, A. (2017). Germination of seeds from two leguminous trees (Acacia karroo and Gleditsia triacanthos) following different pre-treatments. Seed Sci.Technol., 45(1): 259-264, DOI: 10.15258/sst.2017.45.1.21
Kheloufi, A., Mansouri, L.M. & Boukhatem, F.Z. (2017). Application and use of sulfuric acid pretreatment to improve seed germination of three acacia species. Reforesta, 3: 1-10, DOI: https://doi. org/10.21750/REFOR.3.01.25
Kidson, R. & Westoby, M. (2000). Seed mass and seedling dimensions in relation to seedling establishment. Oecologia, 125 (1): 11-17, DOI: 10.1007/PL00008882
Kjemtrup, S., Boyes, D.C., Christensen, C., McCaskill, A.J., Hylton, M. & Davis, K. (2003). Growth stage-based phenotypic profiling of plants. Plant Functional Genomics, pp. 427-441, Part of the Methods in Molecular Biology™ book series (MIMB, Vol. 236), Humana Press, DOI: 10.1385/1-59259-413-1:427
Koornneef, M., Bentsink, L. & Hilhorst, H. (2002). Seed dormancy and germination. Curr.Opin.Plant Biol., 5(1): 33-36, DOI: 10.1016/S1369-5266(01)00219-9
Labed, A. et al. (2016). Compounds from the pods of Astragalus armatus with antioxidant, anticholinesterase, anti-bacterial and phagocytic activities. Pharma.Biol., 54(12): 3026-3032, DOI: 10.1080/13880209.2016.1200 632
Li, X., Qu, L., Dong, Y., Han, L., Liu, E., Fang, S., Zhang, Y. & Wang, T. (2014). A review of recent research progress on the astragalus genus. Molecules, 19(11): 18850-18880, DOI: 10.3390/molecules191118850
Lubbe, A. & Verpoorte, R. (2011). Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod., 34(1), 785-801, DOI: 10.1016/j.indcrop. 2011.01.019
Mohamed-Yasseen, Y., Barringer, S.A., Splittstoesser, W.E. & Costanza, S. (1994). The role of seed coats in seed viability. Bot.Rev., 60(4): 426-439.
Nonogaki, H., Bassel, G.W. & Bewley, J.D. (2010). Germination-still a mystery. Plant Sci., 179(6): 574-581, DOI: 10.1016/j.plantsci.2010.02.010
Orchard, T.J. (1977). Estimating the parameters of plant seedling emergence. Seed Sci.Technol., 5: 61-69.
Ozenda, P. (1991). Flore et végétation du Sahara (3rd ed.). Paris: CNRS éd., DL 2004, 662 p.
Pérez-García, F. & González-Benito, M.E. (2006). Seed germination of five Helianthemum species: effect of temperature and presowing treatments. J. Arid Environ., 65(4): 688-693, DOI: 10.1016/j.jaridenv. 2005.10.008
Rolston, M.P. (1978). Water impermeable seed dormancy. Bot.Rev., 44(3): 365-396.
Saharkhiz, M.J., Kamyab, A.A., Kazerani, N.K., Zomorodian, K., Pakshir, K. & Rahimi, M.J. (2015). Chemical compositions and antimicrobial activities of Ocimum sanctum L. essential oils at different harvest stages. Jundishapur J.Microbiol., 8(1): e13720. 1-7, DOI: 10.5812/jjm.13720
Schopfer, P. & Plachy, C. (1985). Control of seed germination by abscisic acid: III. Effect on embryo growth potential (minimum turgor pressure) and growth coefficient (cell wall extensibility) in Brassica napus L. Plant Physiol., 77(3): 676-686, DOI: https://doi.org/10.1104/pp.77.3.676
Serrato-Valenti, G., Cornara, L., Ferrando, M. & Modenesi, P. (1993). Structural and histochemical features of Stylosanthes scabra (Leguminosae; Papilionoideae) seed coat as related to water entry. Canadian J.Bot., 71(6): 834-840, DOI: 10.1139/b93-095
Shu, K., Liu, X.D., Xie, Q. & He, Z.H. (2016). Two faces of one seed: hormonal regulation of dormancy and germination. Mol. Plant, 9(1): 34-45, DOI: 10.1016/j.molp.2015. 08.010
Simon E.W. & Harun R.M.R. (1972). Leakage during seed imbibition. J.Exp.Bot., 23(4): 1076-1085, DOI: 10.1093/jxb/23.4.1076
Sy, A., Grouzis, M. & Danthu, P. (2001). Seed germination of seven Sahelian legume species. J. Arid Environ., 49(4): 875-882, DOI: 10.1006/jare. 2001.0818
Trivedi, D.R. & Joshi, A.G. (2014). Studies on seed germination of Stereospermum suaveolens with respect to different parameters. Environ.Exp.Biol., 12: 33-37.
van Dongen, J.T., Ammerlaan, A.M.H., Wouterlood, M., van Aelst, A.C. & Borstlap, A.C. (2003). Structure of the developing pea seed coat and the post‐phloem transport pathway of nutrients. Ann.Bot., 91(6): 729-737, DOI: 10.1093/aob/mcg066
Vázquez-Yanes, C. & Orozco-Segovia, A. (1993). Patterns of seed longevity and germination in the tropical rainforest. Annu.Rev.Ecol.Syst., 24(1): 69-87, DOI: 10.1146/annurev. es.24.110193.000441
Rodelas B. & González-López, J. (Eds.) (2013). Beneficial plant-microbial interactions: ecology and applica-tions (1st Ed.), 215-235, DOI: 10.1201/b15251
Zakhia, F., Jeder, H., Domergue, O., Willems, A., Cleyet-Marel, J.C., Gillis, M., Dreyfus, B. & de Lajudie, P. (2004). Characterisation of wild legume nodulating bacteria (LNB) in the infra-arid zone of Tunisia. Syst.Appl.Microbiol., 27(3): 380-395, DOI: 10.1078/0723-2020-00273
Bouafia B., Bouguern Y., Khamari Y., Kheloufi A., Kheloufi H., Mansouri L.M.