Vasile Boghian
ABSTRACT. The study aims to identify the morphoclinical and paraclinical elements useful in the diagnosis of FIP, given that the symptoms are sometimes uncharacteristic, varied and often similar to those of other diseases. The morphoclinical features of 32 patients diagnosed with FIP were evaluated. In 26 patients (81.25%), the predominant symptomatology was similar: intermittent fever, loss of appetite, weakness, dyspnoea and physical signs of peritoneal fluid collection. The peritoneal puncture fluid was inflammatory, with numerous large phagocytes (neutrophils and macrophages), lymphocytes and, in some cases, red blood cells. The cell blood count (CBC) showed the existence of normocytic, hypochromic and hypoplastic anaemia and the presence of an active systemic inflammatory process, confirmed by the presence of aggregated platelets and segmented and vacuolated neutrophils in the stained smear May Grunwald Giemsa (MGG). Biochemical blood examination revealed the evolution of a physiopathological syndrome of hepatocytolysis, increased tissue catabolism and haemolytic anaemia. These results confirm that FIP is usually a systemic disease with polymorphic clinical signs, and biochemical blood tests, unlike CBC, have more prognostic value and lower value for suspecting the disease. However, sometimes, lesions and associated clinical signs in a single organ predominate. Thus, in three patients (9.37%), the predominant symptomatology was hepato-digestive with hepatocellular jaundice; one patient had obvious clinical signs of renal failure, one had signs of cortical syndrome, and one patient showed periosteal lesions (granulomatous osteitis). These results indicate that some less common lesions in cats, such as osteitis granulomatous, should be included in the list of FIP lesions.
Keywords: peritoneal effusion; osteitis granulomatous; cat.
Cite
ALSE and ACS Style
Boghian, V. Morphoclinical and paraclinical features of feline infectious peritonitis (FIP). Journal of Applied Life Sciences and Environment 2023, 56 (1), 115-126.
https://doi.org/10.46909/alse-561089
AMA Style
Boghian V. Morphoclinical and paraclinical features of feline infectious peritonitis (FIP). Journal of Applied Life Sciences and Environment. 2023; 56 (1): 115-126.
https://doi.org/10.46909/alse-561089
Chicago/Turabian Style
Boghian, Vasile. 2023. “Morphoclinical and paraclinical features of feline infectious peritonitis (FIP)” Journal of Applied Life Sciences and Environment 56, no. 115-126.
https://doi.org/10.46909/alse-561089
View full article (HTML)
Morphoclinical and Paraclinical Features of Feline Infectious Peritonitis (FIP)
Vasile BOGHIAN
Department of Clinics, Faculty of Veterinary Medicine, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 8, Mihail Sadoveanu Alley, 700489, Iasi, Romania
*Correspondence: boghian_v@uaiasi.ro
Received: May 29, 2023. Revised: Jun. 27, 2023. Accepted: Jul. 06, 2023. Published online: Jul. 17, 2023
ABSTRACT. The study aims to identify the morphoclinical and paraclinical elements useful in the diagnosis of FIP, given that the symptoms are sometimes uncharacteristic, varied and often similar to those of other diseases. The morphoclinical features of 32 patients diagnosed with FIP were evaluated. In 26 patients (81.25%), the predominant symptomatology was similar: intermittent fever, loss of appetite, weakness, dyspnoea and physical signs of peritoneal fluid collection. The peritoneal puncture fluid was inflammatory, with numerous large phagocytes (neutrophils and macrophages), lymphocytes and, in some cases, red blood cells. The cell blood count (CBC) showed the existence of normocytic, hypochromic and hypoplastic anaemia and the presence of an active systemic inflammatory process, confirmed by the presence of aggregated platelets and segmented and vacuolated neutrophils in the stained smear May Grunwald Giemsa (MGG). Biochemical blood examination revealed the evolution of a physiopathological syndrome of hepatocytolysis, increased tissue catabolism and haemolytic anaemia. These results confirm that FIP is usually a systemic disease with polymorphic clinical signs, and biochemical blood tests, unlike CBC, have more prognostic value and lower value for suspecting the disease. However, sometimes, lesions and associated clinical signs in a single organ predominate. Thus, in three patients (9.37%), the predominant symptomatology was hepato-digestive with hepatocellular jaundice; one patient had obvious clinical signs of renal failure, one had signs of cortical syndrome, and one patient showed periosteal lesions (granulomatous osteitis). These results indicate that some less common lesions in cats, such as osteitis granulomatous, should be included in the list of FIP lesions.
Keywords: peritoneal effusion; osteitis granulomatous; cat.
INTRODUCTION
Feline infectious peritonitis is a severe, usually fatal viral disease caused by a coronavirus (FCoV). There are two types of feline coronavirus that affect cats, the enteric coronavirus (FCoV-1) and the coronavirus that causes FIP (FCoV-2) (Benetka et al., 2004; Gao et al., 2023). The FCoV-1 is transmitted through contact with infected faeces, multiplies in the intestine and mainly causes diarrhoea in young animals that live with other cats and share litter boxes or food bowls (Drechsler et al., 2011; Tekes and Thiel, 2016). The FCoV-2 is considered a mutation of the enteric coronavirus that evolves against the background of immunosuppression induced by other viral infections such as those with feline leukaemia virus (FeLV) and feline immunodeficiency virus (FIV). Approximately 50% of FIP cases are diagnosed in young cats not vaccinated against FeLV and FIV (Richardson et al., 1997; Worthing et al., 2012; Zhou et al., 2020). The coronaviruses that affect cats are alpha-coronaviruses and are different from the beta-coronaviruses found in humans: SARS-CoV-1 (Severe Acute Respiratory Syndrome Coronavirus), identified in 2002 in southern China in the Guangdong region, originating from bats and transmitted to humans by an intermediate host of civet (Paguma larvata) and SARS-CoV-2, identified in 2019 in central China, in the Wuhan region, and responsible for the COVID-19 pandemic (Alberer and von Both, 2021; Gao et al., 2022). At the time of writing this paper, there were no data to show that the FCoV that causes FIP can also cause the disease in humans nor that the SARS-CoV that causes COVID-19 in humans can cause the disease in cats (Stout et al., 2020; Yin et al., 2021).
The clinical picture is the result of vascular and perivascular changes caused by the virus, clinically expressed by two main forms of evolution: “wet” or exudative and “dry”, without cavitary effusions (Felten and Hartmann, 2019; Gao et al., 2023). In the wet form, there are changes in the general condition (fever, apathy, weakness), dyspnoea, exudative peritonitis (abdomen enlarged in volume, positive abdominal ballottement test, inflammatory fluid on peritoneal puncture with total proteins over 3 g/dl) and high mortality (Riemer et al., 2016; Thayer et al., 2022). The dry form has a chronic evolution with atypical and different symptomatology, depending on the main location. In the form with nervous localisation, functional nervous signs predominate, especially changes in behaviour (apathy) and motility (muscle weakness, paresis and paralysis of the hind limbs or convulsions) (Felten and Hartmann, 2019; Rissi, 2018). In the digestive form, the symptomatology is uncharacteristic, with vomiting and persistent diarrhoea sometimes accompanied by jaundice, and is difficult to treat (Kipar and Meli, 2014). Other times, the predominant clinical signs can be respiratory signs (cough, dyspnoea), urinary signs (urinary incontinence) or ocular signs (visual disturbances, uveitis, chorioretinitis), associated with fever and progressive weakness (Addie et al., 2020; Pedersen, 2014). Previously, a case of orchitis-associated FIP has been described (Sigurdardottir et al., 2001), in addition to a case of coronavirus-induced cutaneous vasculitis lesion (Cannon et al., 2005) and a case of syringomyelia suspected to be related with FIP (Kitagawa et al., 2007). These aspects show that the morphoclinical features of FIP vary from case to case.
The clinical diagnosis of FIP is difficult. Rapid antigen/antibody tests (FCoV Ag/Ab Test Kit) can be used to detect Feline Coronavirus. However, rapid tests can only be used to increase the FIP index of suspicion, not FIP. They can show the presence of the virus in the body, but are limited in recognizing the type of coronavirus and cannot confirm whether the cat will get the disease. Therefore, clinical examination data are also necessary for a definitive diagnosis (Felten and Hartmann, 2019; Paltrinieri et al., 2002; Stranieri et al., 2018). In contrast, the polymorphic clinical picture also requires a paraclinical examination when we suspect FIP. Thus, ultrasound examination and the characteristics of the peritoneal puncture fluid are useful, especially in the wet form of the disease, and CBC and biochemical blood examination can provide additional information about the main location of the virus and the functions of the affected organs (Hung et al., 2022; Lewis and O’Brien, 2010; Pedersen, 2014). The results of these investigations are useful to the veterinarian for establishing the subsequent therapeutic conduct.
MATERIALS AND METHODS
The research was conducted on 32 animals (13 male and 19 female) of different ages and breeds that showed clinical signs of FIP (26 with the wet clinical form, 3 with wet form onset and 3 with the dry clinical form). These animals were brought for clinical examination and diagnosis. From a clinical point of view, the constant and predominant signs associated with FIP were identified in each case. Then, the presence or absence of peritoneal fluid collection, as well as the echostructure of the parenchymal organs in the abdominal cavity, especially the liver and kidney, were observed by ultrasound examination. Ultrasound examination was performed using the Acuson NX3 Elite Ultrasound System and the probe 5–8 MHz. In three patients in whom the presumptive diagnosis of FIP was not clear, the disease was confirmed by direct immunological demonstration of the coronavirus antigen in the affected tissues. The samples were collected during necropsy in two patients who had died shortly after starting treatment, one with hepatocellular jaundice and the other with renal failure. In one patient with granulomatous osteitis, the tissue sample was collected by puncture under anaesthesia during the clinical examination. Immunohistochemical analysis used the FCV3-70 monoclonal Ab (Custom Monoclonal Internationals, West Sacramento, USA) and revealed intramacrophagic staining corresponding to the pair of antibodies that recognise the presence of the coronavirus viral antigens. The immunohistochemical technique was a classical one, including negative and positive controls (Kipar and Meli, 2014).
Depending on the presence or absence of peritoneal fluid, the investigations were completed with the collection of fluid by peritoneal puncture and its examination from an organoleptic point of view. Fluid morphology was assessed by using a May Grunwald Giemsa (MGG) stained smear and a direct refractometric exam to determine the protein concentration. Later, blood samples were collected for the cell blood count (CBC), biochemical analyses and blood morphological examination in a panoptical stained smear (MGG). For the CBC examination, blood samples were collected on anticoagulant (EDTA) and examined for RBC (red blood cells), PCV (haematocrit), HGB (haemoglobin), MCV (mean corpuscular volume), MCH (mean corpuscular haemoglobin), MCHC (mean corpuscular haemoglobin concentration), WBC (white blood cells) and PLT (platelets). For these determinations an automatic haematology analyser (Vet Scan HM5) was used. Blood was collected in test tubes without anticoagulant for serum expression and analysed with the same automatic biochemical analyser.
Research continued according to the clinical particularities of each case, with necropsy and radiological examination. Necropsy was performed in two patients with unclear clinical signs for diagnosis who had died shortly after presenting in the clinic: one patient with hepatocellular jaundice as the predominant clinical sign and who had died 5 days after starting symptomatic treatment, and one with renal failure who had died 2 weeks after starting symptomatic treatment. The necropsy helped to diagnose FIP. Radiological examination was also done for diagnostic purposes in one patient with bone lesions and two other patients with predominantly hepato-digestive clinical signs.
RESULTS AND DISCUSSION
In 26 out of 32 patients diagnosed with FIP (81.25%), the predominant symptomatology was characteristic of the exudative (wet) form of disease evolution. The constant clinical manifestations were intermittent fever, capricious appetite, progressive weight loss, dyspnoea, inhibited behaviour (apathy) and physical signs of peritoneal fluid collection (abdomen enlarged in volume, abdominal ballottement). In these situations, ultrasound examination was highly important (Lewis and O’Brien, 2010) (Figure 1).
The peritoneal puncture fluid present in large quantities in the exudative form of the disease was clear or slightly yellowish, very thick, and rich in proteins; the total protein value was 5.5 ± 1.5 g/dL. In the smear obtained from the peritoneal centrifuge and stained MGG, red blood cells and numerous leukocytes (large phagocytes, neutrophils and lymphocytes) were observed, characteristic of inflammatory fluid (Figure 2).
In the haematological examination, lower average values were obtained compared to the reference values of the haemoglobin (HGB), haematocrit (PCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC). The average value of red blood cells (RBC) was within the limits of the average reference values but close to the lower limit, and the mean corpuscular volume (MCV) was within the physiological limits of the species. These results demonstrate the occurrence of normocytic, hypochromic and hypoplastic anaemia (Table 1).
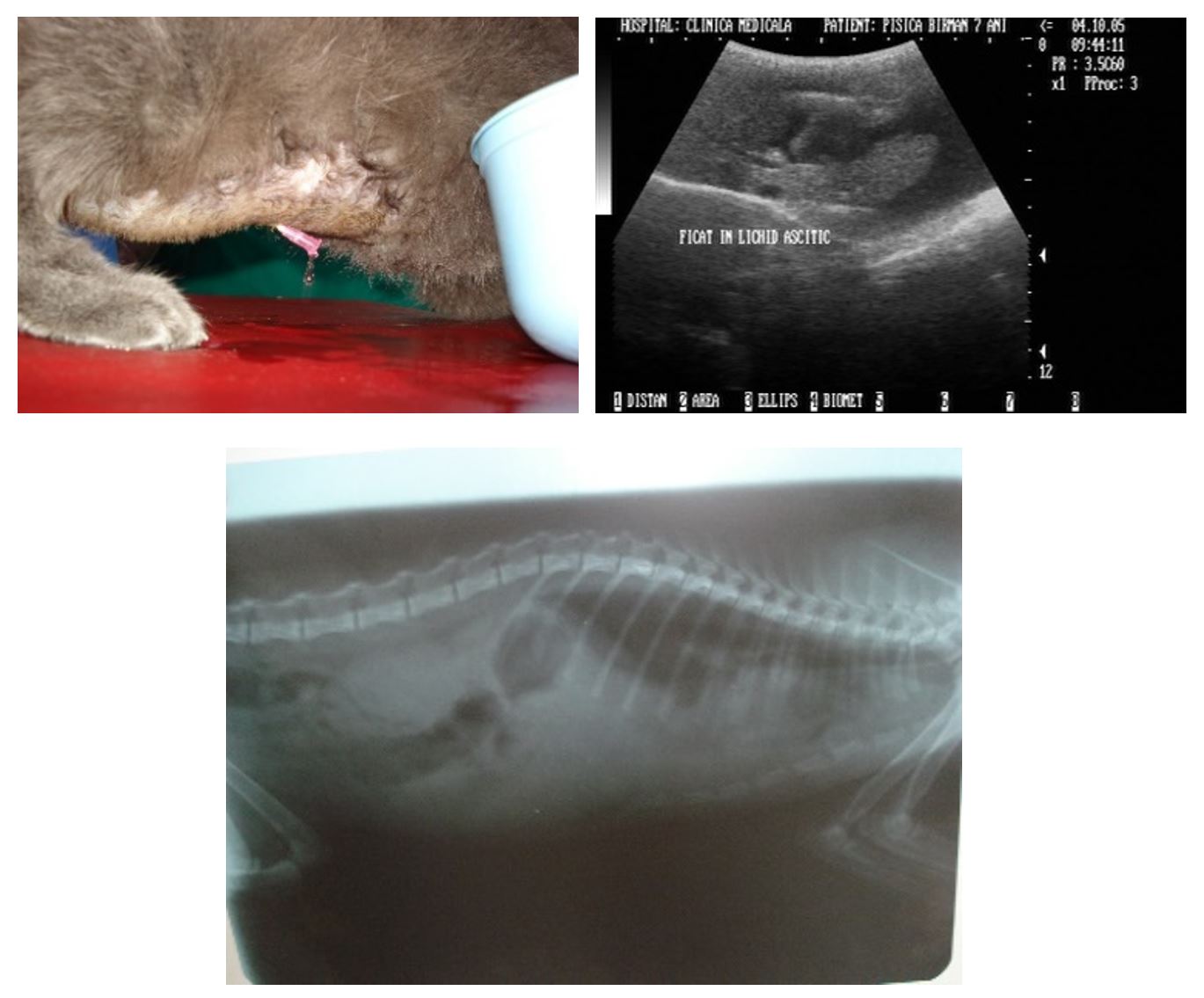
Figure 1 – 2-year-old cat with peritoneal effusion. Liver surrounded by a hypoechoic halo of peritoneal fluid. Diffuse peritoneal radiopacity
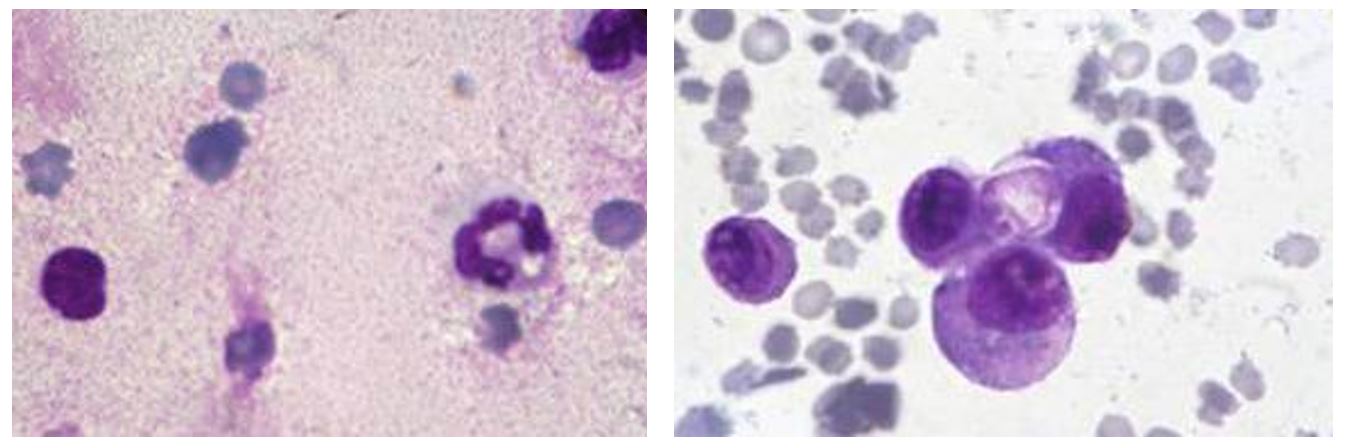
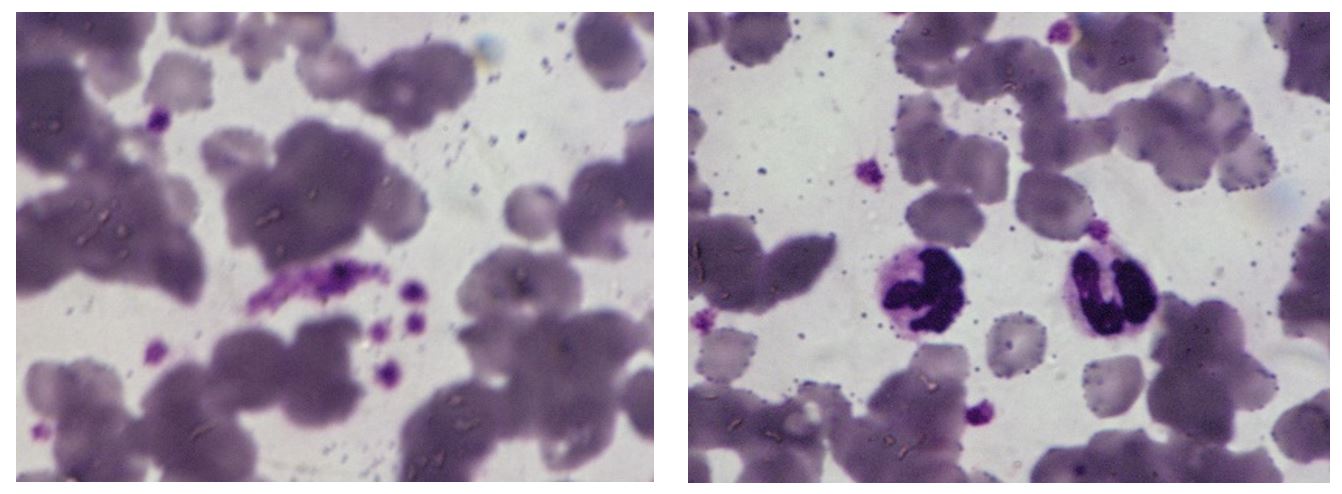
Figure 2 – Peritoneal fluid (MGGx1500) – Red blood cells and numerous leukocytes (neutrophils and lymphocytes). Large phagocytes and erythrophagocytosis
Table 1
Results of the haematological tests for 26 cats with exudative FIP
|
Blood parameter |
ESR |
RBC |
PCV |
HGB |
MCV |
MCH |
MCHC |
|
Unit of measuring |
mm/h |
x106/µL |
% |
g/dL |
µ³ |
pg |
g/dL |
|
Reference values (Aiello and Mays, 1998) |
3.0 |
5.0–10.0 |
30.0–45.0 |
8.0–15.0 |
39.0–55.0 |
13.0–17.0 |
30–36 |
|
Determined values |
9.0 |
6.1 ± 0.9 |
28.3 ± 2.1 |
7.5 ± 1.6 |
46.4 ± 0.4 |
12.3 ± 0.3 |
26.5 ± 0.4 |
Notes: ESR-erythrocyte sedimentation rate; RBC-red blood cells; PCV-haematocrit; HGB-haemoglobin; MCV-mean corpuscular volume; MCH-mean corpuscular haemoglobin; MCHC-mean corpuscular haemoglobin concentration
Table 2
WBC formula in 26 cats with exudative FIP
|
Blood parameter |
PLT |
WBC |
WBC distribution |
||||
|
N |
E |
B |
M |
L |
|||
|
Unit of measuring |
x103/µL |
x103/µL |
% |
% |
% |
% |
% |
|
Reference values (Aiello and Mays, 1998) |
300.0–700.0 |
5.5–19.5 |
35.0–75.0 |
2.0–12.0 |
Rare |
1.0–4.0 |
20.0–55.0 |
|
Determined values |
640.0 ± 2.4 |
11.7 ± 1.3 |
74.0 ± 0.8 |
7.0 ± 0.6 |
2.0 ± 0.1 |
1.0 ± 0.1 |
16.0 ± 0.2 |
Notes: PLT-platelets; WBC-white blood cells; N-neutrophils; E-eosinophils; B-basophils; M-monocytes; L-lymphocytes
The mean values of PLT and WBC were within the reference range. However, regarding the leukocyte formula, a tendency to neutrophilia, lymphopenia and an increase in the number of basophils was observed. This implies the existence of an active inflammatory process at the systemic level (Table 2).
Non-regenerative anaemia and lymphopenia are frequently associated with FIP (Thayer et al., 2022). Instead, microcytosis, thrombocytopenia and neutrophilia were not evident in all cases examined. However, many neutrophils were observed in the peritoneal fluid and aggregated platelets in the blood smear. The active inflammatory process at the systemic level was also confirmed by the increased ESR values (9.0 mm/h) and the presence in the panoptical stained smear of aggregated platelets as well as segmented and vacuolated neutrophils. Reticulocytes were not seen, which confirms hypoplastic anaemia (Figure 3).
The cats with exudative infectious peritonitis had higher mean values of AST, ALT and ALP. At the same time, TP and CRTN had values that fell within the limits of the average reference values. Instead, BUN and TBIL had average values close to the upper limit. These results demonstrate the existence of hepatocytolysis (increased serum transaminases), whereas the renal profile shows the integrity of the renal function (Table 3).
The mean values of Ca, P, Mg, GLU and CHOL were within the reference ranges, and TGL had mean values above the upper limit. Also, the average TBIL values were close to the upper level. This shows the evolution of a process of haemolytic anaemia, which added increased tissue catabolism, demonstrated by the high values of plasma triglycerides (Table 4).
These results show that blood biochemical tests, unlike CBC, have a more prognostic value regarding the disease and are less suitable for suspecting FIP. Perivascular lesions in FIP lead to increased vascular permeability, and therefore, peritoneal effusion is one of the main lesions of the disease. On the other hand, FIP can manifest clinically only with lesions associated with a single organ system, in which pyogranulomatous inflammation develops (Thayer et al., 2022). In this situation, the suspicion and diagnosis of the disease are much more difficult. In the dry form of the disease, peritoneal fluid collection and dyspnoea were absent, and the predominant clinical aspects differed depending on the main location of the chronic pyogranulomatous inflammation and associated lesions (Felten and Hartmann, 2019).
Thus, in 3 out of 32 patients diagnosed with FIP (9.37%), the predominant symptomatology was hepato-digestive with hepatocellular jaundice, inhibited behaviour, loss of appetite and soft faeces (Figure 4).
Necropsy examination revealed inflammatory lesions in the abdominal organs compatible with FIP, pyogranulomatous peritonitis and mesenteric pyogranulomatous lymphadenitis (Sigurdardottir et al., 2001).
In addition, where were slight amounts of inflammatory infiltrate in the intestine, kidney, spleen and Glisson’s capsule and abundant infiltrate in the neutrophils and large phagocytes (Figure 2). The liver was enlarged and friable, with rounded edges, a mottled appearance (passive congestion), a dull yellow colour, a dilated cholecyst and thickened walls.
The immunohistochemical reaction revealed the coronavirus antigen in the affected tissues. In addition, one patient presented predominant clinical signs of renal failure (oliguria and slope oedema, small kidneys with hyperechogenicity, disappearance of the characteristic echostructure and a small hypoechoic halo of peritoneal fluid), one patient presented predominant clinical signs of neurological type (signs of a cortical syndrome), and one patient showed periosteal lesions (granulomatous osteitis) (Figure 5).
Table 3
Results of the blood biochemical tests for 26 cats with exudative FIP
|
Blood parameter |
TP |
AST |
ALT |
ALP |
TBIL |
BUN |
CRTN |
|
Unit of measuring |
g/dL |
IU/L |
IU/L |
IU/L |
mg/dL |
mg/dL |
mg/dL |
|
Reference values (Aiello Susan and Mays, 1998) |
5.7–8.0 |
9.2–39.5 |
8.3–52.5 |
12.0–65.1 |
0.1–0.5 |
15.4–31.2 |
0.5–1.9 |
|
Determined values |
6.8 ± 0.3 |
294.2 ± 4.2 |
72.2 ± 3.1 |
96.6 ± 3.0 |
0.5 ± 0.2 |
28.5 ± 2.6 |
1.6 ± 0.2 |
Notes: TP-total protein; AST-aspartate-aminotransferase; ALT-alanine-aminotransferase; ALP-alkaline phosphatase; TBIL-total bilirubin; BUN-blood urea nitrogen; CRTN-creatinine
Table 4
Ion and energy profile results for 26 cats with exudative FIP
|
Blood parameter |
Ca |
P |
Mg |
GLU |
CHOL |
TGL |
|
Unit of measuring |
mg/dL |
mg/dL |
mg/dL |
mg/dL |
mg/dL |
mg/dL |
|
Reference values (Aiello and Mays, 1998) |
7.9–10.9 |
4.0–7.3 |
1.9–2.8 |
60.8–124.2 |
71.3–161.2 |
50–110 |
|
Determined values |
10.6 ± 0.5 |
5.1 ± 0.4 |
2.5 ± 0.1 |
67.6 ± 1.2 |
146.5 ± 2.8 |
243.2 ± 1.9 |
Notes: Ca-calcium; P-phosphorus; Mg-magnesium; GLU-glucose; CHOL- cholesterol; TGL-triglycerides
During the radiological examination of the granulomatous osteitis, a “sunburst” type periosteal reaction was observed, a bone outline with an irregular appearance at the level of the middle portion of the radial diaphysis. The adjacent muscle tissue showed an increased radiopacity compatible with an inflammatory process. The same lesion was observed in the left frontal bone.
In the tissue collected from this lesion by aspiration puncture under sedation, the coronavirus antigen was highlighted by the immunohistochemical reaction. Incidentally, increased radiopacity was observed at the submandibular level following the inflammatory reaction of the submandibular lymph nodes. Generally, FIP lesions are the consequence of an intense inflammatory reaction in the tissues in response to viral aggression. Type III and IV hypersensitivity reactions play a major role in this case (Kipar and Meli, 2014, Paltrinieri et al., 2002).
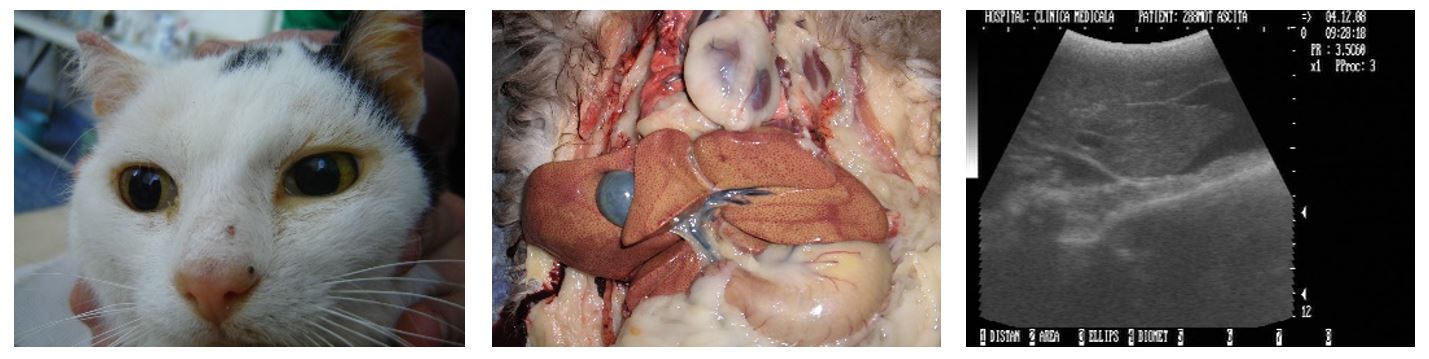
Figure 4 – 2-year-old cat with jaundice. Yellow liver, passive congestion and enlarged gallbladder. Liver with miliary areas of hyperechogenicity and a small amount of peritoneal fluid
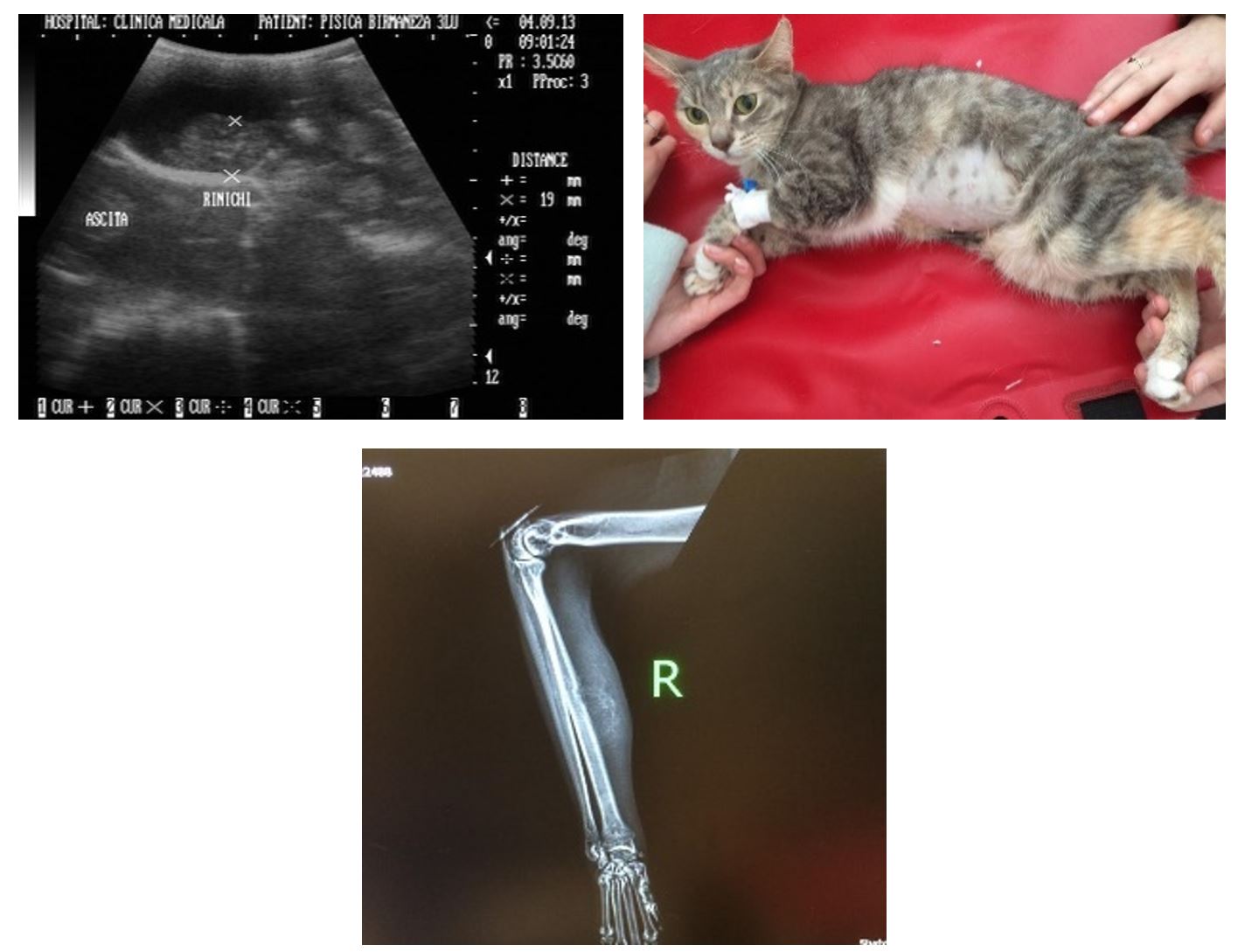
Figure 5 – 3-year-old cat. Small kidney and a fine hypoechoic halo of peritoneal fluid (renal failure). Granulomatous osteitis
In type III hypersensitivity, immune complexes form and deposit around blood vessels, producing vasculitis, disseminated intravascular coagulation and tissue inflammation. Given that osteitis granulomatous is not part of the usual FIP lesions, a local trauma may have favoured this FIP-associated lesion. Thus, granulomatous osteitis, although rarely encountered, could be associated with FIP-induced lesions.
These morphoclinical and paraclinical features of FIP show that the diagnosis of this disease is complex and cannot be specified only by clinical examination (Thayer et al., 2022). On the other hand, the varied clinical features of this disease recommend it to be suspected in all clinical situations where the usual diagnosis and treatment do not yield results.
CONCLUSIONS
Although clinical parameters may assist in the diagnosis of FIP, and the combinations of clinical findings may increase the index of suspicion for FIP, the diagnosis of the disease is clinically difficult. Added to this is the systemic inflammatory process presented by CBC and liver function disorders, which are also common to many diseases.
The ultrasonographic examination and peritoneal puncture were highly useful for the diagnosis of exudative FIP and revealed inflammatory peritoneal fluid collection with numerous large phagocytes, neutrophils and lymphocytes visible in the MGG-stained smear.
Although FIP is usually a systemic disease, sometimes, the predominant clinical signs and lesions may be associated with a single organ system, such as hepatocellular jaundice, cortical syndrome or renal failure. In addition, granulomatous osteitis, although rare, should be included on the list of FIP-induced lesions.
Author Contributions: The author declares that he has read and approved the publication of the manuscript in this form.
Funding: There was no external funding for this study.
Conflicts of Interest: The author declares no conflict of interest.
REFERENCES
Addie, D.D.; Covell-Ritchie, J.; Jarrett, O.; Fosbery, M. Rapid resolution of non-effusive feline infectious peritonitis uveitis with an oral adenosine nucleoside analogue and feline interferon omega. Viruses. 2020, 12. https://doi.org/10.3390/v12111216.
Aiello E.S.; Mays, A.S. The Merck Veterinary Manual, 8th edition, Merck & Co., in cooperation with Merial Ltd., Whitehouse Station, N.J., 1998, 2190-2194.
Alberer, M.; von Both, U. Cats and kids: How a feline disease may help us unravel COVID-19 associated paediatric hyperinflammatory syndrome. Infection. 2021, 49, 191-193. https://doi.org/10.1007/s15010-020-01515-3.
Benetka, V.; Kübber-Heiss, A.; Kolodziejek, J.; Nowotny, N.; Hofmann-Parisot, M.; Möstl, K. Prevalence of feline coronavirus types I and II in cats with histopathologically verified feline infectious peritonitis. Veterinary Microbiology. 2004, 99, 31-42. https://doi.org/10.1016/j.vetmic.2003.07.010.
Cannon, M.J.; Silkstone, M.A.; Kipar, A.M. Cutaneous lesions associated with coronavirus-induced vasculitis in a cat with feline infectious peritonitis and concurrent feline immunodeficiency virus infections. Journal of Feline Medicine Surgery. 2005, 7, 233-236. https://doi.org/10.1016/j.jfms.2004.12.001.
Drechsler, Y.; Alcaraz, A.; Bossong, F.J.; Collisson, E.W.; Diniz, P.P. Feline coronavirus in multicat environments. Veterinary Clinics of North America: Small Animal Practice. 2011, 41, 1133-1169. https://doi.org/10.1016/j.cvsm.2011.08.004.
Felten, S.; Hartmann, K. Diagnosis of feline infectious peritonitis: A review of the current literature. Viruses. 2019, 11, 1068. https://doi.org/10.3390/v11111068.
Gao, Y.Y.; Liang, X.Y.; Wang, Q.; Zhang, S.; Zhao, H.; Wang, K.; Hu, G.X.; Liu, W.J.; Gao, F.S. Mind the feline coronavirus: comparison with SARS-CoV-2. Gene. 2022, 825, 146443. https://doi.org/10.1016/j.gene.2022.146443.
Gao, Y.Y.; Wang, Q.; Liang, X.Y.; Zhang, S.; Bao, D.; Zhao, H.; Li, S.B.; Wang, K.; Hu, G.X.; Gao, F.S. An updated review of feline coronavirus: mind the two biotypes. Virus Research. 2023, 326, 199059. https://doi.org/10.1016/j.virusres.2023.199059.
Hung, L.; Hopper, B.J.; Lenard, Z. Retrospective analysis of radiographic signs in feline pleural effusions to predict disease aetiology. BMC Veterinary Research. 2022, 18, 118. https://doi.org/10.1186/s12917-022-03218-3.
Kipar, A.; Meli, M.L. Feline infectious peritonitis: still an enigma? Veterinary Pathology. 2014, 51, 505-526. https://doi.org/10.1177/0300985814522077.
Kitagawa, M.; Okada, M.; Sato, T.; Kanayama, K.; Sakai, T. A feline case of isolated fourth ventricle with syringomyelia suspected to be related with feline infectious peritonitis. The Journal of Veterinary Medical Science. 2007, 69, 759-762. https://doi.org/10.1292/jvms.69.759.
Lewis, M.K.; O’Brien, R.T. Abdominal ultrasonographic findings associated with feline infectious peritonitis: a retrospective review of 16 cases. Journal of the American Animal Hospital Association. 2010, 46, 152-160. https://doi.org/10.5326/0460152.
Paltrinieri, S.; Comazzi, S.; Spagnolo, V.; Giordano, A. Laboratory changes consistent with feline infectious peritonitis in cats from multicat environments. Journal of Veterinary Medicine Series A. 2002, 49, 503-510. https://doi.org/10.1046/j.1439-0442.2002.00494.x.
Pedersen, N.C. An update on feline infectious peritonitis: diagnostics and therapeutics. The Veterinary Journal. 2014, 201, 133-141. https://doi.org/10.1016/j.tvjl.2014.04.016.
Richardson, J.; Moraillon, A.; Baud, S.; Cuisinier, A.M.; Sonigo, P.; Pancino, G. Enhancement of feline immunodeficiency virus (FIV) infection after DNA vaccination with the FIV envelope. Journal of Virology. 1997, 71, 9640-9649. https://doi.org/10.1128/jvi.71.12.9640-9649.1997.
Riemer, F.; Kuehner, K.A.; Ritz, S.; Sauter-Louis, C.; Hartmann, K. Clinical and laboratory features of cats with feline infectious peritonitis-A retrospective study of 231 confirmed cases (2000–2010). Journal of Feline Medicine and Surgery. 2016, 18, 348-356. https://doi.org/10.1177/1098612X15586209.
Rissi, D.R. A retrospective study of the neuropathology and diagnosis of naturally occurring feline infectious peritonitis. Journal of Veterinary Diagnostic Investigation. 2018, 30, 392-399. https://doi.org/10.1177/1040638718755833.
Sigurdardottir, O.G.; Kolbjornsen, O.; Lutz, H. Orchitis in a cat associated with coronavirus infection. Journal of Comparative Pathology. 2001, 124, 219-222. https://doi.org/10.1053/jcpa.2000.0443.
Stranieri, A.; Giordano, A.; Paltrinieri, S.; Giudice, C.; Cannito, V.; Lauzi, S. Comparison of the performance of laboratory tests in the diagnosis of feline infectious peritonitis. Journal of Veterinary Diagnostic Investigation. 2018, 30, 459-463. https://doi.org/10.1177/1040638718756460.
Tekes, G.; Thiel, H.J. Chapter Six – Feline Coronaviruses: Pathogenesis of Feline Infectious Peritonitis. Advances in Virus Research. 2016, 96, 193-218. https://doi.org/10.1016/bs.aivir.2016.08.002.
Thayer, V.; Gogolski, S.; Felten, S.; Hartmann, K.; Kennedy, M.; Olah, G.A. AAFP/EveryCat feline infectious peritonitis diagnosis guidelines. Journal of Feline Medicine and Surgery. 2022, 24, 905-933. https://doi.org/10.1177/1098612X221118761.
Stout, A.E.; Andre, N.M.; Jaimes, J.A.; Millet, J.K.; Whittaker, G.R. Coronaviruses in cats and other companion animals: where does SARS-CoV-2/COVID-19 fit? Veterinary Microbiology. 2020, 247, 108777. https://doi.org/10.1016/j.vetmic.2020.108777.
Worthing, K.A.; Wigney, D.I.; Dhand, N.K.; Fawcett, A.; McDonagh, P.; Malik, R.; Norris, J.M. Risk factors for feline infectious peritonitis in Australian cats. Journal of Feline Medicine and Surgery. 2012, 14, 405-412. https://doi.org/10.1177/1098612X12441875.
Yin, Y.; Li, T.; Wang, C.; Liu, X.; Ouyang, H.; Ji, W.; Liu, J.; Liao, X.; Li, J.; Hu, C. A retrospective study of clinical and laboratory features and treatment on cats highly suspected of feline infectious peritonitis in Wuhan, China. Scientific Reports. 2021, 11, 5208. https://doi.org/10.1038/s41598-021-84754-0.
Zhou, Q.; Li, Y.; Huang, J.; Fu, N.; Song, X.; Sha, X.; Zhang, B. Prevalence and molecular characteristics of feline coronavirus in southwest China from 2017 to 2020. Journal of General Virology. 2021, 102. https://doi.org/10.1099/jgv.0.001654.
Academic Editor: Prof. Dr. Daniel Simeanu
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Boghian Vasile