Elena Cristina Scutarașu, Valeriu V. Cotea, Camelia Elena Luchian, Lucia Carmen Trincă, Andrei Scutarașu
ABSTRACT. Wine’s quality is influenced both by the grape characteristics and winemaking protocols. Awareness of the significant role of enzymes preparations in beverages technologies contributes to the optimization of the manufacturing process, for improving the chemical composition of the resulting wine and its organoleptic properties. This paper focuses on monitoring the impact of different commercial enzymes (pectinases and β-glycosides) on the main phenolic compounds content of Sauvignon blanc wines. For this experiment, ten phenolic compounds were quantified using a liquid-chromatography (LC) system coupled with ion trap mass spectrometer. The results indicated a significant influence of enzymes on wine’s phenolic fraction. Experimental samples presented high content in protocatechuic acid (9.99 – 13.75 μg/mL) and caftaric acid (2.69 – 9.80 μg/mL). The use of pectinases lead to an increase of phenolic compound’s concentration compared to the control.
Keywords: enzymes, wine, phenolic compounds, pectinases, β-glycosides.
Cite
ALSE and ACS Style
Scutarașu, E.C.; Cotea, V.V.; Luchian, C.E.; Trincă, L.C.; Scutarașu, A. Evaluation of phenolic compounds in white wines treated with enzymes. Journal of Applied Life Sciences and Environment 2021, 54(4), 405-416.
https://doi.org/10.46909/journalalse-2021-035
AMA Style
Scutarașu EC, Cotea VV, Luchian CE, Trincă LC, Scutarașu A. Study of phenotypic variability using the varietal diversity of cultivated forms of naked and hulled oats in the intercropping system. Journal of Applied Life Sciences and Environment. 2021; 54(4): 405-416.
https://doi.org/10.46909/journalalse-2021-035
Chicago/Turabian Style
Scutarașu, Elena Cristina, Valeriu V. Cotea, Camelia Elena Luchian, Lucia Carmen Trincă, and Andrei Scutarașu. 2021. “Evaluation of phenolic compounds in white wines treated with enzymes” Journal of Applied Life Sciences and Environment 54, no. 4: 405-416.
https://doi.org/10.46909/journalalse-2021-035
View full article (HTML)
Evaluation of Phenolic Compounds in White Wines Treated with Enzymes
Elena Cristina Scutarașu1, Valeriu V. Cotea1, Camelia Elena Luchian1*, Lucia Carmen Trincă1, Andrei Scutarașu2
1Iasi University of Life Sciences, 3rd Mihail Sadoveanu Alley, 700490, Romania
2SCDVV Iasi, 48th Mihail Sadoveanu Alley, 700489, Romania
*E-mail: kamelia_luchian@yahoo.com
Received: Mar. 11, 2021. Revised: Apr. 13, 2022. Accepted: Apr. 21, 2022. Published online: June 08, 2022
ABSTRACT. Wine’s quality is influenced both by the grape characteristics and winemaking protocols. Awareness of the significant role of enzymes preparations in beverages technologies contributes to the optimization of the manufacturing process, for improving the chemical composition of the resulting wine and its organoleptic properties. This paper focuses on monitoring the impact of different commercial enzymes (pectinases and β-glycosides) on the main phenolic compounds content of Sauvignon blanc wines. For this experiment, ten phenolic compounds were quantified using a liquid-chromatography (LC) system coupled with ion trap mass spectrometer. The results indicated a significant influence of enzymes on wine’s phenolic fraction. Experimental samples presented high content in protocatechuic acid (9.99 – 13.75 μg/mL) and caftaric acid (2.69 – 9.80 μg/mL). The use of pectinases lead to an increase of phenolic compound’s concentration compared to the control.
Keywords: enzymes, wine, phenolic compounds, pectinases, β-glycosides.
INTRODUCTION
Enzymes play important functions in the winemaking process, improving clarification and filtration process (e.g. pectinases), enriching the volatile fraction (e.g. glycosides), improving sensory characteristics, or in increasing wine stability (Armada et al., 2010; Sui et al., 2020). Wine represents an important source of numerous bioactive constituents, including phenolic compounds (Kammerer and Carle, 2009). Wine phenolic composition is usually influenced by grape characteristics and maturation phase, agro-pedo-climatic conditions, works protocols and fermentation chemical reactions (Kammerer and Carle, 2009).
The relevance of studying phenolic compounds is given by their important benefits that manifest on human health. The mentioned constituents have antibacterial and antioxidative, antiviral and anti-carcinogenic functions (Lorrain et al., 2013; Rentzsch et al., 2009).
The phenolic compounds in wine can come from the raw material, or by an external source, including various oenological treatments. The wine’s composition is generally dependent on many factors, such as plant characteristics, grape variety, geographical location, applied harvesting technique and winemaking protocols (Cotea et al., 2009).
While wine’s composition is in continuous evolution, a progressive research is necessary, even if several papers (Bartwosky et al., 2004; Fernández González et al., 2005; Masino et al., 2008; Bautista-Ortin et al., 2011; Aroca et al., 2022; Haile & Ayele, 2022; Tiraș et al., 2022) already highlighted the advantages of using enzymes (including β-glycosides and pectinases) on wine composition (as well as increasing the concentration of phenolic compounds).
The present study aimed to establish the basic phenolic compounds and how their concentration changes during the fermentation process of white wines that have been previously treated with commercial enzymes. The originality of this experiment consists in the administration of enzymes before the fermentation stage, in must, as manufacturer’s recommendations and most authors analyse their action during other different phases of the winemaking (Table 1).
For this experiment, Sauvignon blanc variety was chose, the wines obtained from this grapes being some of the most worldwide appreciated. There are limited data regarding the effect of enzymes on the phenolic compound’s profile of Sauvignon blanc wines from Romanian vineyards.
MATERIALS AND METHODS
Winemaking process
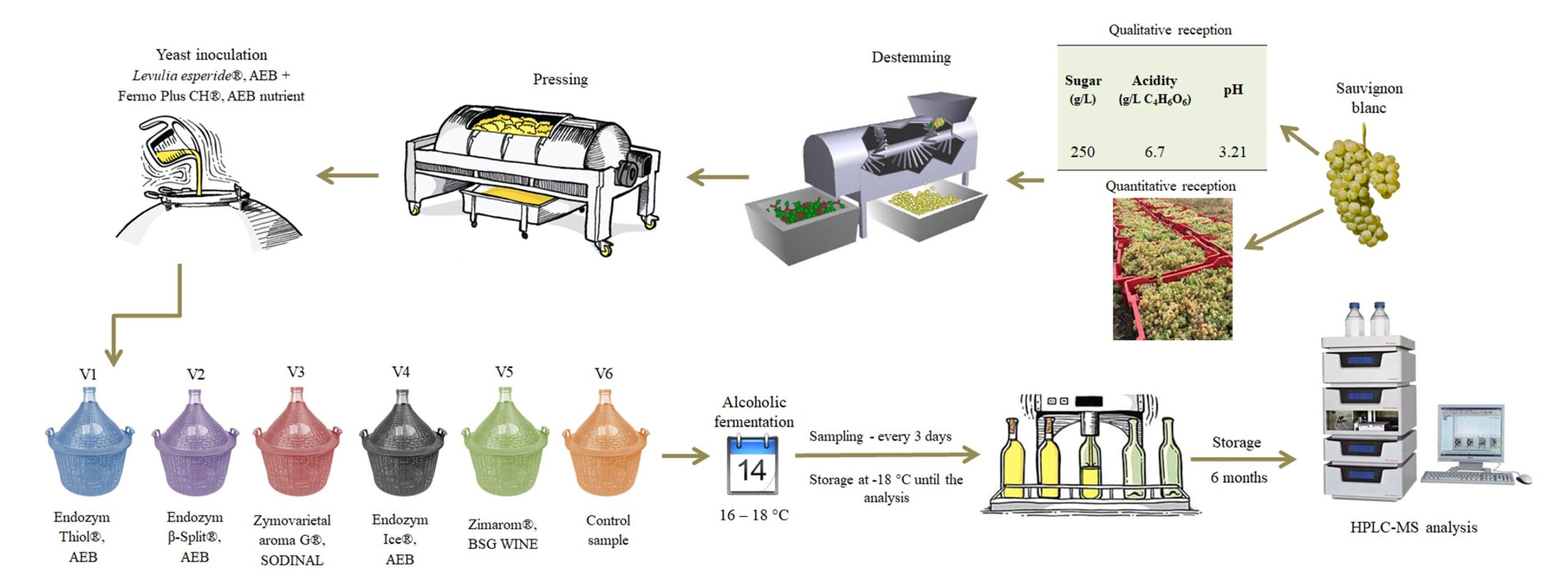
The grapes were harvested in October of 2018 from Copou-Iasi vineyard. The classic working protocol for obtaining white wines is presented in Fig. 1.
The grape juice was placed in 50 L glass containers. Saccharomyces cerevisae yeast (Levulia Esperide®, AEB) – 20 g/hL and yeast nutrient – 30 g/hL (Fermo Plus CH®, AEB) were inoculated in each vessel.
Five enzymes preparations (with pectinase and β-glycoside activities), selected due to availability, were administrated to musts before the installation of alcoholic fermentation. The applied doses were 3 g/hL for powders and 3 mL/hL for liquid preparations according to the manufacturers recommendations. Variants were noted from V1 to V6, as showed in Table 1. V6 represent the control sample (without enzyme treatment).
The fermentation process was carried out at 16 – 18 °C, for 14 days. Every three days, samples were taken to track the evolution of the phenolic component content. These aliquots were stored at -18 °C until the analysis. At the end of the alcoholic fermentation phase, the wines were racked, treated with SO2 (for about 15 – 20 mg free/L), and passed through sterile membrane filters with a fineness of 0.45 μm. Subsequently, the wine samples were bottled and placed in the dark, in an atmosphere with a constant temperature of 8 °C and a relative humidity of 70-80 % for 180 days, after which they were analysed.
Table 1
Manufacturer’s recommendation administration of analysed enzymes
|
Variants |
Enzyme preparations |
Manufacturer |
Moment of administration |
|
V1 |
Endozym® Thiol |
AEB |
Second fermentation day |
|
V2 |
Endozym® β-Split |
AEB |
Around the end of alcoholic fermentation, with a residual sugars content of less than 50 g/L |
|
V3 |
Zymovarietal® aroma G |
SODINAL |
Around the end of alcoholic fermentation, with a residual sugars content of less than 30 g/L |
|
V4 |
Endozym® Ice |
AEB |
During grape’s precessing, recommended to be added directly into the grapes, crushed grapes or must, at the start or during the refilling of the tanks |
|
V5 |
Zimarom® |
BSG WINE |
At the end of fermentation with a residual sugars content of less than 50 g/L |
Phenolic compounds quantification
To detect all phenolic compounds we used the Agilent 1100 HPLC Series system, equipped with degasser, reverse phase analytical column and binary gradient pump.
Data acquisition and processing were performed on specific software (Chemstation, LC / MSD Trap Control, Data Analysis, and Quant Analysis).
The detection of caftaric, caffeic, p-coumaric, ferulic, galic, gentisic, siringic and protocatechuic acids was performed on both UV and MS mode. The UV detector operated for 17.5 minutes at 330 nm and then at 370 nm, and the MS system was equipped with an electrosprey ion source.
A binary gradient of methanol and acetic acid 0.1 percent solution (v/v) was used to simulate the mobile phase. For 35 minutes, the elution began with a linear gradient, starting at 5% methanol and finishing at 42 % methanol; isocratic elution with 42 % methanol followed for the next 3 minutes. A volume of 5 L was injected at a flow rate of 1 mL·min-1. The phenolic compounds eluted in less than 35 minutes.
The concentration of identified phenolic compounds was calculated using calibration curves.
Trans- and cis- resveratrol analysed according to the method presented by Vlase et al. (2019). For the beginning, a methanol standard solution of the trans-resveratrol was used and aliquots of this solution were diluted using bi-distilled water. Two working solutions of 4.9 µg/mL concentration trans-resveratrol were prepared. One of these was used for the calibration curve (10.47 – 837.86 ng/mL range; n = 7) while another was irradiated with UV light and same subsequent dilutions were made. Both dilution series were analysed using chromatography.
Cis-resveratrol was obtained by irradiating a standard trans-resveratrol solution with a 254 nm UV lamp for 10 minutes. The calibration curve of cis-resveratrol was in 9.12 – 730.14 ng/mL range.
The isocratic elution was a mixture of 1 mM ammonium acetate and acetonitrile (73/27, v/v). All solvents were filtered and degassed and the volume injected was 5 mL with 1 mL·min-1 flow rate.
The detection of trans– and cis-resveratrol was carried out using an Agilent Ion Trap VL mass spectrometer and the working conditions were pre-set as follows: 350 oC for atmospheric pressure chemical ionisation ion (ACPI) heater, 60 psi – nebulizer pressure, 5 L/min dry gas flow (nitrogen), 250 oC – heating temperature.
For analysis, wine aliquots were filtered and centrifuged (10.000 rpm, 5 minutes).
All phenolic compounds were analysed in triplicate.
Standard solutions and reagents
All chemicals used (Merck KgaA, Germany) in this experiment were of analytical quality with a purity of over 99%.
Statistics and data visualization
Statistical tests including One Way-ANOVA, Fisher LSD and Principal Components Analysis (PCA) were performed using JMP software® package.
RESULTS AND DISCUSSION
Enzymes preparations are a simple and effective alternative in improving the bioactive compounds’ content, but also the sensory and commercial quality, with a simplistic technology, that is available to any winemaker. In addition, this paper highlights effective results when using enzymes in other stages of the technological process compared to that recommended by the manufacturer or studied by other authors. The results confirm the information sustained by literature (Bautista-Ortin et al., 2011; Espejo, 2020; Aroca et al., 2022; Fernández Haile & Ayele, 2022; Tiraș et al., 2022) and contribute to the improvement and completion of the already existing data.
The experimental wines were dry, with 1.9 – 2.8 g/L residual sugar and alcoholic strength greater than 16 % vol. From previous works, enzymes showed only a minor influence on wine physicochemical characteristics of white wines, in applied working conditions (Scutarașu et al., 2020).
Due to the application of enzymatic treatments, the proportions of phenolic compounds in the wines obtained have significantly differentiated (p < 0.05). The predominant compounds in the analysed Sauvignon blanc wines were protocatechuic acid, caftaric acid, trans- and cis-resveratrol.
Important quantities of protocatechuic acid originate from grapes and new concentrations probably being resulted from pyrocatechol (Cotea et al., 2009). The concentrations of protocatechuic acid was significantly (p < 0.05) influenced by the administered enzymes (Fig. 2). Thus, its level varies from 13.75±0.15 µg/mL in V1 to 9.99±0.02 µg/mL in V3. The results presented by Tian et al. (2009) confirmed that protocatechuic acid is predominant in most of white wines, its concentration being ascendant during fermentation. The values show that under the given conditions, the content of protocatechuic acid is increasing when pectinases are applied, but considerable quantities are originating from grapes.
Caffeic acid is a derivative of cinnamic acid. Caftaric acid is one of the predominant phenolic acids in wines and is the ethyl ester of caffeic acid (Peréz-Navarro, 2020). p-coumaric acid is the precursor of the 4-vinylphenol and may result from the bioconversion reaction under the influence of Saccharomyces or Brettanomyces yeasts (under the action of cinnamate decarboxylase) (Salameh et al., 2008; Waterhouse et al., 2016) or from phenylalanine synthesis, under the action of phenylalanine ammonia-lyase (Moreno & Peinado, 2012). Free forms of caffeic and p-coumaric acids (without being esterified with tartaric acid) can also result from the esterase’s activity. Caftaric and ferulic acids were formed at the end of the alcoholic fermentation, being found in the resulted wine. This phenomenon could be due to possible hydrolysis of hydroxycinnamic acids esters (caftaric, coutaric, fertaric) during fermentation (Budić-Leto & Lovrić, 2002).
Caftaric acid was found in considerable amounts in Sauvignon blanc wines, its values being ranged from 9.80±0.05 µg/mL (V6) to 2.69±0.06 µg/mL (V3) and was not identified in V2. The amount of caffeic acid varied from 4.95±0.08 µg/mL (V2) to 1.14±0.04 µg/mL (V6), highlighting an important positive influence of the applied enzyme (with β-glycosides activities). Ferulic acid was found in the highest amount in the V2 sample (0.37±0.01 µg/mL), followed by the V1 variant (0.35±0.01 µg/mL), and significantly lower concentrations were found in V6 (0.18±0.02 µg/mL). Lengyel & Sikolya (2017) highlighted aproximatelly 1 mg/L ferulic acid and about 28 mg/L caffeic acid in wines obtained from Apold vineyard (Sibiu, Romania).
Resveratrol was identified in both trans- and cis-isomers. The results were depending on the fermentation stage, grape variety, and administered treatment. However, the trans-resveratrol content ranged from 2.20±0.02 µg/mL (V4) to 2.50±0.05 µg/mL (V2), while for cis-isomer, values between 2.55±0.05 µg/mL (V2) and 3.21±0.10 µg/mL (V5) were obtained. The resulted wine samples showed higher values of the cis-resveratrol form than the trans-isomer. Moreover, β-glycosides significantly favoured the formation of cis-resveratrol in analysed wines. Biraruti (2015) reported between 0.07 mg/L and 2.57 mg/L trans-resveratrol for Romanian white wines, depending on the geographical region, analysed variety, and year of production.
Gentisic acid, the isomer of protocatechuic acid (Cotea et al., 2009) registered various fluctuations in its proportion. Its descendent concentration in the first stage of the fermentation process (observed in Fig. 2) may be due to the blockade of the synthesis by pectolytic enzymes and β-glycosides (Moroșanu, 2018). In the final wines, the highest amount of gentisic acid was determined in the V3 variant (0.30±0.01 µg/mL). The control sample showed three times lower values for this compound (0.10±0.00 µg/mL). The administered treatments had a significant influence on the final concentration of gentisic acid. The literature shows that approximately 0.1 – 0.2 mg/L of gentisic acid is usually present in wines (Cotea et al., 2009).
Syringic acid extracted into the must following the winemaking process is usually formed by the esterification of gallic acid with alcohols and the degradation of anthocyanins (Cotea et al., 2009). Therefore, syringic acid concentrations were ascendant in the first phase of the fermentation process, and towards the end, significant decreases were observed.
The resulted wines presented a considerable concentration of syringic acid in the V5 (0.38±0.00 µg/mL) and V1 variants (0.36±0.01 µg/mL), almost three times higher compared to the control sample (0.13±0.00 µg/mL). These results highlight an important contribution of pectinases on the final concentration of this compound. Syringic acid is usually found in low quantities in wines (Stavridou et al., 2016). So, He et al. (2020) identified approximately 13 mg/L syringic acid in Sauvignon blanc wines from New Zealand, while Lengyel & Sikolya (2017) reported 0,1 mg/L in Romanian ones (Apord vineyard, Sibiu).
Gallic acid was predominantly formed during alcoholic fermentation, resulting from chemical reactions that occur during the biochemical process (for example, by hydrolysis of gallate esters). The V1 sample was characterized by significant increases in this compound, recording the highest value (0.35±0.10 µg/mL), while the lowest concentration was presented by the V5 variant (0.17±0.00 µg/mL). According to the literature, Frankel et al. (1995) identified 0.60 – 1.10 mg/100 mL in Californian Sauvignon blanc wines, while Lengyel & Sikolya (2017) reported a level of 1.57 mg/L in Sauvignon blanc wines from Sibiu – Romania (Apold vineyard). Similar to protocatechuic acid, data shows that under the given conditions, the content of gallic acid can be favoured when pectolytic enzymes are used, but important concentrations are coming from grapes.
Depending on the sampling moment but also on the type of administered enzyme the concentration of phenolic compounds showed variations. In this study, the same temperature and commercial yeast were used in the fermentation of experimental wines. The descendant tendency during the fermentation process observed on some phenolic compounds is explained by their oxidation, which took place under the action of different enzymes. Some phenolic compounds may participate in the polymerization reaction with various aroma compounds. The differences in phenolic compounds concentrations between analysed samples and literature data are related to the terroir. Given that no maceration was applied, this explains the low levels of this compound in analysed wines.
In most cases, the highest concentrations of the main phenolic compounds were obtained in V1 variants (Table 2), while the lowest values were shown in the control sample (V6). Although pectinases are usually studied to promote clarification and filtration, a favourable effect on the enrichment of the substrate in phenolic acids was obtained. On the other hand, β-glycosides were effective in increasing caffeic, ferulic, and trans-resveratrol levels, in applied working conditions.
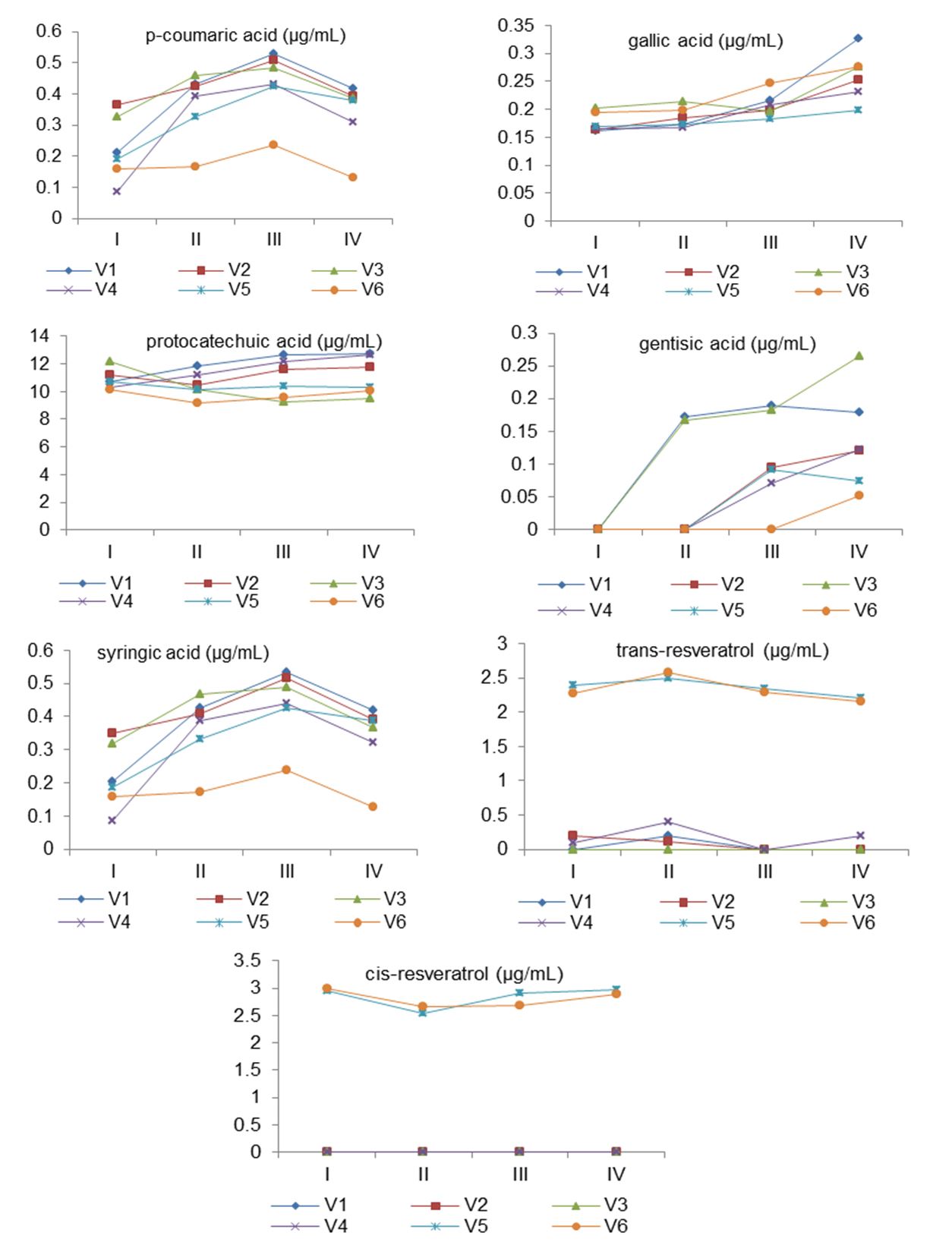
Figure 2 – Variation of the main phenolic compounds on Sauvignon blanc samples
I, II, III, IV – Fermentation stage (Day 1, 3, 6, 9) V1 – Endozym Thiol®, AEB; V2 – Endozym β-Split®, AEB; V3 – Zymovarietal aroma G®, SODINAL; V4 – Endozym Ice®, AEB; V5 – Zimarome®, BSG WINE; V6 – control sample (without enzyme)
Table 2
Concentrations of phenolic compounds on Sauvignon blanc samples
|
C |
Identified levels (μg/mL) |
|||||
|
V1 |
V2 |
V3 |
V4 |
V5 |
V6 |
|
|
1 |
0.36±0.00cd |
0.33±0.08c |
0.34±0.01cd |
0.24±0.04b |
0.39±0.03d |
0.20±0.03ab |
|
2 |
7.34±0.05a |
nd |
2.69±0.06* |
6.93±0.23* |
7.23±0.02ab |
9.80±0.05* |
|
3 |
3.02±0.23d |
4.95±0.08* |
4.49±0.05* |
2.33±0.15bc |
2.55±0.00* |
1.14±0.04a |
|
4 |
0.35±0.01de |
0.37±0.01e |
0.34±0.01cde |
0.26±0.02b |
0.32±0.02cd |
0.18±0.02a |
|
5 |
0.35±0.10e |
0.22±0.02bcd |
0.25±0.02cd |
0.27±0.09d |
0.17±0.00ab |
0.28±0.01de |
|
6 |
0.24±0.00* |
0.26±0.01* |
0.30±0.01* |
0.16±0.00* |
0.12±0.02* |
0.10±0.00a |
|
7 |
0.36±0.01f |
0.30±0.00de |
0.34±0.10ef |
0.23±0.00cd |
0.38±0.00f |
0.13±0.00b |
|
8 |
13.75±0.15* |
12.64±0.12* |
9.99±0.02* |
12.89±0.23* |
10.68±0.01* |
10.47±0.04* |
|
9 |
2.39±0.15de |
2.50±0.05f |
2.35±0.05cd |
2.20±0.02ab |
2.39±0.05de |
2.22±0.05ab |
|
10 |
2.96±0.05cd |
2.55±0.05a |
2.92±0.02cd |
2.98±0.05d |
3.21±0.10e |
2.77±0.05* |
C – identified phenolic compound; 1 – p-coumaric; 2 – caftaric acid; 3 – caffeic acid; 4 – ferulic acid; 5 – gallic acid; 6 – gentisic acid; 7 – syringic acid; 8 – protocatechuic acid; 9 – trans-resveratrol; 10 – cis-resveratrol.
The results are the averages of the three values obtained from the laboratory determinations and the standard deviation. The superscript letters indicate homogeneous groups, between which there is no statistically significant difference (p>0.05) in correlation with the Fisher LSD test;
* – significant difference compared to all the analysed variants.
The results are in concordance with the literature. Moroșanu et al. (2018) confirmed a significant increase in phenolic compound in white wines when applying the clarifying enzymes combined with a short maceration (24 hours) that allowed the extraction of gallic acid from seeds and stems. Cabrita et al. (2008) highlighted an important effect of pectolytic enzymes on phenolic acids formation. Landrault et al. (2001) reported that gallic and caftaric acids are found in the largest proportion of Sauvignon blanc from vineyards in northeastern Europe. Merkytè et al., (2020) confirmed that phenolic acids are important markers for Sauvignon blanc varieties from different wine regions of Romania, but no paper studied the effect of pectinases and β-glycosides on these compounds in the proposed working conditions.
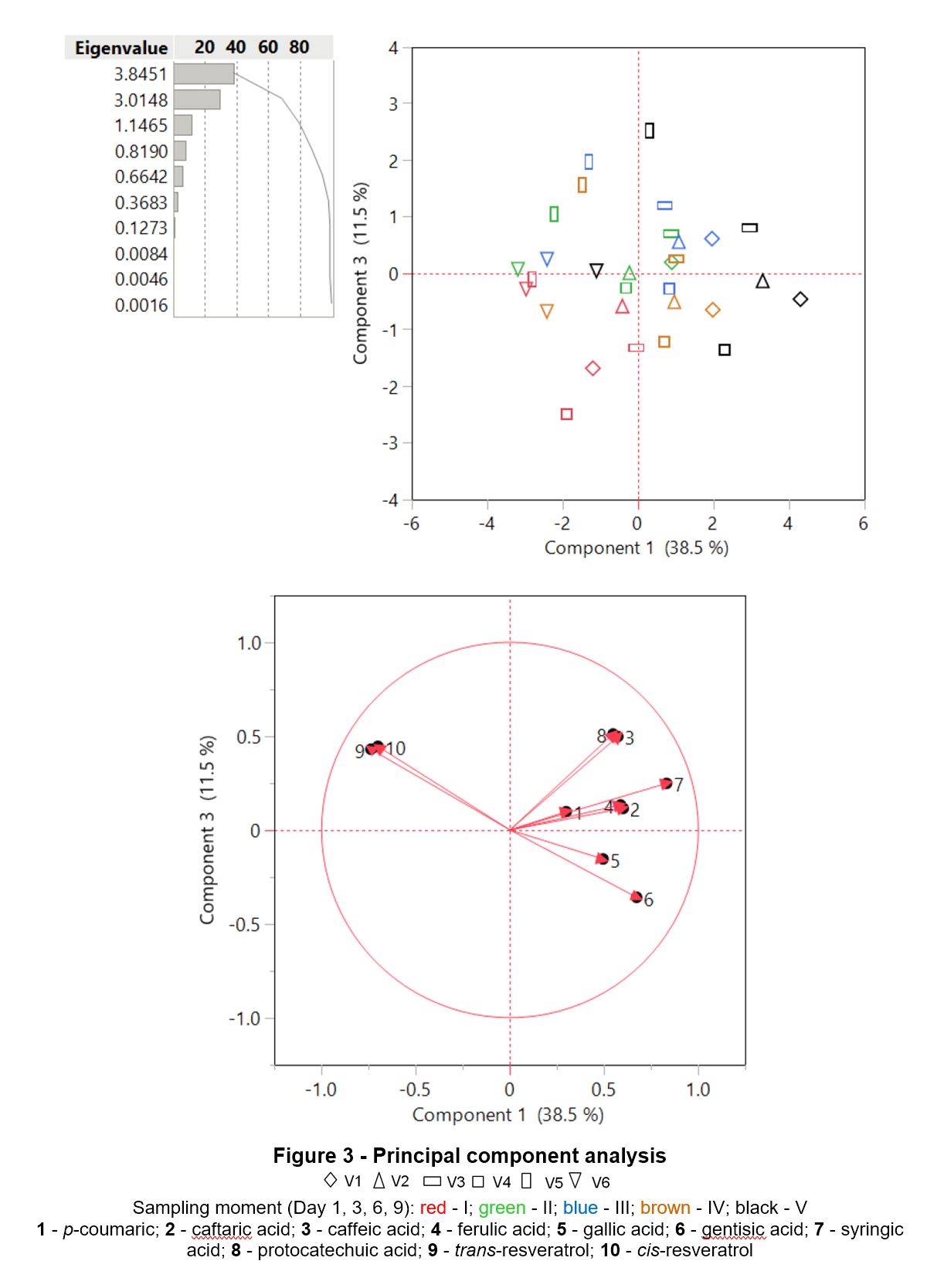
Following the principal component analysis (Fig. 3) corroborated with Pearson test (Table 3), positive correlation was observed between the concentrations of different compounds (variables tendency is to increase when the other increases), such as p-coumaric vs ferulic acid (r = 0.7211), caftaric acid vs ferulic acid (r = 0.9656), caffeic acid vs protocatechuic acid (r = 0.9968), trans-resveratrol vs cis-resveratrol (r = 0.9895), etc. Data suggest that sampling time and administrated treatment manifest a decisive effect on wine phenolic profile The data suggest that the sampling time and the treatment administered show a decisive effect on the phenolic profile of the wine and can be used to optimize the production process in terms of structure and chemical composition.
Table 3
Pearson test correlation
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
|
1 |
1.0000 |
0.5351 |
-0.2194 |
0.7211 |
0.4326 |
0.2201 |
0.2822 |
-0.2166 |
0.1517 |
0.1949 |
|
2 |
0.5351 |
1.0000 |
-0.0373 |
0.9656 |
0.3253 |
0.2844 |
0.6151 |
-0.0716 |
-0.1428 |
-0.1078 |
|
3 |
-0.2194 |
-0.0373 |
1.0000 |
-0.0684 |
-0.0450 |
0.2822 |
0.4335 |
0.9968 |
-0.4766 |
-0.4717 |
|
4 |
0.7211 |
0.9656 |
-0.0684 |
1.0000 |
0.3857 |
0.3202 |
0.5793 |
-0.0973 |
-0.0753 |
-0.0341 |
|
5 |
0.4326 |
0.3253 |
-0.0450 |
0.3857 |
1.0000 |
0.3596 |
0.5581 |
-0.0426 |
-0.1601 |
-0.1130 |
|
6 |
0.2201 |
0.2844 |
0.2822 |
0.3202 |
0.3596 |
1.0000 |
0.3153 |
0.2685 |
-0.5231 |
-0.5047 |
|
7 |
0.2822 |
0.6151 |
0.4335 |
0.5793 |
0.5581 |
0.3153 |
1.0000 |
0.4168 |
-0.4473 |
-0.3956 |
|
8 |
-0.2166 |
-0.0716 |
0.9968 |
-0.0973 |
-0.0426 |
0.2685 |
0.4168 |
1.0000 |
-0.4504 |
-0.4460 |
|
9 |
0.1517 |
-0.1428 |
-0.4766 |
-0.0753 |
-0.1601 |
-0.5231 |
-0.4473 |
-0.4504 |
1.0000 |
0.9895 |
|
10 |
0.1949 |
-0.1078 |
-0.4717 |
-0.0341 |
-0.1130 |
-0.5047 |
-0.3956 |
-0.4460 |
0.9895 |
1.0000 |
1 – p-Coumaric; 2 – Caftaric acid; 3 – Caffeic acid; 4 – Ferulic acid; 5 – Gallic acid; 6 – Gentisic acid; 7 – Syringic acid; 8 – Protocatechuic acid; 9 – Trans-resveratrol; 10 – Cis-resveratrol.
CONCLUSIONS
Enzymes manifest a significant effect in increasing phenolic compounds proportions in wine. The results were depended on the administrated treatment and the sampling moment. Experimental samples were characterised by considerable concentrations in protocatechuic acid, caftaric acid, trans– and cis– resveratrol. The different enzymes generated differential results. The highest content of the majority of identified phenolic compounds was generated by pectinases, while the control sample showed the smallest concentrations.
Enzymatic preparations can give effective results even if they are administered at a different moment of wine production than the one recommended by the producer. Thus, although pectinases are most often studied in order to promote clarification and filtration, they show a considerable effect on the enrichment of the substrate in phenolic acids.
Acknowledgement. This research received no external funding.
REFERENCES
Aroca, A.A., Rollenghem, I., Schneider, R. (2022). Enzymes application in white wine; edited by Morata, A. (2022) – White Wine Technology, Academic Press, https://doi.org/10.1016/B978-0-12-823497-6.00006-5
Bartowski, E.J., Costello, P.J., Villa, A., Henschke, P.A. (2004). The chemical and sensorial effects of lysozyme addition to red and white wines over six months’ cellar storage. Australian Journal of Grape and Wine Research: 10, 143-150, https://doi.org/10.1111/ j.1755-0238.2004.tb00017.x.
Bautista-Ortin, A.B., Jimenez-Pascual, E., Busse-Valverde, N., Lopez-Roca, J.M., Ros-Garcia, J.M, Gomez-Plaza, E. (2011). Effect of wine maceration enzymes on the extraction of grape seed proanthocyanidins. Food Bioprocess Tehnology: 6, 2207-2212, https://doi.org/10.1007/s11947-011-0768-3.
Biraruti, E.I. (2015). Authenticity and quality markers of wines evaluated by advanced analytical techniques. Doctoral thesis, University of Bucharest.
Budić-Leto, I., Lovrić T. (2002). Identification of phenolic acids and changes in their content during fermentation and ageing of white wines Posip and Rukatac. Food Technology and Biotechnology: 40, 221-225.
Cabrita, M., Torres, M., Palma, V., Alves, E., Patão, R., Costa Freitas, A. (2008). Impact of malolactic fermentation on low molecular weight phenolic compounds. Talanta, 74(5), 1281-1286. https://doi.org/10.1016/ j.talanta.2007.08.045.
Cotea, D.V., Zănoagă, C., Cotea, V.V. (2009). Oenochemistry treatise (in Romanian). Romanian Academy Publishing House, Bucharest.
Espejo, F. (2020). Role of commercial enzymes in wine production: a critical review of recent research. Journal of Food Science and Technology, 58(1), 9-21. https://doi.org/10.1007/s13197-020-04489-0.
Fernández-González, M., Úbeda, J.F., Cordero-Otero, R.R., Thanvanthri, G.V., Briones, A.I. (2005). Engineering of an oenological Saccharomyces cerevisiae strain with pectinolytic activity and its effect on wine. International Journal of Food Microbiology: 102, 172-183, https://doi. org/10.1016/j.ijfoodmicro.2004.12.012.
Frankel E.N., Waterhouse A.L., Teissedre P.L. (1995). Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. Journal of Agricultural and Food Chemistry: 43, 890-894.
Haile, S., Ayele, A. (2022). Pectinase from Microorganisms and Its Industrial Applications. The Scientific World Journal, 2022, 1-15. https://doi.org/10. 1155/2022/1881305.
Kammerer, D.R., Carle, R. (2009). Evolution of polyphenols during vinification and wine storage. Functional Plant Science and Biotechnology: 3, 46-59.
Landrault, N., Poucheret, P., Ravel, P., Gasc, F., Cros, G.R., Teissedre, P.-L. (2001). Antioxidant capacities and phenolics levels of french wines from different varieties and vintages. Journal of Agricultural and Food Chemistry: 49, 3341-3348.
Lengyel, E., Silkolya, L. (2014). Authenticity tests of hite wines from the apold depression, management of sustainable development. https://doi.org/10.1515/ msd-2015-0007
Lorrain, B., Ky, I., Pechamat, L., Teissedre, P.L. (2013). Evolution of analysis of polyhenols from grapes, wines, and extracts. Molecules: 18, 1076-1100, https://doi.org/10.3390/ molecules18011076.
Masino, F., Montevecchi, G., Arfelli, G., Antonelli, A. (2008). Evaluation of the combined effects of enzymatic treatment and aging on lees on the aroma of wine from Bombino bianco grapes. Journal of Agricultural and Food Chemistry: 56, 9495-9501, https://doi.org/doi/10.1021/jf8015893
Merkytė, V., Longo, E., Windisch, G., Boselli, E. (2020). Phenolic Compounds as Markers of Wine Quality and Authenticity. Foods: 9, 1785, https://doi.org/10.3390/foods91 21785.
Moreno, J., Peinado, R. (2012). Enological chemistry (2st ed). Editura Academic Press.
Moroșanu, A.M., Luchian, C.E., Niculaua, M., Colibaba, C.L., Tarțian, A.C., Cotea, V.V. (2018). Assessment of major volatile and phenolic compounds from ‘Fetească regală’ wine samples after pre-fermentative treatments using GC-MS Analysis and HPLC analysis. Notulae Botanicae Horti Agrobotanici Cluj-Napoca: 46, 247-259, https://doi. org/10.15835/nbha46110889
Pérez-Navarro, J., Izquierdo-Cañas, P., Mena-Morales, A., Chacón-Vozmediano, J., Martínez-Gascueña J., García-Romero, E., Hermosín-Gutiérrez, I., Gómez-Alonso, S. (2020). Comprehensive chemical and sensory assessment of wines made from white grapes of vitis vinifera cultivars Albillo Dorado and Montonera del Casar: a comparative study with Airén. Foods: 9, 1282, https://doi.org/10.3390/foods 9091282
Rentzsch, M., Wilkens, A., Winterhalter, P. (2009). Non-flavonoid Phenolic Compounds. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, pp. 509–527.
Salameh, D., Brandam, C., Rizk, T., Lteif, R., Strehaiano, P. (2010). Application of a reversed-phase HPLC method for quantitative p-coumaric acid analysis in wine. OENO One: 44, 117, https://doi.org/10.20870/oeno-one.2010.44.2.1465.
Scutarașu, E.C., Luchian, C.E., Vlase, L., Colibaba, L.C., Gheldiu, A.M, Cotea, V.V. (2020). Evolution of phenolic profile of white wines treated with enzymes. Food Chemistry, 340, 127910. https://doi.org/10.1016/j.food chem.2020.127910.
Stavridou, K., Soufleros, E.H., Bouloumpasi, E., Dagkli, V. (2016). The Phenolic Potential of Wines from French Grape Varieties Cabernet Sauvignon, Merlot and Syrah Cultivated in the Region of Thessaloniki (Northern Greece) and Its Evolution during Aging. Food and Nutrition Sciences, 07(02), 122-137. https://doi.org/10.4236/fns.20 16.72014.
Sui, Y., McRae, J., Wollan, D., Muhlack, R., Godden, P., Wilkinson, K. (2020). Use of ultrafiltration and proteolytic enzymes as alternative approaches for protein stabilisation of white wine. Australian Journal of Grape and Wine Research, 27(2), 234-245. https://doi. org/10.1111/ajgw.12475.
Tian, R.R., Pan, Q.H., Zhan, J.C., Li, J.M., Wan, S.B., Zhang, Q.H., & Huang, W.D. (2009). Comparison of phenolic acids and flavan-3-ols during wine fermentation of grapes with different harvest times. Molecules: 14, 827-838. https://doi.org/10.3390/mole cules14020827
Tıraş, Z. E.E., Okur, H.H., Günay, Z., Yıldırım, H.K. (2022). Different approaches to enhance resveratrol content in wine. Ciência e Técnica Vitivinícola, 37(1), 13-28. https://doi. org/10.1051/ctv/ctv20223701013.
Vlase, L., Kiss B., Leucuta, S.E., Gocan, S. (2009). A Rapid Method for Determination of Resveratrol in Wines by HPLC-MS. Journal of Liquid Chromatography & Related Technologies: 32, 2105-2121.
Waterhouse, A.L., Sacks, G.L., Jeffery, D.W. (2016). Understanding wine chemistry (1st ed). Wiley.
Cotea Valeriu V., Luchian Camelia Elena, Scutarașu Andrei, Scutarasu Elena Cristina, Trincă Lucia Carmen