Folusho Anuoluwapo Bankole, Olawale Serifdeen Aboderin
ABSTRACT. Yield trials demand significant time and resources, necessitating efficient data collection on parental lines to optimise breeding programs and reduce costs. This study assessed the correlation between parental traits and hybrid performance, consistency, and predictability of trait expression in F1 hybrids and the heterotic advantage of agronomic traits. A total of 82 parental lines (79 lines and 3 testers) and 237 testcrosses were evaluated alongside 3 standard checks under low- and optimum-soil nitrogen (N) conditions at the Institute for Agricultural Research experimental fields in Zaria and Mokwa during the 2019/20 and 2020/21 growing seasons. Significant genetic variability was observed among parental lines and testcrosses, offering strategic breeding opportunities. Grain yield reductions under low-N conditions (35–95% in inbreds and 1.3–89% in hybrids) highlighted the impact of N stress and the need for N tolerance in maize genotypes. Correlation analysis and repeatability results linked yield improvement in low-N tolerant maize hybrids to the selection of parental lines with superior performance in traits, such as grain yield, stay-green characteristics, and flowering traits. Parental lines P69 and P14, which showed high tolerance to low N and consistent high yields, were identified as valuable genetic resources. Among the hybrids, P65×T2, P66×T3, and P66×T2 stood out, with grain yields exceeding 6000 kg/ha, representing a 42% yield advantage over the best check. These hybrids also demonstrated a high heterotic advantage over their parents and standard checks, indicating their potential for adoption as commercial hybrids in Nigeria.
Keywords: biplot; heterosis; line × tester analysis; maize testcrosses; nitrogen stress; trait correlation.
Cite
ALSE and ACS Style
Bankole, F.A.; Aboderin, O.S. Genetic assessment of yield traits and heterosis in maize testcrosses under different soil nitrogen conditions. Journal of Applied Life Sciences and Environment 2024, 57 (3), 475-491.
https://doi.org/10.46909/alse-573148
AMA Style
Bankole FA, Aboderin OS. Genetic assessment of yield traits and heterosis in maize testcrosses under different soil nitrogen conditions. Journal of Applied Life Sciences and Environment. 2024; 57 (3): 475-491.
https://doi.org/10.46909/alse-573148
Chicago/Turabian Style
Bankole, Folusho Anuoluwapo, and Olawale Serifdeen Aboderin. 2024. “Genetic assessment of yield traits and heterosis in maize testcrosses under different soil nitrogen conditions” Journal of Applied Life Sciences and Environment 57, no. 3: 475-491.
https://doi.org/10.46909/alse-573148
View full article (HTML)
Genetic Assessment of Yield Traits and Heterosis in Maize Testcrosses under Different Soil Nitrogen Conditions
Folusho Anuoluwapo BANKOLE1 and Olawale Serifdeen ABODERIN1,2*
1Department of Agronomy, University of Ilorin, Ilorin, Nigeria; email: bankole.fa@unilorin.edu.ng
2Institute for Agricultural Research, Ahmadu Bello University, Zaria PMB 1044, Nigeria
*Correspondence: olawaleaboderin@yahoo.com
Received: May 23, 2024. Revised: Sep. 19, 2024. Accepted: Sep. 20, 2024. Published online: Nov. 18, 2024
ABSTRACT. Yield trials demand significant time and resources, necessitating efficient data collection on parental lines to optimise breeding programs and reduce costs. This study assessed the correlation between parental traits and hybrid performance, consistency, and predictability of trait expression in F1 hybrids and the heterotic advantage of agronomic traits. A total of 82 parental lines (79 lines and 3 testers) and 237 testcrosses were evaluated alongside 3 standard checks under low- and optimum-soil nitrogen (N) conditions at the Institute for Agricultural Research experimental fields in Zaria and Mokwa during the 2019/20 and 2020/21 growing seasons. Significant genetic variability was observed among parental lines and testcrosses, offering strategic breeding opportunities. Grain yield reductions under low-N conditions (35–95% in inbreds and 1.3–89% in hybrids) highlighted the impact of N stress and the need for N tolerance in maize genotypes. Correlation analysis and repeatability results linked yield improvement in low-N tolerant maize hybrids to the selection of parental lines with superior performance in traits, such as grain yield, stay-green characteristics, and flowering traits. Parental lines P69 and P14, which showed high tolerance to low N and consistent high yields, were identified as valuable genetic resources. Among the hybrids, P65×T2, P66×T3, and P66×T2 stood out, with grain yields exceeding 6000 kg/ha, representing a 42% yield advantage over the best check. These hybrids also demonstrated a high heterotic advantage over their parents and standard checks, indicating their potential for adoption as commercial hybrids in Nigeria.
Keywords: biplot; heterosis; line × tester analysis; maize testcrosses; nitrogen stress; trait correlation.
INTRODUCTION
Maize (Zea mays L.) is one of the most vital cereal crops globally, serving as a staple food and contributing significantly to livestock feed and industrial applications (Bankole et al., 2023). In Nigeria, maize plays a pivotal role in food security and economic sustenance, particularly in Guinea Savanna, a region marked by unique climate and ecological conditions (Kamara et al., 2020). With the demand for maize rising due to population growth, shifting dietary preferences, and increasing industrial needs, improving maize production in this region is imperative.
Heterosis, often termed hybrid vigour, manifests when the offspring of genetically distinct parents exhibit superior performance compared to either (or both) of their parents or a standard check. Estimating heterosis is important for plant breeders, providing a quantifiable measure of genetic gain achieved through hybridisation (Akinwale, 2021; Olayiwola et al., 2021). The determination of heterosis in reference to a standard check (standard heterosis, SH) is essential for assessing the practical benefits of hybrid maize (Sharief et al., 2009; Mogesse et al., 2020). SH serves as a reliable benchmark for evaluating new hybrids, ensuring that only the most economically superior varieties reach the market (Abiy et al., 2019).
Repeatability, a statistical measure indicating the consistency or predictability of a trait’s expression across different environments or years, is vital for predicting selection success (Falconer and Mackay, 1996; Dohm, 2002; Nakagawa and Schielzeth, 2010). It refers to the proportion of total phenotypic variation that is due to genetic factors as opposed to external factors. Traits with high repeatability tend to exhibit stable performance over time, instilling confidence in plant breeders that observed differences among individual plants or lines are primarily due to genetic factors rather than environmental variations or measurement error (Sanchéz et al., 2017; Ferreira et al., 2020). This stability is crucial for maintaining the quality and performance of new varieties in the long term, ensuring their sustained success in agricultural markets.
Moreover, considering the significant costs associated with hybrid yield trials, obtaining information about parental lines that can reliably predict hybrid performance is paramount. One effective approach to obtaining such information is through correlation analysis using statistical functions, such as Pearson, Spearman, and Kendall correlation coefficients, or graphical representations, such as genotype × trait biplots. Correlation coefficients offer numerical measures of the strength and direction of relationships between traits, enabling breeders to quantitatively assess the degree of association between parental traits and hybrid performance. Conversely, genotype × trait biplots visually illustrate patterns and associations between traits and genotypes, aiding breeders in identifying parental lines with desirable trait combinations (Yan and Tinker, 2006). By combining these methods, breeders can effectively identify and prioritise parental lines with superior trait performance, facilitating the development of high-performing hybrids with desired agronomic characteristics.
This study was therefore carried out to: i) evaluate the agronomic performance of the testcrosses; ii) assess the consistency and predictability of trait expression in F1 hybrids across different soil nitrogen (N) conditions; iii) assess the correlation between traits in parental lines and their hybrids; and iv) estimate potential gains in hybrid performance over parents and standard checks.
MATERIALS AND METHODS
Experimental materials
The experimental materials consisted of 82 inbred lines, 237 testcrosses, and 3 standard checks (‘Oba Super 2’, ‘SAMMAZ 50’, and ‘SC619’). The 237 testcrosses were generated from the 82 inbred lines (79 lines and 3 testers) using a line × tester mating design (Kempthrone, 1957) in the 2019 cropping season at the Institute for Agricultural Research, Zaria, Nigeria (Table 1).The inbred lines, which were in their sixth generation of selfing, included both low-N-tolerant and non-tolerant lines. Detailed information regarding the inbred lines, testers, and the development of the testcrosses was provided in a previous report (Aboderin et al., 2024). Checks SAMMAZ50 and Oba Super 2 are high-yielding commercial hybrids released in Nigeria. SAMMAZ50 is adapted to the Southern and Northern Guinea Savanna agroecological zones, while Oba Super 2 is adapted to Forest and Savanna agroecological zones.
Field evaluation
The inbred lines were evaluated in two low-N and two optimum-N environments. The hybrid evaluation trial was conducted in four optimum- and four low-N environments at the Institute for Agricultural Research (IAR) experimental fields in Zaria and Mokwa during the 2020 and 2021 growing season. In this study, an environment denotes the combination of year, location, and soil N level. The IAR experimental field, established through the depletion of available soil N due to continuous maize planting without N fertiliser application for several years, served as the low-N field in the study. These fields were exclusively reserved for evaluating genotypes for tolerance to low soil N. Soil analysis results revealed that the available soil N at the Mokwa and Zaria low-N fields were 0.85 and 1.1 g/kg, respectively, which is below the critical level of N fertiliser requirement for optimum maize growth.
Experimental design
The inbred trial (82 parental lines and 2 inbred checks) was laid out in a 7 × 12 alpha lattice design, while the hybrid trial (237 testcrosses and 3 hybrid checks) was laid out using a 15 × 16 alpha lattice design with 2 replications. The parental lines were planted adjacent to the hybrid trials in the same field in Zaria. In all environments, single rows of plots, each 4 m long, with inter-row and intra-row spacings of 0.75 m and 0.4 m, respectively, were used. To achieve a population of 66,667 plants per hectare, 2 seeds were sown per hole.
Nitrogen treatments
N fertiliser (urea) was evenly applied in 2 split doses at 2 and 5 weeks after sowing (WAS) to achieve an available N level of 30 kg N ha−1 in the low-N fields.
Table 1
List of inbred lines used for the study
|
Code |
Inbred |
Source |
Code |
Inbred |
Source |
Code |
Inbred |
Source |
|
P1 |
SMLW3 |
IAR |
P30 |
SMLW53 |
IAR |
P59 |
SMLW134 |
IAR |
|
P2 |
SMLW4 |
IAR |
P31 |
SMLW57 |
IAR |
P60 |
SMLW135 |
IAR |
|
P3 |
SMLW5 |
IAR |
P32 |
SMLW58 |
IAR |
P61 |
SMLW140 |
IAR |
|
P4 |
SMLW6 |
IAR |
P33 |
SMLW64 |
IAR |
P62 |
SMLW143 |
IAR |
|
P5 |
SMLW7 |
IAR |
P34 |
SMLW69 |
IAR |
P63 |
SMLW144 |
IAR |
|
P6 |
SMLW9 |
IAR |
P35 |
SMLW70 |
IAR |
P64 |
SMLW145 |
IAR |
|
P7 |
SMLW10 |
IAR |
P36 |
SMLW74 |
IAR |
P65 |
SMLW146 |
IAR |
|
P8 |
SMLW11 |
IAR |
P37 |
SMLW75 |
IAR |
P66 |
SMLW147 |
IAR |
|
P9 |
SMLW14 |
IAR |
P38 |
SMLW77 |
IAR |
P67 |
SMLW150 |
IAR |
|
P10 |
SMLW16 |
IAR |
P39 |
SMLW78 |
IAR |
P68 |
SMLW155 |
IAR |
|
P11 |
SMLW17 |
IAR |
P40 |
SMLW84 |
IAR |
P69 |
SMLW156 |
IAR |
|
P12 |
SMLW19 |
IAR |
P41 |
SMLW86 |
IAR |
P70 |
SMLW157 |
IAR |
|
P13 |
SMLW20 |
IAR |
P42 |
SMLW91 |
IAR |
P71 |
SMLW158 |
IAR |
|
P14 |
SMLW21 |
IAR |
P43 |
SMLW93 |
IAR |
P72 |
SMLW160 |
IAR |
|
P15 |
SMLW22 |
IAR |
P44 |
SMLW96 |
IAR |
P73 |
SMLW162 |
IAR |
|
P16 |
SMLW23 |
IAR |
P45 |
SMLW99 |
IAR |
P74 |
SMLW163 |
IAR |
|
P17 |
SMLW24 |
IAR |
P46 |
SMLW100 |
IAR |
P75 |
SMLW165 |
IAR |
|
P18 |
SMLW25 |
IAR |
P47 |
SMLW101 |
IAR |
P76 |
SMLW167 |
IAR |
|
P19 |
SMLW26 |
IAR |
P48 |
SMLW102 |
IAR |
P77 |
SMLW169 |
IAR |
|
P20 |
SMLW27 |
IAR |
P49 |
SMLW104 |
IAR |
P78 |
SMLW183 |
IAR |
|
P21 |
SMLW33 |
IAR |
P50 |
SMLW105 |
IAR |
P79 |
SMLW159 |
IAR |
|
P22 |
SMLW34 |
IAR |
P51 |
SMLW106 |
IAR |
CODE |
TESTERS |
SOURCE |
|
P23 |
SMLW37 |
IAR |
P52 |
SMLW107 |
IAR |
T1 |
IITA 1878 |
IITA |
|
P24 |
SMLW43 |
IAR |
P53 |
SMLW108 |
IAR |
T2 |
IITA 1876 |
IITA |
|
P25 |
SMLW44 |
IAR |
P54 |
SMLW119 |
IAR |
T3 |
SAM 50M |
IAR |
|
P26 |
SMLW48 |
IAR |
P55 |
SMLW120 |
IAR |
CODE |
CHECKS |
SOURCE |
|
P27 |
SMLW50 |
IAR |
P56 |
SMLW121 |
IAR |
C1 |
SAMMAZ 50 |
IAR |
|
P28 |
SMLW51 |
IAR |
P57 |
SMLW122 |
IAR |
C2 |
Oba Super 2 |
Premier Seed |
|
P29 |
SMLW52 |
IAR |
P58 |
SMLW127 |
IAR |
C3 |
SC 619 |
Seedco |
IAR: Institute for Agricultural Research; IITA: International Institute of Tropical Agriculture
The first dose of N fertiliser was applied together with muriate of potash (60 kg P ha−1) and single superphosphate (60 kg K ha−1) at 2 WAS. The second dose of N (urea) was applied 5 WAS. In the optimum field, N fertiliser was applied at a rate of 90 kg N ha−1 in 2 different doses. The first dose involved the application of NPK 15:15:15 at a rate of 60 kg N ha−1, 60 kg P ha−1, and 60 kg K ha−1 at 2 WAS, while the second dose was in the form of urea at 30 kg N ha−1 top-dressed at 4 WAS. Weeds were managed in the field through herbicide application (5 L ha−1 Primextra and Paraquat) during the early phases of maize growth. Subsequent manual weeding was performed when necessary to maintain a weed-free field throughout the growing season.
Data collection and analysis
Agronomic data were collected based on plot and sampled plant bases. Plot-based agronomic data included the assessment of flowering traits in terms of days, the count of ears per plant (EPP), and grain yield (GY). Growth traits were evaluated based on average measurements derived from five randomly selected plants within each plot. Aspect ratings were visually evaluated on a phenotypic scale ranging from 1 to 10, where 1 indicated excellent phenotypic appeal and 10 represented poor phenotypic appeal.
Stay green characteristics (STGR) data were only collected in the low-N field immediately after completing the flowering data and were rated on a scale of 1–10, where 1 = 90–100% of the plant leaves were still green and 10 = virtually all the leaves of the plant were yellow or dead. GY (kg ha−1) under low-N conditions was determined by weighing the shelled ear grain per plot in grams (g), which were subsequently converted to kilograms (kg) and adjusted to a standard moisture level of 15%.
Conversely, under optimum-N conditions, GY was calculated based on the field weight of cobs, measured in kilograms (kg), assuming an 80% shelling percentage, and subsequently adjusted to a 15% moisture content to ensure measurement consistency and comparability.
Combined analyses of variance (ANOVAs) were conducted across environments for GY and other agronomic traits in both trials. Mean comparisons for both hybrid and parent genotypes were performed using the least significant difference (LSD) test. The tolerance level to low soil N of each genotype was assessed using the low-N tolerance index (LNTI), as outlined by Oyekunle and Badu-Apraku (2013a).
Estimation of heterosis
Heterosis was calculated as a percentage for agronomic traits, showing statistically significant differences among genotypes. Better parent (BPH) and mid-parent heterosis (MPH) were estimated using AGD-R software (Rodríguez et al., 2020). Adjusted means of the hybrids and inbred lines from evaluation trials in Zaria only, where both hybrid and inbred lines were planted in adjacent plots, were used for these estimations. SH was computed following the method suggested by Falconer and Mackay (1996):

where F1 = mean of the hybrid.
Repeatability was estimated for each trait under low-N and optimum-N conditions and across both soil-N conditions using the following formula:

where is genotypic variance, is error variance, is G×E interaction variance, and e and r are the numbers of environments and replications within an environment, respectively (Fehr, 1991). Repeatability was classified as follows: high repeatability (r ≥ 0.60); moderate repeatability (0.30 < r < 0.60); and low repeatability (r ≤ 0.30) (Resende, 2002).
Correlation analysis was performed using two methods. In the first method, the Pearson correlation coefficient was calculated between the mid-parent values and their corresponding hybrid means for each trait using SAS software (version 9.2, 2008). Simultaneously, the mid-parent and hybrid values were subjected to genotype × trait biplot analysis to visualise the multivariate relationships between the parental lines and hybrid traits and to assess how different traits in the parental lines relate to the GY of the hybrids.
RESULTS
Analysis of variance
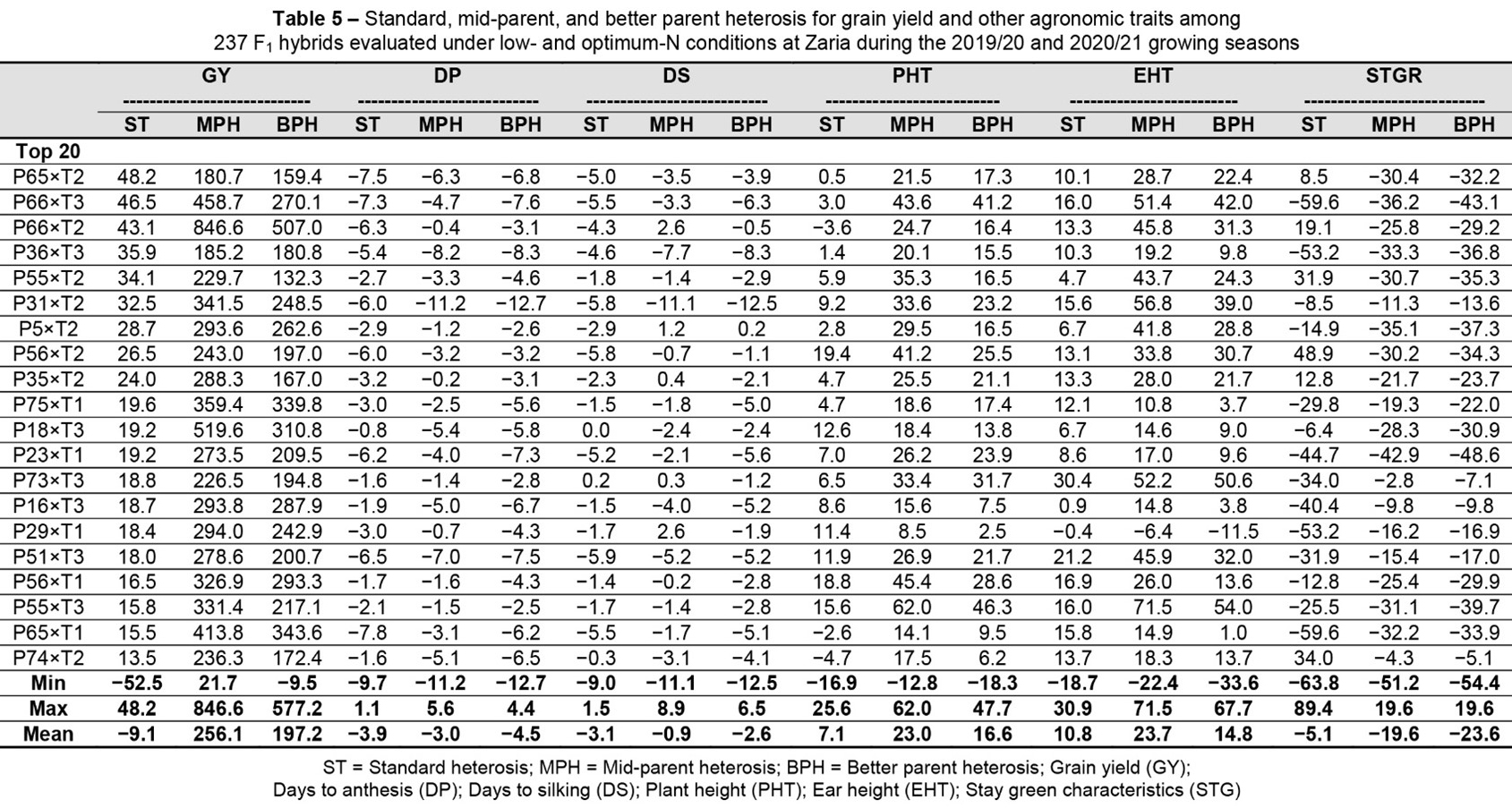
The combined ANOVA across low- and optimum-N environments for GY and other agronomic characters of the parental lines and testcrosses are presented in Table 2.
Environment mean squares were significant (ρ ≤ 0.01 or ρ ≤ 0.05) for all measured traits. Lines and hybrid mean squares were significant (ρ ≤ 0.01 or ρ ≤ 0.05) for all characters, except EPP for the testcrosses. The lines × environment interaction was highly significant (ρ ≤ 0.01) for all characters. Similarly, the hybrid × environment interaction was significant (ρ ≤ 0.05) for all characters, except EPP. Repeatability estimates varied from 0.05 (plant aspect, PA) to 0.92 (STGR) under low-N, 0.001 (ear aspect, EA) to 0.53 (GY) under optimum-N, and 0.001 (EA) to 0.92 (STGR) across the environments. Repeatability estimates for STGR, days to anthesis (DP), days to silking (DS), and anthesis–silking interval (ASI) were high under low-N conditions and across environments, while PA, EA, and EPP consistently displayed low repeatability across environments. GY exhibited moderate repeatability under low-N conditions and slightly higher repeatability under optimum-N conditions and across environments.
Agronomic mean performance of the parental lines and testcrosses
The mean performance and other agronomic traits of the parental line and their testcrosses across the test environments are presented in Table 3. The mean GY among the parental lines was 344 kg ha−1 under low-N conditions, 1803 kg ha−1 under optimum-N conditions, and 1074 kg ha−1 across the environments. The highest yielding inbred lines were P69, P3, and P14 under low-N, P69, P14, and P76 under optimum-N, and P69, P14, and P79 across the environments. The mean GY among the testcrosses was 2473 kg ha−1 under low-N conditions, 5263 kg ha−1 under optimum-N conditions, and 3868 kg ha−1 across the environments. Leading hybrids were P36×T3, P65×T2, and P66×T3 under low-N conditions, P66×T2, P18×T3, and P56×T2 under optimum-N conditions, and P65×T2, P66×T3, and P66×T2 across the environments. Under low-N conditions, parental line P3 and hybrid P36×T3 had the lowest yield reduction (34.87 and 1.26%, respectively), while P32 and P62×T1 had the highest (95.09 and 89.18%, respectively).
DP ranged from 61.3 (P69) to 69.3 days (P48) among the parental lines and from 56.9 (P59×T1) to 66 days (P13×T1) among the hybrids. Parental lines P69, P72, and P64 and hybrids P59×T1, P24×T3, and P22×T3 were the earliest to attain anthesis, with a mean value of 65 (parental lines) and 60.6 days (hybrids). DS ranged from 63.7 (P72) to 72.2 days (P48) for the parental lines and from 59.9 (P59×T1) to 66.8 days (P11×T1) among the hybrids.
ASI among the parental lines ranged from 2 to 4.9 days, with P45 having the shortest interval, followed by P79 and P54. The ASI for the hybrids ranged from 1.9 days in hybrid P13×T3 to 4.2 days in P65×T2, with a mean of 3 days. The maximum plant height (PHT) was recorded in parental line P69 (136 cm) and hybrid P18×T2 (164 cm). Ear height (EHT) ranged from 32 (P42) to 68 cm (P14) and from 45.2 cm (P3×T1) to 72.8 cm (P50×T1). In addition, EPP ranged from 0.7 (P29) to 1.2 (P52) and from 0.74 (P24×T2) to 1.2 (P31×T2). Parental line P52 and hybrid P35×T3 had the best PA ratings (3.4 and 2.7, respectively). The EA rating was highest in parental line P69 and hybrid P25×T1, with values of 2.4 and 2.6, respectively (Table 3).
Inbred line P69 had the best STGR rating (3.9), followed by P41 (4.0) and P3 (4.1), while P23 had the worst rating (7.4). Among the hybrids, STGR ranged from 1.7 for P7×T3 to 8.9 for P52×T2, with a mean of 4.46. The majority of the top-yielding hybrids also had better STGR ratings. Of the parental lines, 37 (including 2 inbred testers) had a positive LNTI. P34 had the lowest LNTI (−7.9), while P69 had the highest (19.6). Of the hybrids, 133 exhibited a positive LNTI, with P66×T3 achieving the highest value (13.5), followed by P36×T3 and P50×T1. Of the 133 hybrids, 19 having a positive LNTI were also ranked among the top 20 highest-yielding hybrids across the environments.
Relationship between the performance of parental inbreds and their hybrids
The correlation analysis between the traits of the lines and hybrids is presented in Table 4. Non-significant positive correlations were observed between the lines and hybrids for GY, PHT, EPP, PA, EA, and STGR. Conversely, negative correlations were noted for DP, DS, ASI, and EHT.
Figure 1 shows the genotype × trait biplot, illustrating the relationship between the traits of the lines and their hybrids. Positive correlations were observed between the parental lines and their hybrids for GY, STGR, DS, EA, and PA. However, negative correlations were noted between the parental lines and their hybrids for ASI, EPP, DP, and EHT. In the biplot (Figure 1), the hybrid GY vector formed an acute angle with each of the DP, DS, STGR, PA, and EA vectors of the lines. Additionally, the hybrid GY vector showed a near-perfect linear relationship with the vectors representing the PHT and EHT of the lines, while being inclined at an almost right angle to the vectors representing the EPP and ASI of the lines.
Heterosis Estimates
The estimates for MPH, BPH, and SH for GY and other agronomic traits among the 237 F1 hybrids are presented in Table 5. All 237 hybrids exhibited a positive MPH for GY, ranging from 21.74 (P61×T2) to 846.56% (P66×T2). Only one hybrid showed a negative BPH for GY, with BPH ranging from −9.47 (P61×T2) to 577.20% (P33×T1). The SH for GY varied from −52.46 (P61×T2) to 48.20% (P65×T2).
The MPH for DP ranged from −11.21% (P31×T2) to 5.57% (P3×T1). BPH ranged from −12.68 (P31×T2) to 4.43% (P64×T1), while SH varied from −9.7% (P59×T1) to 1.1%. For DS, hybrids P31×T2 and P32×T3 had the lowest MPH and BPH, while hybrid P59×T1 exhibited the lowest SH.
Table 3
Agronomic performance of maize inbred lines and their testcrosses (top 20) under low- and optimum-N conditions (Mokwa and Zaria) in 2020 and 2021
|
Genotype |
GY (kg/ha) |
DP |
DS |
ASI |
PHT |
EHT |
EPP |
PA |
EA |
STG |
LNT |
YRD (%) |
||
|
LN |
OPT |
ACC |
||||||||||||
|
P69 |
1280 |
4142 |
2711 |
61.3 |
64.2 |
3.0 |
136 |
62 |
1.1 |
3.6 |
2.4 |
3.9 |
19.5 |
69.1 |
|
P14 |
654 |
4094 |
2374 |
64.6 |
68.3 |
3.8 |
131 |
68 |
0.8 |
3.8 |
2.8 |
5.2 |
2.5 |
84.0 |
|
P79 |
626 |
3344 |
1985 |
63.6 |
65.6 |
2.1 |
115 |
56 |
0.9 |
3.7 |
3.2 |
6.3 |
7.6 |
81.3 |
|
P76 |
286 |
3547 |
1916 |
65.7 |
68.6 |
2.9 |
103 |
45 |
0.9 |
3.7 |
3.2 |
5.8 |
0.5 |
92.0 |
|
P19 |
384 |
2897 |
1641 |
62.7 |
65.9 |
3.2 |
99 |
45 |
1.1 |
3.7 |
3.5 |
6.0 |
1.1 |
86.8 |
|
P20 |
262 |
2883 |
1573 |
63.6 |
66.6 |
3.1 |
97 |
47 |
0.9 |
3.6 |
3.5 |
6.3 |
−3.1 |
90.9 |
|
P10 |
379 |
2737 |
1558 |
66.6 |
69.5 |
2.9 |
114 |
48 |
1.0 |
3.6 |
3.9 |
5.4 |
−0.5 |
86.2 |
|
P17 |
488 |
2589 |
1539 |
61.8 |
65.4 |
3.6 |
113 |
44 |
1.0 |
4.0 |
3.5 |
6.1 |
1.5 |
81.1 |
|
P64 |
337 |
2701 |
1519 |
61.6 |
63.8 |
2.3 |
111 |
40 |
1.0 |
3.5 |
3.5 |
5.6 |
2.1 |
87.5 |
|
P2 |
349 |
2624 |
1487 |
65.8 |
69.1 |
3.4 |
104 |
51 |
1.0 |
3.8 |
3.5 |
5.4 |
0.1 |
86.7 |
|
P23 |
207 |
2744 |
1476 |
66.7 |
69.2 |
2.5 |
111 |
50 |
0.9 |
4.0 |
3.8 |
7.4 |
−5.4 |
92.5 |
|
P45 |
568 |
2377 |
1472 |
67.0 |
69.0 |
2.0 |
89 |
40 |
1.0 |
4.2 |
3.7 |
4.9 |
7.6 |
76.1 |
|
P71 |
248 |
2586 |
1417 |
65.2 |
67.6 |
2.5 |
94 |
45 |
0.9 |
4.2 |
3.9 |
5.4 |
−1.9 |
90.4 |
|
P32 |
132 |
2692 |
1412 |
67.3 |
70.4 |
3.1 |
95 |
46 |
0.8 |
3.7 |
3.5 |
6.5 |
−5.5 |
95.1 |
|
P16 |
272 |
2548 |
1410 |
63.6 |
66.9 |
3.4 |
118 |
55 |
1.1 |
3.8 |
3.3 |
5.1 |
−0.9 |
89.3 |
|
P15 |
204 |
2605 |
1405 |
64.0 |
67.5 |
3.5 |
103 |
51 |
1.0 |
3.9 |
3.6 |
6.7 |
−5.1 |
92.2 |
|
P7 |
600 |
2193 |
1396 |
65.0 |
67.3 |
2.5 |
107 |
46 |
1.0 |
3.5 |
2.8 |
4.9 |
9.9 |
72.7 |
|
P41 |
630 |
2138 |
1384 |
65.6 |
69.4 |
3.9 |
121 |
59 |
1.0 |
4.0 |
3.0 |
4.0 |
6.3 |
70.5 |
|
P77 |
515 |
2159 |
1337 |
61.9 |
64.2 |
2.4 |
109 |
55 |
0.9 |
3.6 |
3.4 |
5.2 |
4.1 |
76.2 |
|
P65 |
641 |
2028 |
1334 |
66.3 |
69.0 |
2.7 |
106 |
44 |
0.9 |
4.1 |
3.7 |
5.6 |
6.9 |
68.4 |
|
Mean |
344 |
1803 |
1074 |
65.0 |
67.9 |
2.9 |
104 |
46 |
0.9 |
3.9 |
3.7 |
5.8 |
−0.1 |
|
|
P65×T2 |
5129 |
7476 |
6303 |
58.3 |
62.5 |
4.2 |
132 |
61 |
0.9 |
3.1 |
3.5 |
5.1 |
6.5 |
31.4 |
|
P66×T3 |
5126 |
7337 |
6232 |
58.4 |
62.2 |
3.9 |
135 |
65 |
0.9 |
2.7 |
2.9 |
1.9 |
13.5 |
30.1 |
|
P66×T2 |
4019 |
8155 |
6087 |
59.0 |
63.0 |
4.0 |
126 |
63 |
0.9 |
2.9 |
3.2 |
5.6 |
7.7 |
50.7 |
|
P36×T3 |
5742 |
5815 |
5779 |
59.6 |
62.8 |
3.2 |
133 |
61 |
1.0 |
3.1 |
3.2 |
2.2 |
13.0 |
1.3 |
|
P55×T2 |
4236 |
7174 |
5705 |
61.3 |
64.6 |
3.2 |
139 |
58 |
0.9 |
3.2 |
3.5 |
6.2 |
6.2 |
40.9 |
|
P31×T2 |
4125 |
7144 |
5635 |
59.2 |
62.0 |
2.8 |
143 |
64 |
1.2 |
3.2 |
3.1 |
4.3 |
8.5 |
42.3 |
|
P5×T2 |
4551 |
6399 |
5475 |
61.2 |
63.9 |
2.7 |
135 |
59 |
1.0 |
3.1 |
3.2 |
4.0 |
8.5 |
28.9 |
|
P56×T2 |
2958 |
7802 |
5380 |
59.2 |
62.0 |
2.8 |
156 |
63 |
1.0 |
3.1 |
3.2 |
7.0 |
4.2 |
62.1 |
|
P35×T2 |
3918 |
6629 |
5273 |
61.0 |
64.3 |
3.3 |
137 |
63 |
0.9 |
3.1 |
3.5 |
5.3 |
3.4 |
40.9 |
|
P75×T1 |
3287 |
6889 |
5088 |
61.1 |
64.8 |
3.7 |
137 |
62 |
0.9 |
3.0 |
3.2 |
3.3 |
4.2 |
52.3 |
|
P18×T3 |
2138 |
8003 |
5071 |
62.5 |
65.8 |
3.3 |
147 |
59 |
0.9 |
3.2 |
3.5 |
4.4 |
0.8 |
73.3 |
|
P23×T1 |
3240 |
6896 |
5068 |
59.1 |
62.4 |
3.2 |
140 |
60 |
1.0 |
3.0 |
3.2 |
2.6 |
7.3 |
53.0 |
|
P73×T3 |
3352 |
6755 |
5053 |
62.0 |
65.9 |
3.9 |
139 |
73 |
0.9 |
3.3 |
3.3 |
3.1 |
2.6 |
50.4 |
|
P16×T3 |
2667 |
7432 |
5050 |
61.8 |
64.8 |
3.0 |
142 |
56 |
1.0 |
3.3 |
3.6 |
2.8 |
4.1 |
64.1 |
|
P29×T1 |
2604 |
7464 |
5034 |
61.1 |
64.7 |
3.6 |
146 |
55 |
0.9 |
2.9 |
3.3 |
2.2 |
3.7 |
65.1 |
|
P51×T3 |
3892 |
6144 |
5018 |
58.9 |
61.9 |
2.9 |
147 |
67 |
0.9 |
2.9 |
3.5 |
3.2 |
8.7 |
36.7 |
|
P56×T1 |
3314 |
6595 |
4955 |
61.9 |
64.9 |
3.0 |
156 |
65 |
1.0 |
2.9 |
3.2 |
4.1 |
7.4 |
49.7 |
|
P55×T3 |
4028 |
5822 |
4925 |
61.7 |
64.7 |
3.0 |
151 |
65 |
1.0 |
3.0 |
3.5 |
3.5 |
7.8 |
30.8 |
|
P65×T1 |
4044 |
5782 |
4913 |
58.1 |
62.2 |
4.1 |
128 |
64 |
0.8 |
2.9 |
3.3 |
1.9 |
6.3 |
30.1 |
|
P74×T2 |
2560 |
7095 |
4827 |
62.0 |
65.6 |
3.6 |
125 |
63 |
0.9 |
3.1 |
4.0 |
6.3 |
−1.5 |
63.9 |
|
C1 |
1730 |
6335 |
4032 |
62.3 |
65.3 |
3.0 |
121 |
57 |
0.9 |
3.2 |
3.4 |
5.7 |
−2.6 |
|
|
C2 |
980 |
6088 |
3534 |
61.9 |
64.7 |
2.9 |
137 |
60 |
0.9 |
3.0 |
3.5 |
4.0 |
−3.3 |
|
|
C3 |
2185 |
6321 |
4253 |
63.0 |
65.8 |
2.8 |
131 |
56 |
0.9 |
3.1 |
3.3 |
4.7 |
−0.8 |
|
|
Mean |
2473 |
5263 |
3868 |
60.6 |
63.8 |
3.2 |
140 |
62 |
0.9 |
3.1 |
3.4 |
4.5 |
0.9 |
|
Grain yield (GY); Days to anthesis (DP); Days to silking (DS); Anthesis silking interval (ASI); Plant height (PHT); Ear height (EHT); Ears per plant (EPP); Plant aspect (PA) Ear aspect (EA); Stay green characteristics (STG); Low N tolerance index (LNT); Percentage yield reduction (YRD); Low-N (LN); Optimum-N (OPT); Across both low- and optimum-N conditions (ACC)
Table 4
Pearson correlation coefficients between traits of maize inbred lines and their hybrids under low- and optimum-N conditions
|
Trait |
Correlation coefficient |
|
Grain yield |
0.03ns |
|
Days to anthesis |
−0.07ns |
|
Days to silking |
−0.09ns |
|
Anthesis–silking interval |
−0.04ns |
|
Plant height |
0.06ns |
|
Ear height |
−0.05ns |
|
Number of ears per plant |
0.03ns |
|
Plant aspect |
0.03ns |
|
Ear aspect |
0.01ns |
|
Stay green characteristics |
0.001ns |
ns means non- significant; N = 237; Parental lines value for each trait = ½ (P1 + P2), where P1 and P2 represent the two parental lines involved in hybridization.
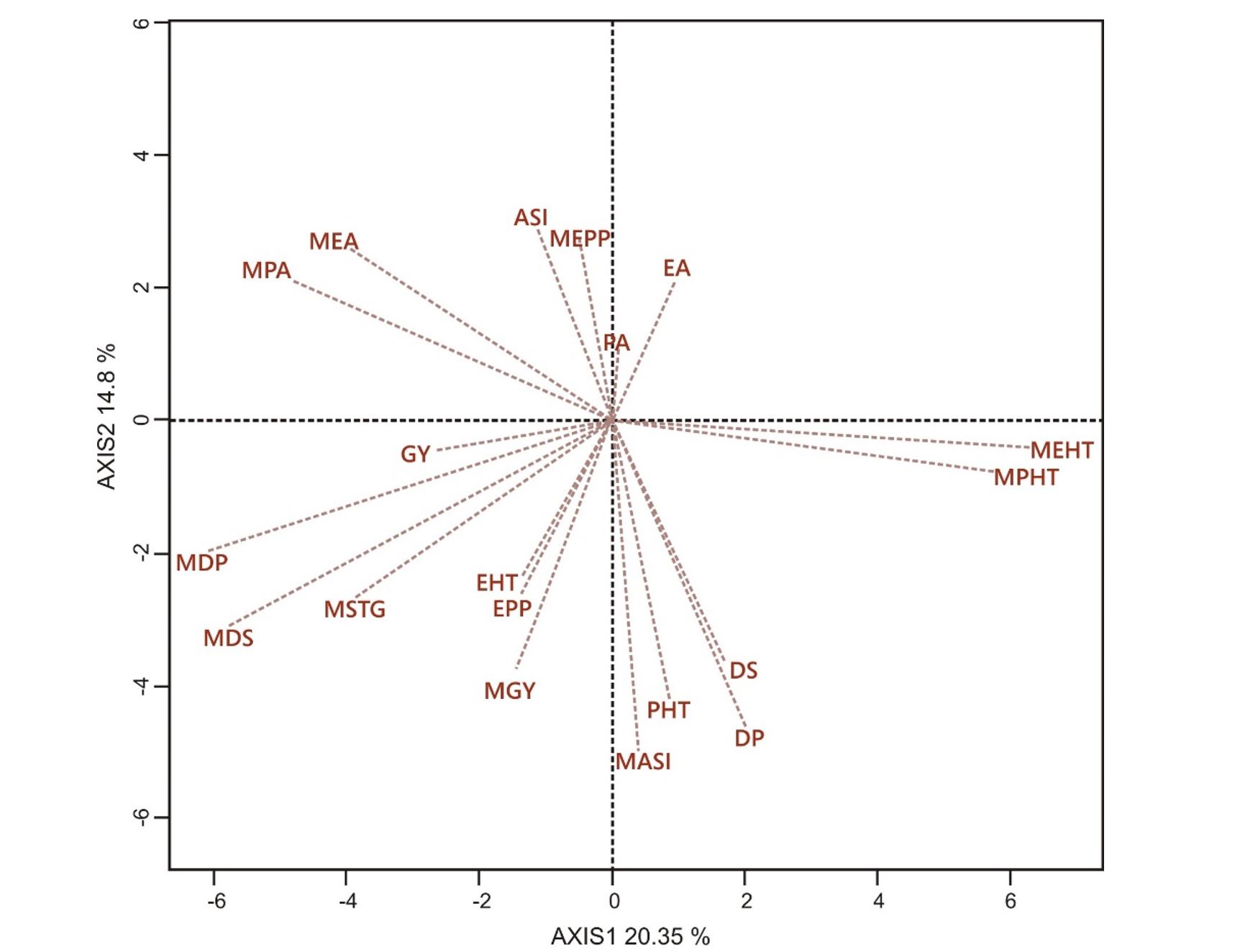
(GY, Hybrid grain yield; PHT, Hybrid plant height; EHT, Hybrid ear height; EPP, Hybrid number of ears per plant; PA, Hybrid plant aspect; EA, Hybrid ear aspect; DP, Hybrid days to anthesis; DS, Hybrid days to silking; ASI, Hybrid anthesis silking interval; STG, Hybrid stay green characteristics; MGY, Mid-parent grain yield; MPHT, Mid-parent plant height; MEHT, Mid-parent ear height; MEPP, Mid-parent number of ears per plant; MPA, Mid-parent plant aspect; MEA, Mid-parent ear aspect; MDP, Mid-parent days to anthesis; MDS, Mid-parent days to silking; MASI, Mid-parent anthesis silking interval; MSTG, Mid-parent stay green characteristics).
Figure 1 – A vector view of the genotype-by-trait biplot showing interrelationships between mid-parent values and their hybrid values for grain yield and other agronomic traits
Regarding ASI, hybrids P21×T2 and P21×T3 showed the lowest MPH and BPH, but P13×T3 had the lowest SH. Concerning growth traits (PHT), hybrids P1×T2 and P14×T2 had the lowest BPH and MPH, while P68×T3 exhibited the lowest SH.
For EHT, hybrids P14×T2 and P9×T1 displayed the lowest MPH and BPH, with P3×T1 recording the lowest SH. Hybrid P37×T1 showed the lowest MPH and BPH for STGR, while hybrid P24×T1 had the lowest SH (Table 5).
DISCUSSION
The significance of environmental mean squares for all measured traits indicates the influence of environmental conditions on trait expression, highlighting the importance of considering environmental factors in breeding programs (Abu et al., 2021; Obeng-Bio et al., 2020). Significant mean squares for lines and hybrids across most traits suggest substantial genetic variability among the parental lines and their hybrids, emphasising the potential for genetic improvement through hybridisation. The highly significant genotype × environment interaction underscores the differential responses of both parental lines and hybrids to varying environmental conditions, indicating the need for environment-specific breeding strategies to optimise trait expression. This interaction’s significance for all characteristics suggests that the same genotype may produce differential trait expression under different environmental conditions, highlighting the complexity of trait inheritance and the need for multilocational trials to assess performance stability across diverse environmental conditions (Abu et al., 2021; Badu-Apraku et al., 2016).
The repeatability estimates offer valuable insights into the consistency and stability of trait expression across different environmental conditions. Traits, such as STGR, DP, DS, and ASI, demonstrated high repeatability across environments, indicating a strong genetic influence and minimal impact from environmental variations. These traits are characterised by stable performance, making them suitable targets for direct phenotypic selection. In contrast, traits, such as PA, EA, and EPP, exhibited low repeatability, suggesting a greater susceptibility to environmental influences. Consequently, direct phenotypic selection for these traits may not be effective. GY, displaying moderate repeatability under low-N conditions and slightly higher repeatability under optimum-N conditions and across environments, highlights the complex interaction between genetic and environmental factors. This suggests that variety selection based solely on GY may be inefficient under low-N conditions (Badu-Apraku et al., 2011). Traits with high repeatability can be used in conjunction with GY to make indirect selections in such conditions (Adu et al., 2021; Monneveux et al., 2006).
The observed GY reductions, ranging from 35 to 95% among inbreds and from 1.3 to 89% among hybrids under low-N conditions, emphasise the significant impact of N stress on the performance of maize genotypes (Makumbi et al., 2011; Ribeiro et al., 2020).
Notably, the low yield reduction percentage and positive LNTI values observed in parental lines P69 and P14 as well as hybrids P65×T2, P66×T3, and P66×T2, indicate their potential tolerance to low-N conditions. These parental lines represent valuable genetic resources for developing maize varieties with improved N use efficiency and resilience to N stress. Similarly, the identified hybrids offer practical solutions for farmers facing challenges with varying soil fertility levels. With their inherent genetic potential to consistently produce good yields under N stress conditions, these hybrids hold promise for enhancing maize productivity in environments characterised by limited N availability.
Additionally, accurate prediction of hybrid performance, informed by the traits of their parental lines, is pivotal for making significant progress in selection (Oyekunle and Badu-Apraku, 2013b; Reif et al., 2013; Schrag et al., 2018; Zhao et al., 2013). Although the correlations observed between the traits of inbred lines and their hybrids may not have reached statistical significance, the consistency in results from multiple analyses conducted in the study suggests a degree of reliability in using inbred lines’ performance to predict hybrid performance (Betràn et al., 2003). The positive correlation observed for traits, such as GY, PA, EA, and STGR, indicates a certain level of similarity in performance between the parental lines and their hybrids. This suggests that when parental lines exhibit favourable performance for these traits, there is a likelihood that their hybrids will also display similar favourable traits (Betràn et al., 2003).
Moreover, the orientation of the hybrid GY vector relative to the parental trait vectors on the biplot elucidated significant positive and negative effects for certain parental traits on hybrid yield performance. Traits, such as DP, DS, STGR, PA, and EA, positively influenced hybrid GY, whereas the PHT and EHT of the parents exerted negative effects on hybrid GY performance. Consequently, breeders aiming to develop high-yielding hybrids resilient to N stress should prioritise selecting parental lines with superior GY, prolonged leaf greenness, favourable PA and EA ratings, moderate PHT and EHT, and shorter DP and DS (Badu-Apraku et al., 2012, 2023).
Furthermore, it is crucial to consider all three forms of heterosis (BPH, MPH, and SH) to identify combinations that not only outperform the parents but also surpass existing standard varieties. Positive heterosis values for GY are highly desirable, as they indicate favourable gene combinations for improving the yield potential of hybrids. Crosses, such as P65×T2, P66×T3, and P66×T2, which exhibited high positive heterotic effects over both the parents and standard checks, are promising candidates for potential adoption in maize breeding programs. Negative heterosis is particularly desirable for flowering traits, as it signifies early flowering in hybrids compared to the parents (Dagne et al., 2013). This is especially advantageous under low-N stress conditions, representing the hybrids’ ability to expedite their reproductive processes before nutrient deficiency becomes more limiting. In this study, numerous crosses exhibited high negative values for all three heterosis parameters related to flowering traits. Among these hybrids, P31×T2 and P36×T3 also recorded high GYs, making them promising candidates for commercial use or as valuable parents in the development of early maturing maize varieties.
Similarly, hybrids displaying negative values for all three heterosis parameters related to both PHT and EHT offer practical advantages in terms of lodging resistance, mechanical harvesting, and yield stability (Olakojo and Olaoye, 2005; Salami et al., 2007). Combinations in the study exhibiting high negative heterosis for STGR are indispensable for developing hybrids with enhanced resilience to stress conditions, such as drought and nutrient deficiency. Among these combinations, P65×T1, P66×T3, P29×T1, and P36×T3 also exhibited high positive heterosis estimates for GY. These hybrids not only exhibit desirable stress-tolerant traits but also possess the genetic potential to produce high yields under optimal conditions.
CONCLUSIONS
In conclusion, parental lines P69 and P14 emerged as valuable genetic resources for developing maize varieties with improved tolerance to low-N stress. Among the hybrids, P65×T2, P66×T3, and P66×T2 displayed outstanding performance in GY and other key traits across varying N environments, positioning them as priority candidates for further evaluation and potential adoption. Additionally, correlation analysis and repeatability results indicated that yield improvement in low-N tolerant maize hybrids is linked to selecting parental lines with superior performance in traits, such as GY, STGR, and flowering traits.
Author Contributions: Conceptualisation, methodology, analysis, data curation, writing, review: FAB and OSA. The authors declare that they have read and approved the publication of the manuscript in this present form.
Funding: The authors gratefully acknowledge the financial support provided by the Accelerating Genetic Gains for Maize (AGG) Project and the Institute for Agricultural Research (IAR), Zaria.
Acknowledgments: We extend our sincere appreciation to the technical staff at the IAR Maize Improvement Unit in Zaria and Mokwa, Nigeria, for their valuable assistance.
Conflicts of Interest: There was no conflict of interest.
REFERENCES
Abiy, B.G.; Hussein, M.; Demissew, A. Standard heterosis of hybrids maize (Zea mays L.) for grain yield and yield related traits at Kulumsa, southeastern Ethiopia. International Journal of Research Studies in Agricultural Sciences 2019, 5 (9), 1-7. https://doi.org/10.20431/2454-6224.0509001.
Aboderin, O.S.; Oyekunle M.; Bankole, F.A.; Olaoye, G. Combining ability and Heterotic Grouping of Maize (Zea mays L.) Inbred Lines for Tolerance to Low Soil Nitrogen in Nigeria. Peruvian Journal of Agronomy 2024, 8 (1), 1-18. https://doi.org/10.21704/pja.v8i1.2101.
Abu, P.; Badu-Apraku, B.; Tongoona, P.; Ifie, B.E.; Ribeiro, P.F.; Obeng-Bio, E.; Offei, S.K. Genetics of extra-early maturing yellow and orange quality protein maize inbreds and derived hybrids under low soil nitrogen and Striga infestation. Crop Science 2021, 61 (2), 1052-1072. https://doi.org/10.1002/csc2.20384.
Adu, G.B.; Badu-Apraku, B.; Akromah, R. Strategies for selecting early maturing maize inbred lines for hybrid production under low soil nitrogen and Striga infestation. Agronomy 2021, 11 (7), 1309. https://doi.org/10.3390/agronomy11071309.
Akinwale, R.O. Heterosis and heterotic grouping among tropical maize germplasm. Cereal Grains 2021, 2, 59. https://doi.org/10.5772/intechopen.98742.
Badu-Apraku, B.; Fakorede, M.A.B.; Oyekunle, M.; Akinwale, R.O. Selection of extra-early maize inbreds under low N and drought at flowering and grain-filling for hybrid production. Maydica 2011, 56, 1721.
Badu-Apraku, B.; Akinwale, R.O.; Franco, J.; Oyekunle, M. Assessment of reliability of secondary traits in selecting for improved grain yield in drought and low-nitrogen environments. Crop Science 2012, 52 (5), 2050-2062. https://doi.org/10.2135/cropsci2011.12.0629.
Badu-Apraku, B.; Fakorede, M.A.; Talabi, A.O.; Oyekunle, M.; Akaogu, I.C.; Akinwale, R.O.; Annor, B.; Melaku, G.; Fasanmade, Y.; Aderounmu, M. Gene action and heterotic groups of early white quality protein maize inbreds under multiple stress environments. Crop Science 2016, 56 (1), 183-199. https://doi.org/10.2135/cropsci2015.05.0276.
Badu-Apraku, B.; Fakorede, M.A.B.; Annor, B.; Adu, G.B.; Obeng-Bio, E.; Abu, P.; Bhadmus, O.; Nelimor, C. Genetic enhancement of early and extra early maturing maize for tolerance to low-soil nitrogen in Sub-Saharan Africa. Crop Breeding, Genetics and Genomics 2023, 5 (1), 1-44. https://doi.org/10.20900/cbgg2023000.
Bankole, F.A.; Olajide, O.O.; Olaoye, G. Performance and yield stability of quality protein maize (Zea mays L.) hybrids under rainfed condition. Agriculture 2023, 69 (2), 66-76. https://doi.org/10.2478/agri-2023-0006.
Betràn, F.J.; Beck, D.; Bänziger, M.; Edmeades, G.O. Genetic analysis of inbred and hybrid grain yield under stress and nonstress environments in tropical maize. Crop Science 2003, 43 (3), 807-817. https://doi.org/10.2135/cropsci2003.8070.
Dagne, W.; Vivek, B.; Labuschagne, M. Association of parental genetic distance with heterosis and specific combining ability in quality protein maize. Euphytica 2013, 191 (2), 205-216. https://doi.org/101007/s10681-012-0757-2.
Dohm, M. Repeatability estimates do not always set an upper limit to heritability. Functional Ecology 2002, 16 (2), 273-280. https://doi.org/10.1046/j.1365-2435.2002.00621.x.
Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics. Longman Group Ltd, 1996.
Ferreira, F.; Rocha, J.; Alves, R.; Elizeu, A.; Benites, F.; de Resende, M.D.; Souza Sobrinho, F.; Bhering, L. Estimates of repeatability coefficients and optimum number of measures for genetic selection of Cynodon spp. Euphytica 2020, 216 (5), 70. https://doi.org/10.1007/s10681-020-02605-x.
Fehr, W.R. Principles of cultivar development. Theory and Technique. New York, Macmillan, 1991.
Kamara, A.Y.; Kamai, N.; Omoigui, L.O.; Tongola, A.; Ekeleme, F.; Onyibe, J.E. Guide to maize production in Northern Nigeria. Ibadan, Nigeria: IITA, 2020, pp 26.
Kempthrone, O. An introduction to genetic statistics. John Willey and Sons, Inc, New York, 1957.
Makumbi, D.; Betrán, J.F.; Bänziger, M.; Ribaut, J.M. Combining ability, heterosis and genetic diversity in tropical maize (Zea mays L.) under stress and non-stress conditions. Euphytica 2011, 180 (2), 143-162. https://doi.org/10.1007/s10681-010-0334-5.
Mogesse, W.; Zelleke, H.; Nigussie, M. Standard heterosis for grain yield and yield related traits in maize (Zea mays L.) inbred lines in Haramaya District, Eastern Ethiopia. East African Journal of Sciences 2020, 14, 51-64. https://doi.org/10.20372/eajs.v14i1.977
Monneveux, P.; Sanchez, C.; Beck, D.; Edmeades, G.O. Drought tolerance improvement in tropical maize source populations: Evidence of progress. Crop Science 2006, 46 (1), 180-19126. https://doi.org/10.2135/cropsci2005.04-0034.
Nakagawa, S.; Schielzeth, H. Repeatability for Gaussian and non-Gaussian data: A practical guide for biologists. Biological Reviews of the Cambridge Philosophical Society 2010, 85 (4), 935-956. https://doi.org/10.1111/j.1469-185X.2010.00141.x.
Obeng-Bio, E.; Badu-Apraku, B.; Ifie, B.E.; Danquah, A.; Blay, E.T.; Dadzie, M.A.; Noudifoulè, G.T.; Talabi, A.O. Genetic diversity among early provitamin A quality protein maize inbred lines and the performance of derived hybrids under contrasting nitrogen environments. BMC Genetics 2020, 21 (1), 78. https://doi.org/10.1186/s12863-020-00887-7.
Olakojo, S.A.; Olaoye, G. Combining ability for grain yield, agronomic traits and Striga lutea tolerance of maize hybrids under artificial Striga infestation. African Journal of Biotechnology 2005, 4 (9), 984-988.
Olayiwola, M.O.; Ajala, S.O.; Ariyo, O.J.; Ojo, D.K.; Gedil, M. Heterotic grouping of tropical maize inbred lines and their hybrid performance under stem borer infestation and low soil nitrogen condition in West and Central Africa. Euphytica 2021, 217, 14. https://doi.org/10.1007/s10681-020-02739-y.
Oyekunle, M.; Badu-Apraku, B. Hybrid performance and inbred-hybrid relationship of early maturing tropical maize under drought and well-watered conditions. Cereal Research Communications 2013a, 43 (2), 314-325. https://doi.org/10.1556/CRC.2013.0052.
Oyekunle, M.; Badu-Apraku, B. Genetic analysis of grain yield and other traits of early-maturing maize inbreds under drought and well-watered conditions. Journal of Agronomy and Crop Science 2013b, 200 (2), 92 -107. https://doi.org/10.1111/jac.12049.
Reif, J.; Zhao, Y.; Würschum, T.; Gowda, M.; Hahn, V. Genomic prediction of sunflower hybrid performance. Plant Breeding 2013, 132, 107-114. https://doi.org/10.1111/pbr.12007.
Resende, M.D.V. Genética biométrica e estatística no melhoramento de plantas perenes. Brasília: Embrapa, 2002.
Ribeiro, P.F.; Badu-Apraku, B.; Gracen, V.; Danquah, E.Y.; Afriyie-Debrah, C.; Obeng-Dankwa, K.; Toyinbo, J.O. Combining ability and testcross performance of low N tolerant intermediate maize inbred lines under low soil nitrogen and optimal environments. The Journal of Agricultural Science 2020, 158 (5), 1-20. https://doi.org/10.1017/S0021859620000702.
Rodríguez, F.S.; Alvarado, G.; Pacheco, Á.; Crossa, J.; Burgueño, J. AGD-R (Analysis of Genetic Designs with R for Windows) Version 5.0. 2020.
Salami, A.E.; Adegoke, S.A.O.; Adegbite, O.A. Genetic variability among maize cultivars grown in Ekiti State, Nigeria. Middle-East Journal of Scientific Research 2007, 2, 9-13.
Sanchez, C.F.B.; Alves, R.S.; Garcia, A.D.P.; Teodoro, P.; Peixoto, L.A.; Silva, L.A.; Bhering, L.; Resende, M.D.V. Estimates of repeatability coefficients and the number of the optimum measure to select superior genotypes in Annona muricata L. Genetics and Molecular Research 2017, 16 (3), 1-8. https://doi.org/10.4238/gmr16039753.
SAS Institute. SAS system for windows. Release 9.2. Cary, NC: SAS Institute, 2008.
Schrag, T.; Westhues, M.; Schipprack, W.; Seifert, F.; Thiemann, A.; Scholten, S.; Melchinger, A. Beyond genomic prediction: Combining different types of omics data can improve prediction of hybrid performance in maize. Genetics 2018, 208 (4), 1373-1385. https://doi.org/10.1534/genetics.117.300374.
Sharief, A.E.; El-Kalla, S.E.; Gado, H.E.; Abo-Yousef, H.A.E. Heterosis in yellow maize. Australian Journal of Crop Science 2009, 3 (3), 146-154.
Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Canadian Journal of Plant Science 2006, 86 (3), 623-645. https://doi.org/10.4141/P05-169.
Zhao, Y.; Zeng, J.; Fernando, R.; Reif, J. Genomic prediction of hybrid wheat performance. Crop Science 2013, 53 (3), 802-810. https://doi.org/10.2135/cropsci2012.08.0463
Academic Editor: Dr. Iulian GABUR
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.