Usman Habu Taura, Muhammad Abbagoni Abubakar, Abdulhalim Musa Abubakar, Mohammed Umar Kurgiya
ABSTRACT. Awareness of the need to protect the environment and people’s health has led to an intensification of concerns for obtaining sustainable products and processes. Toxic waste created during the production and use of synthetic dyes has an impact on both human and environmental health. As a result, natural dyes are more secure and safer than synthetic dyes. This study is significant because it has the potential to help develop sustainable and environmentally friendly textile dyeing techniques. In this study, a natural dye was successfully extracted from orange peel (Citrus recticulata Rutaceae) which was applied on textile fibre and was found to be partly effective for eco-friendly dyeing applications. The extracted dye does not have a good wash fastness, which is the ability of the fabric to retain its colour after washing. This weakness is demonstrated by carrying out a Fourier transform infrared (FTIR) analysis where the potential binding mechanisms between the dye and the textile fibres was revealed. Different functional groups can interact with the fibre’s functional groups, affecting the dye’s affinity for the fabric and its overall colour fastness properties. Findings show that dyes from orange peels showed promise in this study, but need to be improved further. Therefore, the study suggests that further research is needed to optimize the dyeing process and improve the fabric’s resistance to washing and other environmental factors.
Keywords: mordant; natural dye; orange peel; synthetic dye; textile industry.
Cite
ALSE and ACS Style
Taura, U.H.; Abubakar, M.A.; Abubakar, A.M.; Kurgiya, M.U. Extraction and characterisation of natural dye from orange peel for textile applications. Journal of Applied Life Sciences and Environment 2024, 57 (1), 169-181.
https://doi.org/10.46909/alse-571130
AMA Style
Taura UH, Abubakar MA, Abubakar AM, Kurgiya MU. Extraction and characterisation of natural dye from orange peel for textile applications. Journal of Applied Life Sciences and Environment. 2024; 57 (1): 169-181.
https://doi.org/10.46909/alse-571130
Chicago/Turabian Style
Taura, Usman Habu, Muhammad Abbagoni Abubakar, Abdulhalim Musa Abubakar, and Mohammed Umar Kurgiya. 2024. “Extraction and characterisation of natural dye from orange peel for textile applications” Journal of Applied Life Sciences and Environment 57, no. 1: 169-181.
https://doi.org/10.46909/alse-571130
View full article (HTML)
Extraction and Characterisation of Natural Dye From Orange Peel for Textile Applications
Usman Habu TAURA1*, Muhammad Abbagoni ABUBAKAR2, Abdulhalim Musa ABUBAKAR3 and Mohammed Umar KURGIYA2
1Oil and Gas Research Centre, Sultan Qaboos University, Muscat, Oman, 123
2Department of Chemical Engineering, University of Maiduguri, P.M.B 1069 Maiduguri, Borno State, Nigeria; email: magoni@unimaid.edu.ng; mohdumar461@yahoo.com
3Department of Chemical Engineering, Modibbo Adama University, P.M.B 2076, Yola, Adamawa State, Nigeria; email: abdulhalim@mau.edu.ng
*Correspondence: usman@squ.edu.om
Received: Feb. 05, 2024. Revised: Feb. 24, 2024. Accepted: Mar. 01, 2024. Published online: Apr. 02, 2024
ABSTRACT. Awareness of the need to protect the environment and people’s health has led to an intensification of concerns for obtaining sustainable products and processes. Toxic waste created during the production and use of synthetic dyes has an impact on both human and environmental health. As a result, natural dyes are more secure and safer than synthetic dyes. This study is significant because it has the potential to help develop sustainable and environmentally friendly textile dyeing techniques. In this study, a natural dye was successfully extracted from orange peel (Citrus recticulata Rutaceae) which was applied on textile fibre and was found to be partly effective for eco-friendly dyeing applications. The extracted dye does not have a good wash fastness, which is the ability of the fabric to retain its colour after washing. This weakness is demonstrated by carrying out a Fourier transform infrared (FTIR) analysis where the potential binding mechanisms between the dye and the textile fibres was revealed. Different functional groups can interact with the fibre’s functional groups, affecting the dye’s affinity for the fabric and its overall colour fastness properties. Findings show that dyes from orange peels showed promise in this study, but need to be improved further. Therefore, the study suggests that further research is needed to optimize the dyeing process and improve the fabric’s resistance to washing and other environmental factors.
Keywords: mordant; natural dye; orange peel; synthetic dye; textile industry
INTRODUCTION
Ecological alarms and cognizance have favoured a change to sustainable products and processes that are less impactful to the environment and living organisms’ well-being (Muniz et al., 2020). Natural colourants have been utilized, mainly for textile colouration, since very old times (Helmy, 2020). Natural dyes are currently in demand not only in the textile industry but also in the cosmetics, leather, food and pharmaceutical industries (Chungkrang et al., 2021). In the field of obtaining textile materials, the consequences of processes and products that raised suspicions were evaluated, and in many cases improved solutions or even new alternatives, friendly to the environment and people, were found (Tayyab et al., 2020). Neglect of natural dye utilization started in 1856 due to the advent of synthetic dyes that yielded uniform colour shades (Naveed et al., 2020). Its current revival can be attributed to worldwide environmental awareness (Edeen, 2015; Pizzicato et al., 2023; Talib et al., 2023). About half (50-65%) of the total orange fruit weight is orange peels (Citrus recticulata Rutaceae), which is highly rich in pigments (Devi and Saini, 2020; Kodal and Aksu, 2017). Indonesia recorded more than 2 million tons of orange production in 2019 (Kusumawati et al., 2020); China’s annual yield is around 15 million tons, ranked third in the world (Li et al., 2021; Wei et al., 2013) and Nigeria produces about 1.626 million tons annually (Akpan et al., 2014). Globally, the production figure is put at over 100 million tons yearly (Brezo-Borjan et al., 2023; Che et al., 2024).
There are over 500 dye-yielding plant species, and China occupied the top seat in dyestuff exports, followed by India (Parikh et al., 2019). Thus, sustainability of the feedstock is not an issue.
Natural dyes are usually less allergenic and toxic (Gupta, 2020; Kumar and Dhinakaran, 2017; Parikh et al., 2019) than synthetic dyes and generate wastewater that can be treated by biodegradation (Baaka et al., 2017), all reasons why these kinds of colourants have been preferable as environmentally less impactful alternatives to certain synthetic dyes (Khan et al., 2018). Knowing the potential of local plant-based substrates for natural dye extraction is critical for their use and value in native species (Muniz et al., 2020). Toxic waste generated during the synthesis and application of synthetic dyes affects the health condition of humans and the environment (Sayem et al., 2021). As such, the use of natural dyes is safer and more secure compared to artificial synthetic dyes. The aim of this study is to develop a healthy, sustainable and environmentally friendly alternative for artificial dye production and application. The objectives are to extract natural dye from orange peels, use a pH meter to measure the degree of acidity or alkalinity, determine the important functional groups using Fourier transform infrared (FTIR) spectroscopy and test the effectiveness of the produced dye on textiles. Already, quality shades and fastness have been achieved using lemon and orange peel waste in dyeing (Aishwariya, 2020). FTIR was previously tested on wool dyeing using combinations of walnut husk and myrobalan (Hosseinnezhad et al., 2023) and mordanted cotton with Syzygium cumini fruits (Periyasamy, 2022) as bio-mordants. Kim et al. (2014) examined the rubbing fastness, water fastness, alkaline colour change, dry cleaning fastness, light fastness and washing fastness of citrus peel extract on cotton, wool, rayon and silk fabrics. There is a need to experiment with the forgone properties to compare amongst onion peel, Areca concinna peel, walnut shell, pine nut shell, olive fruit peel, tangerine peel, onion peel, orange peel, avocado seed and lemon peel in one study. The present study concentrated only on the effect of pH, functional group, temperature, acting-mordant concentration and textile weight after dyeing with orange peel pigment. Conventional mordants are iron sulphate, alum, copper sulphate, stannous chloride and potassium dichromate (Zubairu and Mshelia, 2015).
MATERIALS AND METHODS
Orange Peel Collection and Pretreatment
Fresh orange peel waste samples were collected from a local fruit seller at Gamboru Market in Jere Local Government, Borno State, Nigeria. The orange peels were washed rigorously with water to free them of contaminants and spread on a tray to dry under sunlight. The dried peels were crushed into powder form using a grinder (mortar and pestle and grinding machine), as carried out by Kumar and Dhinakaran (2017). This was later screened into different sizes by a vibrating screen to obtain fine dried powder.
Dye Extraction
About 200 mL of distilled water was poured into a 400 mL beaker and 12 g of dried and pulverized citrus peels (puree) were soaked in the distilled water for 48 h, as described by Kusumawati et al. (2020), before heating to 60°C for 30 min to yield what is called ‘dye extract’. Next, the dye extract was left for 30 min at ambient temperature and filtered (Parikh et al., 2019). Coloured crude dye solution (150 cm3) was diluted with distilled water (50 cm3) and immediately used for dyeing. Normally, the extraction efficiency is improved by soaking the dried powder in the cold distilled water before the extraction step.
pH Determination
An electronic device called a pH meter was used to measure the quantifiable parameter ‘pH’ in liquids or semisolid compounds in certain situations. Calibration of pH meters was done using buffer solutions with known hydrogen ion activity before the pH of the sample was measured.
FTIR Spectroscopy Analysis
A UV-1800 FTIR spectrometer was used to determine the functional groups within the samples taken.
Dyeing and Mordanting
Initially, a cloth was cleansed with ‘Good Mama powder’ detergent and rinsed with cold distilled water to remove impurities. The cotton fabric has a yarn count of 40, making it finer and smoother with high density compared to a coarser yarn count. Such a high-density plain weave pattern cloth contributes to its smoother texture and a more compact fabric structure. Rinsing was carried out by gentle soaking of the cotton fabric and swirling at room temperature to remove impurities with a sufficient volume of water inside a small container. In this experiment, three dyeing and mordanting procedures were followed. First, dyeing without mordant, where a cotton cloth is immersed in dye at temperatures of 60 and 90°C for 45 min before it is dried and analysed.
In the absence of conventional mordants, sodium hydroxide (NaOH) was used to increase the solubility of the dyes as it acts like a colour modifier by changing the pH of its environment. However, it does not act as a mordant in the traditional sense of fixing the dye to the substrate. Second in line is mordanting before dyeing, where the cloth is treated with varying molarity (0.5, 1.0 and 1.5%) of NaOH solutions at 60 and 90°C for 30 min. It was then dyed for 45 min at the same temperatures and analysed after drying. Third, or last, in the list is dyeing followed by mordanting, where the cotton cloth was dyed for 45 min at 60°C and 90°C, dried, and then treated with NaOH solutions (0.5, 1.0 and 1.5%) for 45 min at the same temperatures. After drying, the fabric was stored for analysis. The treated cloth’s weight was measured before and after dyeing, and various tests were conducted, including washing, exposure to sunlight, and manual rubbing, to evaluate its performance. ISO 105 methods for testing a few fastness properties were used, as followed by Periyasamy (2022).
RESULTS AND DISCUSSION
Extracted Dye
The procedure described above is summarized in Figure 1.

Figure 1 – Laboratory steps followed
As observed in Figure 1e, the orange peel was heated and filtered for the extraction of dye. It was found that the colour quality of the dye solution was comparable to that of powdered colouring materials because its colour did not change following the 30 min heating process, leading to high quality components. Orange peels were found to be the best plant part for extracting natural colour. Berry et al. (1971) advised that colour extract from the peel should be stored at 40 (4.4 ) at all times. For sustainability, the abundance of oranges is a great merit for dye production, which would help reduce waste associated with its consumption and processing and spur economic growth.
Effect of pH
pH is a measurement of the acidity or alkalinity of a solution and it ranges from 0 to 14, with 7 being neutral. Values below 7 are acidic, while those above 7 are alkaline. The pH of the extract was measured in triplicate with the pHS-3C precise pH meter. Table 1 displays the pH values of the 3 tests carried out.
Table 1
pH level measurements
|
Sample |
1st Test |
2nd Test |
3rd Test |
|
Orange peels dye extract |
3.34 |
3.36 |
3.34 |
A slightly acidic pH, showcased by Table 2, indicates that the sample is somewhat stable. The findings’ dependability was further supported by the repeat measurements carried out in the laboratory. A pH of 3 was recorded previously by Che et al. (2024) as optimal after a response surface design run. Dyeing of fabrics at acidic pH was also reported by Baaka et al. (2017).
FTIR Functional Group
Absorption peaks are displayed in Figure 2 with wavenumbers ranging from 4000-500 cm−1 and percentage transmittance from 40-80%. A broad, moderate and sharp absorption bands are located at 3446.9657 cm−1. Sharp, moderate and weak absorption bands are located at 1728.2182 cm−1, 1551.3697 cm−1, 1360.6465 cm−1, 1212.8404 cm−1, 1156.1859 cm−1, 1062.3111 cm−1, 851.8788 cm−1 and 700.6141 cm−1.
The frequency at which the IR radiation interacts with the sample is indicated by the wavenumber. The given range, spanning from 4000 to 500 cm-1, includes a broad variety of frequencies that correlate to various chemical bonds. The existence of N-H stretching vibrations are indicated by the broad and moderate signal at 3446.965 cm-1, pointing to the presence of compounds or molecular fragments containing amine or amide functional groups. A sharp moderate signal (2388.1051 cm-1) suggests the presence of C≡C stretching vibrations, demonstrating the presence of certain alkyne functional groups in the dye. Sharp and weak signals at a wavenumber of 1728.2182 cm-1 show the existence of C=O stretching vibrations, which identifies compounds or functional group containing carbonyl groups (e.g., esters). A wavenumber of 1551.3697 cm-1 defines the presence of C=C stretching vibration and also describe either a low concentration or less intense vibration of these bonds. The value at 1360.6465 cm-1 was caused by stretching vibrations of N-O bonds within the nitro group (NO2). Those at 1212.8404 cm-1, 11556.1859 cm-1 and 1062.3111 cm-1 signal C-F bonds, fluorine or other compounds containing fluorine. Bands such as, 851.8788 cm-1 and 700.6141 cm-1 indicate the presence of different C-H chemical bond bending vibrations in alkenes and aromatics.
Weight After Dyeing
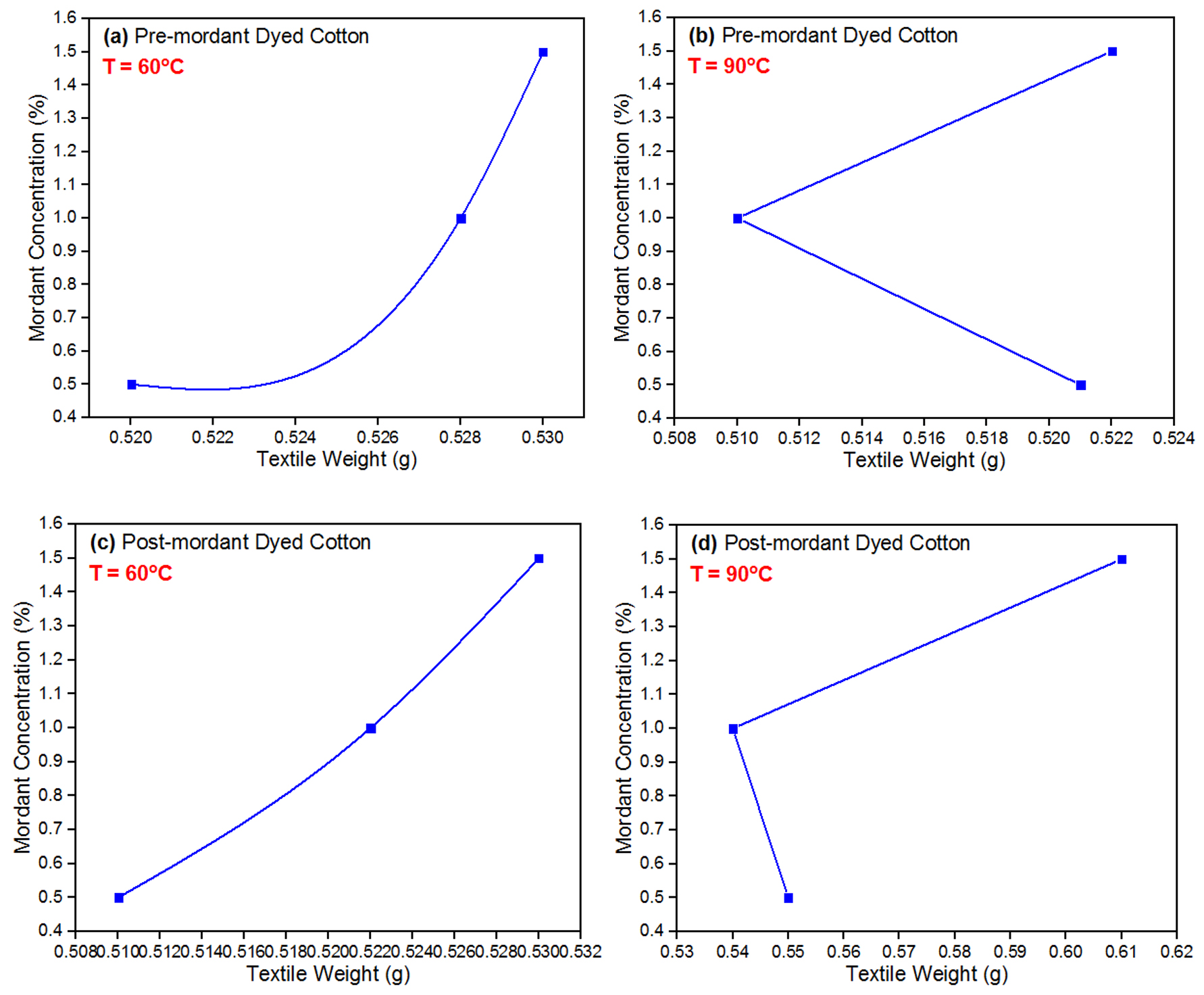
The results of weighing cotton fabrics both before and after dyeing at various temperatures and concentrations of mordant are given in Table 2. ‘Plain cotton’ weight is the initial weight of the cotton (0.42 g) before any dyeing or treatment that is used for comparing weight changes following dyeing procedures. ‘Free-mordant dyed cotton’ refers to cotton dyed without any mordant and dyed at two different temperatures; the weight increased in the absence of a mordant, perhaps because of moisture or a little amount of dye adhesion. ‘Pre-mordant dyed cotton’ was dyed after being treated with a mordant at varying concentrations and varying temperatures; the weight increases with higher NaOH concentrations at both temperatures, suggesting that the NaOH was facilitating better dye absorption. ‘Post-mordant dyed cotton’ is similar to pre-mordant dyeing, but the cotton was dyed first and then treated with mordant at varying concentrations and temperatures; at higher temperatures there was a considerable increase in weight, which was particularly visible with the maximum concentration of NaOH, indicating improved dye absorption. Li et al. (2021) stated that, at a temperature > 110 °C, greater than the maximum used in this study (90°C) in Table 2, the orange peel pigment ceases to be stable. The optimum dyeing temperature for most textile fabrics is 100°C (Hou et al., 2013).
It is evident that the weight of the fabric varies depending on the NaOH concentration and temperature. The data reveals a clear trend where increasing the temperature leads to an increase in the weight of the fabric after dye application. For the “free-mordant dyed cotton” material, fabric weights rose from 0.42-0.49 g at 60°C and further to 0.50 g at 90°C. Similarly, for the “pre-mordant dyed cotton” material, fabric weights increased with higher temperatures, reaching 0.520, 0.528 and 0.530g at 60°C for mordant concentrations of 0.5, 1.0 and 1.5%, respectively (Figure 3a). A temperature of 60°C is also the temperature tagged as optimum in Che et al. (2024) and Kim et al. (2014) during orange peel extraction and utilization as dye. At 90°C (Figure 3b), fabric weights were 0.521, 0.510 and 0.522 g for the same mordant concentrations. In the case of “post-mordant dyed cotton” material, fabric weights also showed an increasing trend with temperature, with weights ranging from 0.510-0.610 g at 90°C for mordant-like NaOH concentrations of 0.5-1.5%, as shown in Figure 3 (c-d). These results suggest that higher temperatures likely enhance dye uptake and binding to the fabric, resulting in increased fabric weight post-dyeing. Such weight gains represent the dye and NaOH binding to the cotton fibres, enhancing colour fastness and the overall quality of the dyed textiles. It is found in Ivanovska et al. (2022) that pH, time, temperature and extract concentration affect the cloth colour depth, colour hues and colour fastness. Therefore, controlling temperature during the dyeing process is crucial for achieving optimal dye uptake and fabric properties.
Table 2
Textile Weight Before and After Dye Application
|
S/N |
Material |
Acting-Mordant concentration (%) |
Temperature |
Weight (g) |
|
1. |
Plain cotton |
– |
– |
0.42 |
|
2. |
Free -mordant dyed cotton |
– |
60oC |
0.49 |
|
|
|
|
90oC |
0.50 |
|
3. |
Pre-mordant dyed cotton |
0.5 |
60oC |
0.520 |
|
|
1.0 |
0.528 |
||
|
1.5 |
0.530 |
|||
|
0.5 |
90oC |
0.521 |
||
|
1.0 |
0.510 |
|||
|
1.5 |
0.522 |
|||
|
4. |
Post-mordant dyed cotton |
0.5 |
60oC |
0.510 |
|
|
|
1.0 |
|
0.522 |
|
|
|
1.5 |
|
0.530 |
|
|
|
0.5 |
90o C |
0.550 |
|
|
|
1.0 |
0.540 |
|
|
|
|
1.5 |
0.610 |
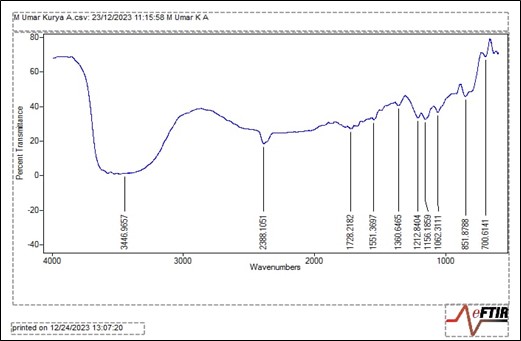
Figure 2 – FTIR spectra of liquid dye extracts
Data in Table 2 also show that the weight of the fabric after dye application increases with increasing NaOH concentration. For the “pre-mordant dyed cotton” material, fabric weights increased from 0.520-0.530 g at 60°C and from 0.521-0.522 g at 90°C when the mordant/modifier concentration was increased from 0.5% to 1.5%.
Similarly, for the “post-mordant dyed cotton” material, fabric weights increased from 0.510-0.530 g at 60°C and from 0.550-0.610 g at 90°C when the mordant/modifier concentration was increased from 0.5-1.5%. These results suggest that increasing the NaOH concentration can enhance dye uptake and binding to the fabric, leading to an increase in fabric weight post-dyeing. However, it is important to note that excessive mordant/modifier concentrations can lead to negative effects on the fabric, such as reduced fabric strength and stiffness. Therefore, it is crucial to optimize the mordant concentration to achieve the desired dye uptake and fabric properties while minimizing any negative effects.
Effect of rubbing and sunlight on the dyed fabric
Rubbing did not affect the dyed fabrics in both cases. Islam et al. (2020) reported that fastness properties were not affected by the use of orange peel extract (Citrus aurantium Dulcis). Washing affected it in both cases in this study. This is because the cotton fabrics turned pale after washing. It was also found that the dyed cotton clothes were not affected by sunlight at all. The pale colour after washing indicates that the dye did not have good wash fastness, which is the ability of the dye to resist fading or washing out during laundering. A good dye should have good wash fastness, which means that it should retain its colour even after repeated washing. Since the study did not extensively evaluate the wash fastness of the extracted dye, it is unclear how well the dye performed in this regard. Given the fact that Kumar and Dhinakaran (2017)’s dye from orange and lemon peel gave better fastness to rubbing and washing, the property demonstrated by orange peel in the present study needs to be improved, as it is mildly satisfactory. It wouldn’t be a surprise since Naveed et al. (2020) and Katyal et al. (2021) affirmed that natural dyes’ drawbacks are their poor fastness property and low affinity for textile fibres (caused by weak bonding). In agreement with the current study, Edeen (2015) reported almost zero colour left on a cotton fabric washed at 60°C for 30 min. In this study, the level of adherence of the dye to the cloth, leading to colour changes from plain white, is shown in Figure 4.
By mere observation, it is difficult to distinguish the colour intensity of the fabric dyed in Figure 4. It can best be differentiated by measuring their colour strength.
Dyeing and Mordanting
The dye and the ingredients used for the dye are chemically bound by the mordant-acting chemical. This might also be the reason for colour alteration. To improve absorption and fixing efficiency and guard against fading from washing and light exposure, a mordanting chemical is utilized.

Figure 4 – Shades of colour on the cloth: I. plain cloth; II-III. free mordant; IV-IX. pre-mordant; X-XV. simultaneous mordant and XVI-XXI. post-mordant
Typically, NaOH was used as part of the dyeing ingredients both prior to and following the dyeing process (pre- and post-mordanting), as used on Tencel fabric (Naveed et al., 2020). For instance, a honey-milk colour was observed using NaOH on white cotton material and dyeing it with Citrus sinensis peel dye solution. Additionally, cotton white fabric was first dyed with a citrus peel dye solution (post-mordanting) and then NaOH was used as a mordant in a subsequent step. Parikh et al. (2019) used NaOH to remove starch and impurities from the cloth while shades of yellow colour were observed by Tejavathi and Niranjan (2018) when different mordants were used on Celastrus paniculatus Willd dye extract.
CONCLUSIONS
Primarily, this study successfully extracted a natural dye from orange peel that was found partly effective for dyeing clothes. The extracted dye was characterized using FTIR, its pH was measured and a favourable pH within 3.34-3.36 was discovered. FTIR analysis identified various functional groups within the dye solution, such as N-H stretching vibrations, C≡C stretching vibrations, C=O stretching vibrations, C=C stretching vibrations, N-O bond stretching vibrations and C-H chemical bond bending vibrations in alkenes and aromatics. These functional groups are indicative of the chemical constituents present in the dye solution. Furthermore, the effects of temperature and acting-mordant concentration on cotton fabric weight during the dyeing process were evaluated. The data showed that higher temperatures and NaOH concentrations generally resulted in increased fabric weights after dye application, indicating enhanced dye uptake and binding to the fabric. Sunlight and rubbing effects are not influential in the dyed cloth, but only washing. The modifier-like mordant used (NaOH) proved to be effective at preventing quick fading of the dyed fabric. Other factors should be considered in future findings such as the dye stability, lightfastness and wash fastness. The effects of temperature-time profile, colour strength and actual use of mordant, as well as the liquor ratio were not considered in this study. To determine how environmentally friendly this natural dye is, a life cycle review comparing the effects of utilizing it vs. synthetic alternatives would be necessary.
Author Contributions: Conceptualization and methodology: UHT; Supervision, review and editing: MAA; Analysis, data curation and writing: AMA; Resources and investigation: MUK. All authors declare that they have read and approved the publication of the manuscript in the current form.
Funding: There was no external funding for this study.
Conflicts of Interest: The authors declare no competing interests.
REFERENCES
Aishwariya, S. Textiles from orange peel waste. Science and Technology Development Journal. 2020, 23, 508-516. https://doi.org/10.32508/stdj.v23i2.1730
Akpan, P.; Akpan, E.G.; Ozor, P.A. An estimation of orange oil (bio-diesel) quantity from orange peels in Nigeria. NIIE 2014 International Conference on Waste to Wealth in national Transformation at Awka, Anambra State Nigeria. 2014, 138-146. https://doi.org/10.13140/RG.2.1.3796.2721
Baaka, N.; Mahfoudhi, A.; Mhenni, M.F. Orange peels waste as a low cost cotton natural dye. Moroccan Journal of Chemistry. 2017, 5, 259-265. https://doi.org/10.48317/IMIST.PRSM/morjchem-v5i2.7028
Berry, R.E.; Bissett, O.W.; Kew, T.J. Orange peel color extract: Its use and stability in citrus products. Journal of Food Science. 1971, 36, 367-369. https://doi.org/10.1111/j.1365-2621.1971.tb06364.x
Brezo-Borjan, T.; Svarc-Gajic, J.; Morais, S.; Delerue-Matos, C.; Rodrigues, F.; Loncarevic, I.; Pajin, B. Chemical and biological characterisation of orange (Citrus sinensis) peel extracts obtained by subcritical water. Processes. 2023, 11, 1-15. https://doi.org/10.3390/pr11061766
Che, J.; Dannenberg, J.M.; Yu, M.; Yang, X.; Liu, Y. Identification, extraction, and application of orange peel color extracts for silk fabric coloration. Heliyon. 2024, 10, 1-10. https://doi.org/10.1016/j.heliyon.2023.e23836
Chungkrang, L.; Bhuyan, S.; Phukan, A.R. Natural dyes: Extraction and applications. International Journal of Current Microbiology and Applied Sciences (IJCMAS). 2021, 10, 1669-1677. https://doi.org/10.20546/ijcmas.2021.1001.195
Devi, O.R.; Saini, H. Utilization of orange peel waste in textile industry: A review. International Journal of Chemical Studies (IJCS). 2020, 8, 5-8. https://doi.org/10.22271/chemi.2020.v8.i4a.9808
Edeen, A.B. Dyeing of Egyptian cotton fabrics with orange peel using padding technique. International Design Journal. 2015, 5, 511-516. https://doi.org/10.21608/IDJ.2015.101805
Gupta, V.K. Fundamentals of natural dyes and its application on textile substrates, In Chemistry and Technology of Natural and Synthetic Dyes and Pigments. 1st edition, Publishing house IntechOpen, London, United Kingdom, 2020. https://doi.org/10.5772/intechopen.89964
Helmy, H.M. Extraction approaches of natural dyes for textile coloration. Journal of Textile Color and Polymer Science. 2020, 17, 65-76. https://doi.org/10.21608/jtcps.2020.30990.1037
Hosseinnezhad, M.; Gharanjig, K.; Iamin, H.; Rouhani, S.; Adeel, S. Environmentally dyeing of wool yarns using of combination of myrobalan and walnut husk as bio-mordants. Progress in Color, Colorants and Coatings. 2023, 16, 197-205. https://doi.org/10.30509/PCCC.2022.167001.1177
Hou, X.; Chen, X.; Cheng, Y.; Xu, H.; Chen, L.; Yang, Y. Dyeing and UV-protection properties of water extracts from orange peel. Journal of Cleaner Production. 2013, 52, 410-419. https://doi.org/10.1016/j.jclepro.2013.03.004
Islam, M.T.; Mazumder, N.-U.-S.; Asaduzzaman, S. Optimization of vat dyeing with an orange peel extract reducing agent using response surface methodology. AATCC Journal of Research. 2020, 7. https://doi.org/10.14504/ajr.7.1.1
Ivanovska, A.; Gajic, I.S.; Ladarevic, J.; Milosevic, M.; Savic, I.; Mihajlovski, K.; Kostic, M. Sustainable dyeing and functionalization of different fibers using orange peel extract’s antioxidants. Antioxidants. 2022, 11, 1-15. https://doi.org/10.3390/antiox11102059
Katyal, R.; Kakkar, P.; Kaur, T.; Tyagi, T.; Sharma, P.; Vats, S.; Wadhwa, N.; Mathur, R. Colouring properties of plant pigments on fabric: Survey on preference for antimicrobial naturally dyed mask. Current Trends in Biotechnology and Pharmacy. 2021, 15, 52-57.
Khan, B.; Sindhyan, R.; Divan, A.; Rathod, S. Extraction, characterization & applications of natural dyes. Annals of Plant Sciences. 2018, 7, 2463-2467. https://doi.org/10.21746/aps.2018.7.11.4
Kim, K.; Kim, H.; Lim, H. Research on the dyeability and functional property of citrus peel extract as a natural dye. The Research Journal of the Costume Culture. 2014, 22, 431-439. https://doi.org/10.7741/rjcc.2014.22.3.431
Kodal, S.P.; Aksu, Z. Phenolic pigment extraction from orange peels: Kinetic modeling. 15th International Conference on Environmental Science and Technology. 2017, Rhodes, Greece, 1-5.
Kumar, C.S.S.; Dhinakaran, M. Extraction and application of natural dyes from orange peel and lemon peel on cotton fabrics. International Research Journal of Engineering and Technology (IRJET). 2017, 4, 237-238.
Kusumawati, N.; Santoso, A.B.; Wijiastuti, A.; Muslim, S. Extraction, optimization, and dyeing standardization using fresh orange citrus peel on cotton fabrics. International Journal on Advanced Science Engineering Information Technology. 2020, 10, 1278-1283. https://doi.org/10.18517/ijaseit.10.3.3430
Li, K.; Ding, Q.; Zhang, H. Eco-friendly dyeing of cotton fabric using natural dye from orange peel. The Journal of The Textile Institute. 2022, 113. https://doi.org/10.1080/00405000.2021.1881226
Muniz, P.; Rossi, T.; Santiago, R.; Queiroz, D.; Freeman, H.S.; Aparecida, S.; Leo, P.; Maria, S. Natural dye from Croton urucurana Baill. bark : Extraction, physicochemical characterization, textile dyeing and color fastness properties. Dyes and Pigments. 2020, 173. https://doi.org/10.1016/j.dyepig.2019.107953
Naveed, T.; Babar, A.A.; Rashdi, S.Y.; Rehman, F.; Naeem, M.A.; Wang, W.; Abbas, M.; Ramzan, B. Dyeing and colorfastness properties of tencel fabric treated with natural dye extracted from orange peel. Surface Review and Letters (SRL). 2021, 28. https://doi.org/10.1142/S0218625X20500559
Parikh, M.; Patel, J.; Chauhan, A. Optimization of natural dye from citrus sinensis. International Research Journal of Engineering and Technology (IRJET). 2019, 6, 658-661.
Periyasamy, A.P. Natural dyeing of cellulose fibers using syzygium cumini fruit extracts and a bio-mordant: A step toward sustainable dyeing. Sustainable Materials and Technologies. 2022, 33. https://doi.org/10.1016/j.susmat.2022.e00472
Pizzicato, B.; Pacifico, S.; Cayuela, D.; Mijas, G.; Riba-Moliner, M. Advancements in sustainable natural dyes for textile applications: A review. Molecules. 2023, 28. https://doi.org/10.3390/molecules28165954
Sayem, A.N.M.; Ahmed, F.; Saha, P.; Talukder, B. A review on natural dyes: Raw materials, extraction process, and their properties. Advance Research in Textile Engineering. 2021, 6.
Talib, A.; ur-Rehman, F.; Adeel, S.; Ali, A.; Ahmad, T. Sustainable isolation and application of plant extract-based natural dye for bio-dyeing of silk fabric. Coatings. 2023, 13. https://doi.org/10.3390/coatings13010112
Tayyab, N.; Sayed, R.Y.; Faisal, R.; Wang, W.; Javeed, A.A.; Mudassar, A.; Ahmad, F.; Muhammad, A. Dyeing and colour fastness of natural dye from Citrus aurantium on Lyocell fabric. Industria Textila. 2020, 71, 350-356. https://doi.org/10.35530/IT.071.04.1686
Tejavathi, D.H.; Niranjan, R. Extraction and characterization of natural dye from arils of Celastrus paniculatus Willd. for textile application. International Journal of Scientific Research in Biological Sciences (IJSRBS). 2018, 5, 83-89. https://doi.org/10.26438/ijsrbs/v5i5.8389
Wei, G.; Yang, X.; Guangju, Z.; Caodezhen, Xiayang. Method for extracting pigment from tangerine peel (Patent No. CN102911514B). 2018.
Zubairu, A.; Mshelia, Y.M. Effects of selected mordants on the application of natural dye from onion skin (Allium cepa). Science and Technology. 2015, 5, 26-32. https://doi.org/10.5923/j.scit.20150502.02
Academic Editor: Dr. Iuliana Motrescu
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Abubakar Abdulhalim Musa, Abubakar Muhammad Abbagoni, Kurgiya Mohammed Umar, Taura Usman Habu