Mansoor Javed, Akbar Ali, Muhammad Kashif, Muhammad Ali, Saif Ullah, Ayesha Alam
ABSTRACT. To find out genetic variability, heritability, and trait association among yield and yield-related traits among bread wheat genotypes, an alpha lattice design was used in triplicate manner where 50 wheat genotypes were evaluated at the University of Agriculture Peshawar during rabbi growing season 2021-22 along with a regional check genotype. Data were taken on ten parameters. All the genotypes showed significant variation among them, signifying the possibility of enhancing genetic improvement through breeding programs. Highly significant differences were found in days to heading, days to maturity, plant height, spike length, grain filling duration, number of grains per spike, biological yield and grain yield these traits indicating diversity in yield potential. Moderate to low heritability values were noted for most of the traits. The study exhibits positive correlations for plant height with grain yield, spike length with biological yield and harvest index with grain yield. On the basis of high heritability and positive correlation of grain yield with other traits, it is recommended that G-41, G-3, G-12, G-37, G-34 and G-14 genotypes which have the potential to be incorporate in further breeding programs.
Keywords: correlation; crop improvement; heritability; quantitative genetics; variability; wheat breeding; wheat genotypes.
Cite
ALSE and ACS Style
Javed, M.; Ali, A.; Kashif, M.; Ali, M.; Rahman, S.U. Estimation of heritability, genotypic variability and correlations analysis for yield and yield attributing traits among bread wheat (Triticum aestivum L.) genotypes. Journal of Applied Life Sciences and Environment 2024, 57 (1), 91-106.
https://doi.org/10.46909/alse-571125
AMA Style
Javed M, Ali A, Kashif M, Ali M, Rahman SU. Estimation of heritability, genotypic variability and correlations analysis for yield and yield attributing traits among bread wheat (Triticum aestivum L.) genotypes. Journal of Applied Life Sciences and Environment. 2024; 57 (1): 91-106.
https://doi.org/10.46909/alse-571125
Chicago/Turabian Style
Javed, Mansoor, Akbar Ali, Muhammad Kashif, Muhammad Ali, Saif Ullah, and Ayesha Alam. 2024. “Estimation of heritability, genotypic variability and correlations analysis for yield and yield attributing traits among bread wheat (Triticum aestivum L.) genotypes” Journal of Applied Life Sciences and Environment 57, no. 1: 91-106.
https://doi.org/10.46909/alse-571125
View full article (HTML)
Estimation of Heritability, Genotypic Variability and Correlations Analysis for Yield and Yield Attributing Traits Among Bread Wheat (Triticum aestivum L.) Genotypes
Mansoor JAVED1*, Akbar ALI2, Muhammad KASHIF1, Muhammad ALI3, Saif ULLAH2 and Ayesha ALAM4
1Department of Agronomy, University of Agriculture Peshawar, Pakistan
2Department of Plant Breeding and Genetics, University of Agriculture Peshawar, Pakistan
3Department of Horticulture, University of Sargodha, Pakistan
4Department of Botany, Government Postgraduate College Dargai, Malakand, Pakistan
*Correspondence: mansoor92@aup.edu.pk
Received: Jan. 29, 2024. Revised: Feb. 16, 2024. Accepted: Feb. 19, 2024. Published online: Mar. 13, 2024
ABSTRACT. To find out genetic variability, heritability, and trait association among yield and yield-related traits among bread wheat genotypes, an alpha lattice design was used in triplicate manner where 50 wheat genotypes were evaluated at the University of Agriculture Peshawar during rabbi growing season 2021-22 along with a regional check genotype. Data were taken on ten parameters. All the genotypes showed significant variation among them, signifying the possibility of enhancing genetic improvement through breeding programs. Highly significant differences were found in days to heading, days to maturity, plant height, spike length, grain filling duration, number of grains per spike, biological yield and grain yield these traits indicating diversity in yield potential. Moderate to low heritability values were noted for most of the traits. The study exhibits positive correlations for plant height with grain yield, spike length with biological yield and harvest index with grain yield. On the basis of high heritability and positive correlation of grain yield with other traits, it is recommended that G-41, G-3, G-12, G-37, G-34 and G-14 genotypes which have the potential to be incorporate in further breeding programs.
Keywords: correlation; crop improvement; heritability; quantitative genetics; variability; wheat breeding; wheat genotypes.
INTRODUCTION
Bread wheat (Triticum aestivum L.), is an important cereal crop with a long history of cultivation dating back thousands of years. Wheat grains has been domesticated by humans approximately 10,000 years B.C during the Neolithic period and remains an important crop globally. Bread wheat is widely consumed cereal and is a staple food in worldwide.
Pakistan occupies 6th position in production of wheat worldwide. Total cultivated area with wheat crop during the 2021–2022 cropping season was 9.2 million hectares and production was recorded 27.5 million tons (PBS, 2022) It contributes 1.8% to the country’s GDP and 9.2% value added in agriculture (Khan et al., 2022).
In Pakistan the population growth rate is 2.4% per year, due to the increase in population a rise is occur in food demand requiring an increase in wheat production to meet the rising demand for food. However, in Pakistan, due to various factors there is a significant gap between the potential yield of wheat (7 metric tons) and the average yield (2.5 metric tons per hectare) (Kirby et al., 2017).
Genetic variability plays an important role to meet the present and future crop breeding challenges such as breeding for increasing yield, wider adaptation, desirable quality, drought tolerance, pest and disease resistance (Begna, 2021). The greater the genetic variation among plants, the higher will be likelihood of achieving productive recombinants and broad heritability during genetic improvement.
Therefore, having precise knowledge of germplasm variability, heritability, genetic advance and genetic relationships among yield component traits is a prerequisite for crop improvement programs. This knowledge can aid in the development of superior recombinants for all desired traits (Tilahun et al., 2020).
Continuous processing of new genetic materials carrying various genes which are essential for enhancing cereal crops yield and productivity. To fulfill the food requirements of increasing population.
Utilization of new genetic resources in genetic improvement programs is essential for increasing wheat crop productivity (Khan et al., 2013). Therefore, most plant breeding programs all over the world have main objective of developing high-yielding wheat varieties as these programs generates unique genetic material that carries high ratio of high-yielding genotypes.
Yield components plays important role for enhancing grain yield of wheat crop because these characteristics have a high genetic correlation with the yield of wheat (Bashir et al., 2010). Wheat traits containing high heritability, making selection in early generations possible. To overcome the yield gap between potential and actual yield, breeders can make new crosses and develop high-yielding varieties for farmers to grow (Reynolds et al., 2011).
To select desirable genotypes and identify independent traits that affect the grain yield, researchers emphasized the use of correlations and path coefficient (Chowdhury et al., 2019). Correlation coefficients are crucial for determining the similarity between different traits and their dependence on grain yield.
However, analyzing correlation coefficients alone is not enough to explain the relationships between various traits, as it cannot determine the direct and indirect effects of independent variables on dependent variables. Therefore, path coefficient analysis tools may also be utilized to study the impact of each independent variable on the dependent variable (Alogaidi, 2018).
In light of the significance of these analytical tools, the current study was conducted to evaluate the variability of genotypes and to perform correlation analysis of yield attributing traits in bread wheat genotypes.
MATERIALS AND METHODS
Total 50 genotypes were obtained from CIMMYT (International Wheat and Maize Improvement Center), including a regional check genotype (PS-2015) listed in (Table 1).
The experiment was conducted at The University of Agriculture Peshawar, having longitude 34.026°N, latitude 71.4814°E with altitude 359 m /1178 feet above sea level.
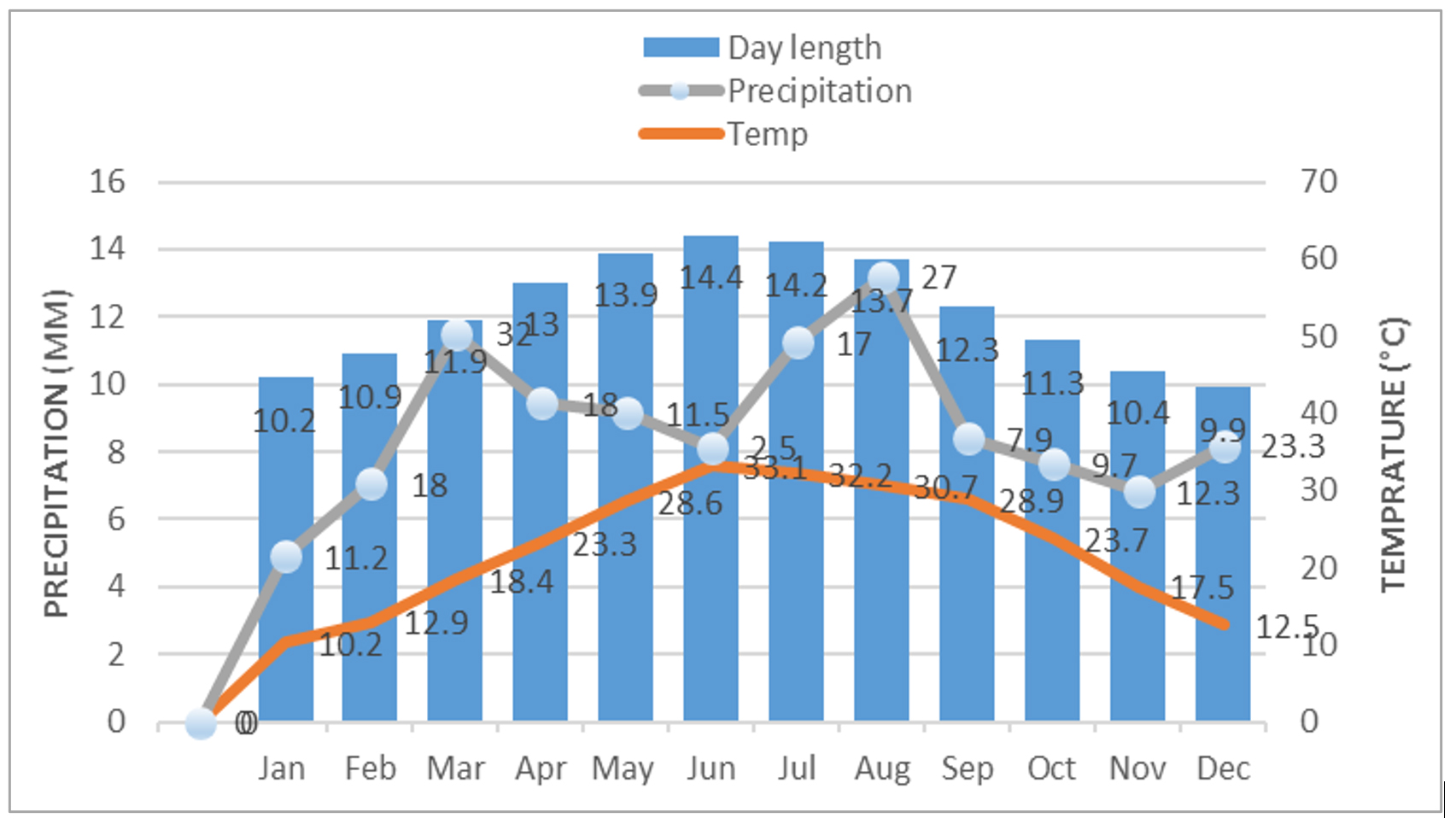
Annual rainfall, average temperature and day length of the area are presented in Figure 1.
Experiment was conducted using an alpha lattice design having three replications which were further divided into five sub-blocks and ten genotypes were allocated to each sub-block. Each genotype was sown in four rows, with 5 meters in length and 25 cm gap between each row. Sowing was done in November 2021.
Sampling techniques were used for recording plant height (cm), dpike length, grain filling duration, number of grains per spike, 1000-grain weight (g), biological yield, grain yield and harvest index (%) while days to heading and days to maturity were recorded on plot basis. Seed bed preparation was done by mechanical methods. Seeds sowing was done manually.
Irrigation was applied as per the crop water demand through the flood irrigation system. Fertilizers (DAP and Urea) were applied at the time of seedbed preparation.
Statistical Analysis
The data collected for various parameters underwent an analysis of variance (ANOVA) procedure, utilizing a suitable model for an alpha lattice design. This approach was similar to the one described by (Barreto et al., 1997). Least significant test was also performed to check significance among different genotypes of wheat.
The computation of genotypic ( g) and phenotypic ( p) correlations between yield and yield attributing components involved the utilization of the formula proposed by (Singh and Chaudhary, 1985).
These correlations were derived from the analysis of phenotypic, genotypic and environmental covariance.
Heritability
Heritability in broad sense (h2) was estimated by using formula suggested by (Allard, 1960) (Equation 1).
h2BS = (δ2g)/δ2p (1)
where δ2g = genetic variance for a specific trait; δ2p = phenotypic variance for a specific trait; h2BS = heritability in broad sense for specific trait
Correlation
The genetic correlation ( g) and phenotypic correlation ( p) between two characters, X1 and X2, was estimated according to Kwon and Torrie (1964).
Table 1
List of genotypes used in the experiment
| S.No | Genotype | Name | Origin |
| 1 | G-1 | PerSabaq 2015 | PERSABAQ 2015 |
| 2 | G-2 | FITIS | MX11617 |
| 3 | G-3 | MUNAL *2/WESTONIA | MX11617 |
| 4 | G-4 | 2*WBLLI*2/KKTS/SHORTENED | MX11617 |
| 5 | G-5 | ATTILA/PSN/SERI/3/MILAN/4/BOW | MX11617 |
| 6 | G-6 | ATTILA*2/MURGA/PBW65*2 | M38ES25SA16 |
| 7 | G-7 | FRANCOLIN#1/YANAC | M38ES25SA16 |
| 8 | G-8 | SHER6/PBW343*2 | M38ES25SA16 |
| 9 | G-9 | WHEAR/2*PRL/WAXBI/4/COPOI | M38ES25SA16 |
| 10 | G-10 | WBLLI*2/OTUS/TUKURU/WBLLI*2 | MX11617 |
| 11 | G-11 | WBLLI*2/4/PBW65/YACO | M38ES25SA16 |
| 12 | G-12 | F2001/BRAMBLING/PVN | M38ES25SA16 |
| 13 | G-13 | F2001/KIRITATI/C80 1/3* BATAVIA | M38ES25SA16 |
| 14 | G-14 | WBLLI*2/KKTS/PASTOR/KUKUNA | MX11617 |
| 15 | G-15 | ROLFO07/YANAC/BRAMBLING*2 | M38ES25SA16 |
| 16 | G-16 | KIRITATI/TURKU | M38ES25SA16 |
| 17 | G-17 | FRET2*2/SHAMA/KUKUNA*2 | MX11617 |
| 18 | G-18 | TRCH/SRTU/MILAN | M38ES25SA16 |
| 19 | G-19 | TRCH/SRTU | MX11617 |
| 20 | G-20 | PBW343*2/KUKUNA/KHVAKI | M38ES25SA16 |
| 21 | G-21 | UP2338*2/SHAMA | M38ES25SA16 |
| 22 | G-22 | SAUAL/YANAC/SAUL*2 | M38ES25SA16 |
| 23 | G-23 | TACUETO F2001/SAUAL | MX11617 |
| 24 | G-24 | KFA/S/REH/HARE/2* BCN/PGO | MX11617 |
| 25 | G-25 | SAUAL/MUTUS/KUKUNA/WBLL*2 | M38ES25SA16 |
| 26 | G-26 | SITE/MO/PASTOR/WAXWING/KIRI | MX11617 |
| 27 | G-27 | BOROL14/KFA/2*KACHU/2*KACHU | M38ES25SA16 |
| 28 | G-28 | TILILA/TURK/BOW/4/SERI.1B*2 | MX11617 |
| 29 | G-29 | WAXWING/2*ROLF07/BORL14 | MX11617 |
| 30 | G-30 | BECARD/FRNCLN/KACHU*1 | M38ES25SA16 |
| 31 | G-31 | CHEWINK/CHYAK/5/UP2338*2/ | MX11617 |
| 32 | G-32 | CHEWINK#1/CHYAK/S/UP2338*2/ | M38ES25SA16 |
| 33 | G-33 | MUU/KBIRD/3/PRL/2*PASTOR 2 | MX11617 |
| 34 | G-34 | BECARD/ND643/ATTILA*2/PASTOR | MX11617 |
| 35 | G-35 | 2*ATTILA 2PASTOR/3/WBLL1*2/ | MX11617 |
| 36 | G-36 | FRET2/TUKURU/FRET2/MUNAL#1/ | MX11617 |
| 37 | G-37 | /4/BAV92*2/S/HAR311/6/PASTOR | MX11617 |
| 38 | G-38 | KIRITATI*2/3/C801/3*BATAVIA/4/ | M38ES25SA16 |
| 39 | G-39 | 2*BAV92/MUNAL#1//5/KIRITATI/4 | M38ES25SA16 |
| 40 | G-40 | /WBLL1*2/3/COPIO/BRAMBUNG*2/ | M38ES25SA16 |
| 41 | G-41 | PBW343*2/FRANCOLI | MX11617 |
| 42 | G-42 | /NADI/3/PBW343*2/KUKUNA *2/ | M38ES25SA16 |
| 43 | G-43 | NADI/COPIO/NADI/CMSS 11B00944T | MX11617 |
| 44 | G-44 | WBLL1*2/KUKURI//HEILO/3/ | M38ES25SA16 |
| 45 | G-45 | YAV3/SCO/JO69/CRA/3/YAV 79/4/AE | MX11617 |
| 46 | G-46 | NIGHAR/BABAX//3/ER 2000/4/ | M38ES25SA16 |
| 47 | G-47 | PASTOR//2 *BAU/4/PANDION//FLIN/ | MX11617 |
| 48 | G-48 | MEX94.27.1.20/3/3*BCN/5/ | MX11617 |
| 49 | G-49 | 8/BOW/VEE/5/ND/KAL/BB/3/YACO | MX11617 |
| 50 | G-50 | 5*BORL95/CNDO/R143/ENTE/MEX1 | MX11617 |
Genetic correlation
(Equation 2)
(2)
where CovG(X1X2) = Genetic covariance among trait X1 and X2; VG(X1) and VG(X2) = Genetic variance for trait X1 and X2, respectively.
Phenotypic correlation
(Equation 3)
(3)
where COVP (X1X2) = phenotypic covariance among traits X1 and X2; VP(X1) and VP(X2) = phenotypic variance for traits X1 and X2, respectively.
RESULTS AND DISCUSSION
Days to heading and maturity
Days to heading and maturity are important agronomic traits that determine the adaptability of wheat cultivars to different environments. Different wheat genotypes exhibited significant differences for days to heading and maturity (Table 2). The data indicated that the number of days to heading ranged from 103 to 110 days, with an average of 105 days. Among the genotypes, genotype G-30 exhibited the minimum number of days to heading (103), while maximum number of days to heading (110) was noted for genotype G-50. The mean duration for days to maturity ranged from 143 to 147 days, with an average of 145.24 days. G-38 exhibited the minimum days to maturity (143 days), while G-11, G-21, and G-35 recorded the maximum days to maturity (147 days) (Table 3). The observed significant variation among days to heading and maturity suggested that such cultivars of wheat genotypes with different heading and maturity times can be developed by breeders which are suitable for specific environments.
At genotypic level days to heading exhibited a significant positive correlation with plant height (rg=0.398) and grain yield (rg=0.37) as indicated in (Table 4). However, there was a negative correlation observed between days to heading and grain filling duration (rg=-0.87) at the genotypic level. Similarly, at the phenotypic level, days to heading displayed a significant negative correlation with grain filling duration (rp=-0.79). Similarly, at genotypic level, days to maturity displayed a significant positive correlation with grain filling duration (rg=0.59) and grains spike-1 (rg=0.589), while exhibiting a negative correlation with thousand grain weight (rg=-0.49). Baye et al., (2020) also reported a positive correlation between days to heading with plant height and grain yield. Days to heading and grain filling duration at both genotypic and phenotypic levels showed negative correlation suggested that early-heading genotypes have shorter grain filling durations, which may negatively affect grain yield of wheat genotypes (Slafer et al., 2015).
The moderate broad sense heritability estimated (Table 5) for days to heading (0.46) indicated that a significant proportion of the phenotypic variation is genetically controlled and can be used for the selection of superior genotypes (Falconer and Mackay, 1996). On the other hand, the low heritability estimated for days to maturity (0.21) indicated that environmental factors played a major role in controlling this trait, and genetic improvement may be limited (Talebi and Fayyaz, 2012).
Plant height
Plant height is important trait to avoid lodging in wheat genotypes, which is a major yield-limiting factor in wheat production. A thorough analysis of variance indicated noteworthy variations (P≤0.01) in plant height among different wheat genotypes (Table 2). The observed plant heights ranged from 82.7 cm to 104.9 cm. Genotype G-41 exhibited shorter plants (82.8 cm), while genotype G-43 had taller plants (105.0 cm) (Table 3).
The observed significant variation for plant height among the studied genotypes suggested that there is potential for development of such cultivars with different plant height which are suitable for different environments. Our results are in line with (Khan et al., 2010) who also reported considerable disparity among wheat genotypes for plant height.
Genotypic and phenotypic correlation result showed that at the genotypic level, plant height exhibited significant positive correlations with spike length (rg = 0.52), biological yield (rg = 0.74), grain yield (rg = 0.49) and days to heading (rg = 0.39).
Plant height displayed a significant negative relationship with grain filling duration (rg = -0.32) (Table 4). Phenotypically, plant height demonstrated a significant positive correlation with spike length (rp = 0.34) and biological yield (rp = 0.35).
The observed positive correlation between plant height and grain yield at both genotypic and phenotypic levels is similar with previous findings of (Githinji, 2016) who reported a positive interaction between plant height and grain yield.
Table 2
ANOVA Table for various genotypes of bread wheat
| DH | DM | PH | GF | SL | |
| Replication | 18.621 | 2.541 | 73.7868 | 24.09 | 5.28668 |
| Genotypes | 4.115** | 2.557* | 68.459** | 7.2699** | 1.118** |
| Error | 1.19216 | 1.450274 | 7.7937 | 2.647436 | 0.422544 |
| Total df 149 | |||||
| GS | TGW | BY | GY | HI | |
| Replication | 39.5001 | 19.3401 | 17648030.3 | 2303344.87 | 47.141601 |
| Genotypes | 58.462** | 70.018** | 6065583.5* | 994330.99** | 23.593307** |
| Error | 13.815976 | 10.47835 | 3590340.8 | 443114.9 | 12.52796 |
| Total df 149 |
*, ** = Significant at 0.05 and 0.01 probability level, respectively.
DY=Days to heading, DM=Days to maturity, PM=Plant height, GF=Grain filling, GS=Grains spike-1,
SL=Spike length, TGW=Thousand grain weight, BY= Biological yield, GY=Grain yield, HI= Harvest index
Table 3
Average performance of wheat genotypes for various traits in bread wheat
| Geno-types | DH | DM | PH | GF | GS | SL | TGW | BY | GY | HI |
| G-1* | 105 | 145 | 90.6 | 41 | 49 | 10.3 | 36.34 | 17239 | 4918 | 29 |
| G-2 | 106 | 146 | 98.9 | 40 | 53 | 10.7 | 37.48 | 19874 | 4858 | 25 |
| G-3 | 106 | 144 | 96 | 39 | 40 | 10.8 | 42.67 | 18232 | 5099 | 28 |
| G-4 | 106 | 146 | 91.8 | 42 | 44 | 10 | 40.51 | 18791 | 5324 | 29 |
| G-5 | 105 | 146 | 97.4 | 42 | 41 | 10.2 | 41. 05 | 20523 | 5121 | 26 |
| G-6 | 106 | 144 | 96 | 39 | 49 | 10.6 | 36.19 | 17988 | 4388 | 25 |
| G-7 | 104 | 146 | 90.8 | 42 | 48 | 10 | 36.99 | 18368 | 5353 | 30 |
| G-8 | 109 | 145 | 103.6 | 37 | 46 | 9.9 | 40. 07 | 19931 | 5384 | 28 |
| G-9 | 107 | 144 | 103.3 | 39 | 47 | 10.2 | 40. 05 | 18074 | 4607 | 26 |
| G-10 | 105 | 146 | 85.3 | 42 | 50 | 9.2 | 38.71 | 16244 | 3703 | 23 |
| G-11 | 106 | 147 | 90.7 | 42 | 44 | 9.7 | 35.39 | 15182 | 3662 | 25 |
| G-12 | 105 | 146 | 94 | 43 | 58 | 9.5 | 33.8 | 18065 | 4284 | 24 |
| G-13 | 104 | 144 | 98.1 | 41 | 49 | 10.3 | 40.47 | 16988 | 3732 | 22 |
| G-14 | 105 | 145 | 89.8 | 40 | 49 | 9.6 | 34.55 | 20560 | 5517 | 27 |
| G-15 | 105 | 146 | 92.7 | 42 | 47 | 10.2 | 37.27 | 16074 | 4470 | 28 |
| G-16 | 106 | 144 | 93.5 | 40 | 41 | 10.3 | 43. 07 | 17204 | 3573 | 22 |
| G-17 | 105 | 145 | 95 | 41 | 47 | 9.9 | 39.25 | 18232 | 4578 | 26 |
| G-18 | 107 | 146 | 93.3 | 39 | 57 | 10.4 | 36.48 | 19313 | 5110 | 27 |
| G-19 | 106 | 145 | 94.8 | 40 | 51 | 9.5 | 39.91 | 17688 | 5435 | 31 |
| G-20 | 105 | 146 | 94.7 | 43 | 50 | 9.5 | 38.94 | 18949 | 5254 | 28 |
| G-21 | 106 | 147 | 92.7 | 41 | 52 | 10.3 | 37.81 | 18873 | 5502 | 30 |
| G-22 | 106 | 144 | 93.9 | 40 | 42 | 10 | 32.77 | 16860 | 4224 | 25 |
| G-23 | 104 | 144 | 91.6 | 40 | 41 | 9.6 | 51.1 | 17231 | 4346 | 26 |
| G-24 | 107 | 145 | 99.6 | 39 | 53 | 8.9 | 37.24 | 19255 | 5186 | 28 |
| G-25 | 105 | 145 | 91.2 | 40 | 49 | 8.6 | 49.22 | 16955 | 4230 | 25 |
| G-26 | 106 | 144 | 93.9 | 39 | 42 | 10.2 | 46.22 | 18654 | 5352 | 29 |
| G-27 | 106 | 144 | 93.4 | 38 | 48 | 8.6 | 40.25 | 15375 | 4906 | 33 |
| G-28 | 107 | 144 | 92 | 39 | 50 | 9.0 | 49.32 | 15945 | 4343 | 28 |
| G-29 | 104 | 146 | 86.8 | 43 | 45 | 8.3 | 40.99 | 17946 | 4534 | 26 |
| G-30 | 103 | 146 | 85.7 | 43 | 46 | 10.1 | 42.25 | 17531 | 4495 | 26 |
| G-31 | 105 | 145 | 94 | 41 | 52 | 9.8 | 42.62 | 16350 | 3991 | 25 |
| G-32 | 104 | 145 | 96.2 | 41 | 53 | 10.4 | 45.11 | 16555 | 4457 | 27 |
| G-33 | 106 | 145 | 92.8 | 40 | 39 | 9.8 | 39.6 | 17045 | 4896 | 29 |
| G-34 | 107 | 146 | 90.8 | 40 | 52 | 9.7 | 40. 0 | 20507 | 3993 | 21 |
| G-35 | 104 | 147 | 101.1 | 39 | 56 | 10.0 | 38.28 | 18369 | 3389 | 19 |
| G-36 | 106 | 145 | 92.4 | 40 | 44 | 9.0 | 42.49 | 18568 | 5068 | 28 |
| G-37 | 104 | 145 | 90.9 | 42 | 45 | 9.9 | 52. 97 | 1892 | 4678 | 25 |
| G-38 | 105 | 143 | 86.9 | 39 | 46 | 9.2 | 35.51 | 17178 | 4483 | 27 |
| G-39 | 105 | 145 | 83.2 | 41 | 40 | 8.5 | 36.82 | 14874 | 4683 | 32 |
| G-40 | 106 | 146 | 85.8 | 41 | 47 | 9.5 | 34.41 | 18993 | 3625 | 20 |
| G-41 | 104 | 146 | 82.8 | 41 | 43 | 8.7 | 37.92 | 15750 | 3448 | 22 |
| G-42 | 105 | 146 | 90.0 | 42 | 48 | 7.9 | 36.41 | 15726 | 4207 | 28 |
| G-43 | 106 | 144 | 105.0 | 39 | 48 | 10.3 | 51.30 | 18884 | 5254 | 29 |
| G-44 | 107 | 146 | 94.7 | 40 | 47 | 9.4 | 30.63 | 17313 | 4056 | 24 |
| G-45 | 104 | 146 | 91.7 | 42 | 49 | 9.1 | 40. 04 | 17221 | 3592 | 21 |
| G-46 | 105 | 144 | 100.6 | 38 | 47 | 9.2 | 39.52 | 20269 | 4875 | 25 |
| G-47 | 106 | 146 | 95.4 | 38 | 46 | 8.6 | 41.67 | 16030 | 4493 | 28 |
| G-48 | 106 | 146 | 98.9 | 41 | 47 | 9.1 | 46.19 | 17383 | 4607 | 27 |
| G-49 | 105 | 146 | 101.6 | 42 | 53 | 9.3 | 43.49 | 19883 | 5309 | 27 |
| G-50 | 110 | 145 | 94.5 | 38 | 40 | 9.4 | 29.18 | 19792 | 5124 | 26 |
| Means | 105 | 145.24 | 93.57 | 40.42 | 47.4 | 9.62 | 39.61 | 17875 | 4595 | 26 |
| LSD (0.05) | 1.7722 | 1.9547 | 4.53 | 2.641 | 6.033 | 1.06 | 5.254 | 3075.6 | 1080.50 | 5.745 |
DY = Days to heading, DM = Days to maturity, PM = Plant height, GF = Grain filling, GS = Grains spike-1, SL = Spike length, TGW = Thousand grain weight, BY = Biological yield, GY = Grain yield, HI = Harvest index
*check PS. 2015
Table 4
Phenotypic (rP) and Genotypic (rG) correlation coefficients for various traits in wheat
| TS | DH | DM | GF | PH | SL | GS | TGW | BY | GY | HI |
| DH | – | -0.111 | -0.87** | 0.398* | -0.128 | -0.11 | -0.28 | 0.239 | 0.37* | 0.259 |
| DM | -0.04 | – | 0.59** | -0.286 | -0.129 | 0.589** | -0.49** | 0.11 | -0.29 | -0.36 |
| GF | -0.79** | 0.67** | – | -0.6** | 0.038 | 0.38* | -0.039 | -0.19 | -0.44 | -0.40 |
| PH | 0.28 | -0.18 | -0.32 | – | 0.52** | 0.312 | 0.18 | 0.74** | 0.49* | 0.056 |
| SL | 0.04 | -0.06 | -0.07 | 0.34* | – | 0.115 | 0.162 | 0.35* | 0.175 | -0.099 |
| GS | -0.09 | 0.109 | 0.14 | 0.17 | 0.034 | – | -0.09 | 0.37* | -0.012 | -0.23 |
| TGW | -0.18 | -0.09 | 0.08 | 0.09 | 0.00 | -0.11 | – | -0.013 | 0.068 | 0.064 |
| BY | 0.005 | -0.09 | 0.02 | 0.35* | 0.45* | 0.13 | -0.05 | – | 0.662** | 0.08 |
| GY | 0.04 | -0.10 | -0.09 | 0.23 | 0.24 | 0.002 | 0.13 | 0.44** | – | 0.788** |
| HI | 0.046 | -0.11 | -0.11 | -0.03 | -0.10 | -0.09 | 0.17 | 0.26 | 0.75** | – |
*, ** = significant at 0.05 and 0.01 probability level, respectively. TS = Traits, DY = Days to heading, DM = Days to maturity, PM = Plant height, GF = Grain filling, GS = Grains spike-1, SL = Spike length, TGW = Thousand grain weight, BY = Biological yield, GY = Grain yield, HI = Harvest index
However, (Slafer et al., 2015) noted negative correlation between plant height and grain filling duration which indicated that taller plants have shorter grain filling durations, which may negatively affect grain yield. Genetic variance (Vg = 20.23) for plant height among the wheat genotypes exceeded environmental variance (Ve = 7.79), resulting in a high heritability estimate (0.73) for plant height (Table 5). The high heritability estimated for plant height (0.73) suggested that genetic improvement of this trait can be effective (Falconer and Mackay, 1996).
Spike length
Spike length plays an important role in yield determination of wheat. Analysis of variance showed significant differences (P≤0.01) in spike length among the various wheat genotypes (Table 2). The recorded spike length data ranged from 7.9 cm to 10.8 cm. G-42 produced shorter spikes measuring 7.9 cm, while G-3 exhibited longer spikes measuring 10.8 cm (Table 3). Like Thorne (1965) who also discovered a lot of variation among wheat genotypes. The observed considerable disparity for this trait among the wheat genotypes suggested the potential for genetic improvement.
There was a significant positive genetic correlation between spike length and plant height (rg = 0.52) as well as biological yield (rg = 0.45), while a phenotypic correlation was observed between spike length and plant height (rp = 0.34) and biological yield (rp = 0.35) (Table 4). The observed positive correlation between spike length and biological yield at both genotypic and phenotypic levels is correlated with previous finding of (Rao et al., 2022) who reported a positive correlation between these two traits. The genetic variance for spike length in wheat genotypes was relatively lower in magnitude compared to the environmental variance (Vg = 0.24, Ve = 0.422). Consequently, the broad sense heritability for spike length was low, amounting to 0.36 (Table 5).
The low heritability estimated for spike length (0.36) indicated that this trait is dominantly under the control of environmental factors. And genetic improvement may be limited (Talebi and Fayyaz, 2012).
Grain filling duration
Grain filling duration plays a crucial role in maximizing bread wheat production as it directly influences grain size and weight. The duration of grain filling showed statistically significant variations (P≤0.01) among different wheat genotypes, as presented in (Table 2). The data collected for grain filling duration ranged from 37 to 43 days. Among the genotypes, G-8 exhibited the shortest duration of 37 days, while G-12, G-20, G-29, and G-30 had the longest duration of 43 days (Table 3). The considerable disparity among wheat genotypes for grain filling duration suggested for genetic improvement. Considerable disparity for grain filling duration among wheat genotypes were also observed by (Bhushan et al., 2013; Malbhage et al., 2020).
Table 5
Heritability, genotypic and phenotypic variance estimates for various traits of bread wheat
| Traits | Vg | Ve | Vp | h²(BS) |
| Days to heading | 0.98 | 1.193 | 2.18 | 0.46 |
| Days to maturity | 0.38 | 1.451 | 1.83 | 0.21 |
| Grain filling duration | 1.55 | 2.648 | 4.20 | 0.38 |
| Plant height | 20.23 | 7.7937 | 28.03 | 0.73 |
| Spike length | 0.24 | 0.4226 | 0.66 | 0.36 |
| Grains spike-1 | 14.89 | 13.816 | 28.71 | 0.53 |
|
1000 grain Wt. Biological yield |
19.86 825080.91 |
10.479 3590340.8 |
30.33 4415421.7 |
0.66 0.20 |
| Grain yield | 183738.73 | 443114.9 | 626853.53 | 0.30 |
| Harvest index% | 3.70 | 12.527955 | 16.23 | 0.24 |
Grain filling duration demonstrated significant positive genotypic and phenotypic correlations with days to maturity (rg= 0.59, rp= 0.67) and a negative genotypic correlation with days to heading (rg=-0.87), as shown in (Table 4). The observed positive correlation between grain filling duration and days to maturity suggested that genotypes with a longer grain filling period allowed for extended grain development and filling, leading to potentially higher grain yields. This relationship could be attributed to the availability of a longer time for resource accumulation and allocation to develop grains, resulting in increased grain weight and yield. The genetic variance for grain filling duration among wheat genotypes was relatively lower in magnitude compared to the environmental variance (Vg = 1.55, Ve = 2.648), resulting in a heritability of 0.38, indicating a moderate heritable component for grain filling duration. However, the low heritability estimates for grain filling duration (0.38) mentioned in (Table 5) indicated that environmental factors play a dominant role in controlling this trait, and genetic improvement may be limited (Farooq et al., 2014).
Grains spike−1
Grains spike−1 is different in wheat genotypes. Statistical analysis of data showed considerable disparity (P≤0.01) among wheat genotypes regarding grains spike-1 (Table 2). The number of grains spike-1 ranged from 39 to 58, with G-33 producing the least (39) and G-12 producing the most (58) grains spike-1 (Table 3). The significant difference observed among wheat genotypes for grains spike-1 suggested genetic variations that influence this trait. Considerable disparity among wheat genotypes for grain-related traits was also reported by (Fan et al., 2020; Yang et al., 2020). The variation in grains spike-1 could be attributed to various factors, including differences in genetic backgrounds, breeding programs, and environmental conditions during plant growth and development.
Correlation analysis results revealed significant positive correlations between grains spike-1 and days to maturity (rg=0.589), grain filling duration (rg=0.38) at the genotypic level and a positive correlation with biological yield (rp=0.37) at phenotypic level as shown in (Table 4). The positive correlation with days to maturity suggests that genotypes producing more grains spike-1 tend to have a longer maturation period. (Ullah et al., 2018) also observed that longer maturation periods provide more time for grain development and filling. The positive correlation with biological yield suggests that genotypes with higher grain production also exhibit greater overall biomass production. This relationship indicates the importance of a well-developed plant structure and resource allocation for achieving higher grain yield. The broad sense heritability for grains spike-1 (Table 5) was estimated to be 0.53. A heritability value of 0.53 indicated that genetic factors contributed moderately to the observed variation in grains spike-1. Bhanu et al. (2018) also reported similar heritability estimates for other wheat traits, such as grain yield. These findings imply that selection based on grains spike-1 can be effective in improving the trait in future breeding programs.
Thousand grain weight
An investigation into the thousand grain weight among various wheat genotypes demonstrated significant variations (P≤0.01) as determined by the analysis of variance (Table 2). The mean values for the thousand grain weight ranged from 29.18 to 52.97 g. G-50 exhibited the lowest recorded thousand grain weight (29.18 g), while G-37 had the highest (52.97 g) (Table 3). Previous finding of (Arya et al., 2017; Kumar et al., 2017; Mecha et al., 2016, 2017) also reported considerable disparity among wheat genotypes in terms of thousand grain weight.
Correlation analysis indicated a genetic association between thousand grain weight and days to maturity (rg=-0.49) (Table 4). These results contradicted the conclusions of Rajput, (2018) and Savadi et al., (2017) who reported a significant correlation between thousand grain weight and grain yield.
The genetic variance for the thousand grain weight of the wheat genotypes (Vg = 19.86) exceeded the environmental variance (Ve = 10.47). Consequently, the broad sense heritability for the thousand grain weight was determined to be moderate (0.66) (Table 5). Similar findings were documented by (Preeti et al., 2018; Ullah et al., 2018).
Biological yield
Biological yield among different wheat genotypes showed considerable disparity among wheat genotypes (Table 2). This finding is also consistent with previous studies that have reported significant genotype differences in biological yield (Avinashe et al., 2015; Singh et al., 2019). The data ranged for biological yield 14,874 kg.hac-1 to 20,560 kg.hac-1. The lowest biological yield of 14874 kg ha-1 was observed in G-39, while the highest yield of 20560 kg ha-1 was recorded in G-14 (Table 3). Dabi et al., (2016) also reported similar ranges of biological yield in other wheat studies which suggested that the observed variation is not unique to this particular study but reflects the inherent genetic variability in wheat populations.
Regarding genotype associations, biological yield exhibited significant genotypic correlations with plant height (rg = 0.74), grain yield (rg = 0.44), spike length (rg = 0.35), and grains per spike (0.37) (Table 4).
Similarly, phenotypically, biological yield showed correlations with plant height (rp = 0.35), spike length (rp 0.45), and grain yield (rp = 0.662) (Table 4). The positive genotypic correlation between biological yield and plant height, grain yield, spike length, and grains per spike suggested that these traits contribute to higher yield potential. Similar trait correlations have been reported in previous studies by Avinashe et al., (2015) supporting the robustness of these associations.
The genetic variance recorded for biological yield in wheat genotypes was lower than the environmental variance, with Vg = 825080 and Ve = 3590340. Singh et al., (2019) also reported higher environmental variance than genetic variance for yield-related traits in wheat. Consequently, the broad-sense heritability for biological yield was calculated to be low at 0.20 (Table 5). This implies that environmental factors, plays a significant role in influencing biological yield. It also highlights the importance of breeding for stress tolerance and resilience to maximize yield potential under varying environmental conditions. The low broad sense heritability value of 0.20 indicated that genetic factors explain only a small proportion of the observed variation in biological yield. This study makes an agreement with the previous studies that have reported low heritability values for yield-related traits in wheat (Singh et al., 2019; Jamil et al., 2017).
Grain yield
Grain yield revealed significant variation among wheat genotypes (Table 2). The data range from 3448 to 5517 kg ha-1 for grain yield. Lowest grain yield 34478kg ha-1 was reported for G-41, while highest yield of 5517 kg ha-1 was reported for G-14 (Table 3), suggested that there are substantial differences in productivity among the tested genotypes. This finding is consistent with previous studies that have demonstrated genetic variability in grain yield among wheat varieties (Imadud et al., 2018).
Correlation study revealed several significant relationships between grain yield and other agronomic traits (Table 4). Genotypic correlations (rg) indicated a strong positive association between grain yield and harvest index (rg = 0.75) and biological yield (rg = 0.662) and plant height (0.49) (Table 4), these findings suggested that genotypes with higher harvest index, plant height and biological yield tend to exhibit higher grain yields. This is consistent with previous research demonstrating the importance of these traits in determining grain yield potential (Fellahi et al., 2013). Phenotypic correlations (rp) also showed significant associations between grain yield and harvest index (rp = 0.788) as well as biological yield (rp = 0.44). These correlations indicated that phenotypic performance in terms of harvest index and biological yield can be reliable indicators of grain yield potential. However, it is important to note that phenotypic correlations can be influenced by environmental factors, whereas genotypic correlations provide a more direct measure of the underlying genetic relationships.
The genetic variance for grain yield (Vg = 183738.73) was smaller than the environmental variance (Ve = 443114.9), suggesting that environmental factors have a greater influence on grain yield than genetic factors. The estimated broad-sense heritability for grain yield was relatively low 0.30 (Table 5), implies that approximately 29% of the observed variation in grain yield can be attributed to genetic differences, while the remaining variation is influenced by environmental factors and their interactions. This suggests that improving grain yield through traditional breeding approaches may be challenging due to the relatively low heritability (Savadi et al., 2017; Wang et al., 2018).
Harvest index
Considerable disparity in harvest index was observed among various wheat genotypes (P≤0.01), as shown in (Table 2). The range of harvest index data varied from 20% to 32%, with an average of 26% (Table 3). Among the genotypes, G-34 had the lowest harvest index of 21%, while G-27 exhibited the highest harvest index of 33%. A correlation analysis revealed a significant correlation between harvest index and grain yield at both the phenotypic and genotypic levels (rp = 0.788, rg = 0.75) (Table 4). In a study conducted by Dabi et al., (2016) was also reported that there was a strong positive correlation between harvest index and grain yield, which holds true at both the genotypic and phenotypic levels. The genetic variance (Vg = 3.70) for harvest index in wheat genotypes was relatively lower compared to the environmental variance (Ve = 12.52). The broad sense heritability for harvest index was determined to be low at 0.24 (Table 5).
CONCLUSIONS
One of the main goal of wheat breeding programs is to develop high-yielding superior lines. The current study’s findings indicated that there was considerable disparity among genotypes for most of the parameters studied. This suggests that there is ample opportunity for effective selection in future breeding programs. For certain traits such as plant height, there was high estimates of broad sense heritability (0.73), indicating that early generation selection would be effective in improving these traits. At the phenotypic level, grain yield showed significant correlation with days to heading, plant height, and biological yield. At the genotypic level, it exhibited a significant correlation with biological yield. Therefore, it is recommended to consider these traits in the selection process to enhance grain yield in bread wheat. Based on the current study, G-41, G-3, G-12, G-37, G-34, and G-14 are identified as potential lines for further breeding program in different ecological conditions.
Author Contributions: Conceptualization, MJ, AA, Data Collection, MJ, AA, SU and MK, Data Analysis, MJ, MA and AA, Manuscript writing; MJ, AA, Writing review and editing, MJ and AA. All authors have read and agreed to the published version of the manuscript.
Funding: This study was supported by The University of Agriculture Peshawar, Plant Breeding and Genetics Department.
Conflicts of Interest: All authors declared no conflict of interest.
REFERENCES
Allard, R.W. Principles of Plant Breeding. John Wiley and Sons. Inc. New York, U.S.A., 1960, p 430.
Alogaidi, F.F. Studying of genetic and phenotypic variances, correlation and path coefficient analysis of yield and its component in some wheat varieties. Journal of Kerbala for Agricultural Sciences. 2018, 5, 151-63. https://doi.org/10.59658/jkas.v5i3.641
Arya, V.K.; Singh, J.; Kumar, L.; Kumar, R.; Kumar, P.; Chand, P. Genetic variability and diversity analysis for yield and its components in wheat (Triticum aestivum L.). Indian Journal of Agriculture Research. 2017, 51, 128-34. https://doi.org/10.18805/ijare.v0iOF.7634
Avinashe, H.A.; Shukla, R.S.; Jaiwar, N.D.S. Correlation and path analysis for yield and yield contributing characters in bread wheat (Triticum aestivum L.). Electronic Journal of Plant Breeding. 2015, 6, 555-559.
Barreto, H.J.; Edemeades, G.O.; Chapman, S.C.; Crossa, S. The alpha lattice design in plant breeding and agronomy: Generation and analysis. Agricultural and Food Sciences, Computer Science. 1997, 25, 544.
Bashir, M.; Khalil, I.H.; Iqbal, M.; Rahman, H. Genotypic and phenotypic correlation among yield components in bread wheat under normal and late plantings. Sarhad Journal of Agriculture. 2010, 26, 259-265.
Begna, T. Role and economic importance of crop genetic diversity in food security. International Journal of Agriculture Science and Food Technology. 2021, 7, 164-169. https://doi.org/10.17352/2455-815X.000104
Bhanu, A.N.; Arun, B.; Mishra, V.K. Genetic variability, heritability and correlation study of physiological and yield traits in relation to heat tolerance in wheat (Triticum aestivum L.). Biomedical Journal of Scientific and Technology Research. 2018, 2, 112-2116. https://doi.org/10.26717/BJSTR.2018.02.000636
Bhushan, B.; Bharti, S.; Ojha, A.; Pandey, M.; Gourav, S.S.; Tyagi, B.S.; Singh, G. Genetic variability, correlation coefficient and path analysis of some quantitative traits in bread wheat. Journal of Wheat Research. 2013, 5, 21-26.
Baye, A.; Berihun, B.; Bantayehu, M.; Derebe, B. Genotypic and phenotypic correlation and path coefficient analysis for yield and yield-related traits in advanced bread wheat (Triticum aestivum L.) lines. Cogent Food & Agriculture. 2020, 6, 1752603. https://doi.org/10.1080/23311932.2020.1752603
Chowdhury, M.M.; Haque, M.A.; Malek, M.A.; Rasel, M.; Ahamed, K.U. Genetic variability, correlation and path coefficient analysis for yield and yield components of selected lentil (M.) genotypes. Fundamental and Applied Agriculture. 2019, 4, 769-776. https://doi.org/10.5455/faa.21740
Dabi, A.; Mekbib, F.; Desalegn, T. Estimation of genetic and phenotypic correlation coefficients and path analysis of yield and yield contributing traits of bread wheat (Triticum aestivum L.) genotypes. International Journal of Natural Research. Ecology and Management. 2016, 1, 145-154. https://doi.org/10.11648/j.ijnrem.20160104.11
Falconer, D.S.; Mackay, T.F. Introduction to quantitative genetics (4th ed.). Pearson Education Limited. 1996.
Fan, X.; Xu, Z.; Wang, F.; Feng, B.; Zhou, Q.; Cao, J.; Wang, T. Identification of colored wheat genotypes with suitable quality and yield traits in response to low nitrogen input. PloS one. 2020, 15, e0229535. https://doi.org/10.1371/journal.pone.0229535
Farooq, M.; Hussain, M.; Siddique, K.H. Drought stress in wheat during flowering and grain-filling periods. Critical reviews in plant Science. 2014, 33, 331-49. https://doi.org/10.1080/07352689.2014.875291
Fellahi, Z.E.A.; Hannachi, A.; Bouzerzour, H.; Boutekrabt, A. Line × Tester mating design analysis for grain yield and yield-related traits in bread wheat (Triticum aestivum L.). International Journal of Agronomy. 2013, 1, 9. https://doi.org/10.1155/2013/201851
Githinji, G.G. Effects of Mutagenesis on Drought Tolerance and Agronomic Traits of Selected Bread Wheat (Triticum aestivum L.). MSc Thesis, University of Eldoret, 2016.
Imadud, D.: Fazal, M.; Irfan, A.S.; Hamayoon, K.; Fahad, U.K.; Ibrarullah, I.; Tauqir I. Genetic variability and heritability for yield and yield associated traits of wheat genotypes in Nowshera Valley, Pakistan. Pakistan Journal of Agriculture Research. 2018, 216-222. http://dx.doi.org/10.17582/journal.pjar/2018/31.3.216.222
Jamil, A.; Khan, S.; Sayal, O.U.; Waqas, M.; Ullah, Q.; Ali, S. Genetic variability, broad sense heritability and genetic advance studies in bread wheat (Triticum aestivum L.) germplasm. Pure and Applied Biology (PAB). 2017, 6, 538-543. http://dx.doi.org/10.19045/bspab.2017.60055
Khan, A.A.; Alam, M.A.; Alam, M.K.; Alam, M.J.; Sarker, Z.I. Genotypic and phenotypic correlation and path analysis in durum wheat (Triticum turgidum L. var. durum). Bangladesh Journal of Agriculture Research. 2013, 38, 219-225. https://doi.org/10.3329/bjar.v38i2.15885
Khan, A.J.; Azam, A.; Ali, A. Relationship of morphological traits and grain yield in recombinant inbred wheat lines grown under drought conditions. Pakistan Journal of Botnoy. 2010, 42, 259-267.
Khan, I.; Amanullah; Jamal, A.; Mihoub, A.; Farooq, O.; Farhan Saeed, M.; Roberto, M.; Radicetti, E.; Zia, A.; Azam, M. Partial substitution of chemical fertilizers with organic supplements increased wheat productivity and profitability under limited and assured irrigation regimes. Agriculture. 2022, 12, 1754. https://doi.org/10.3390/agriculture12111754
Kirby, M.; Ahmad, M.U.D.; Mainuddin, M.; Khaliq, T.; Cheema, M.J.M. Agricultural production, water use and food availability in Pakistan: historical trends, and projections to 2050. Agriculture and Water Management. 2017, 179, 34-46. https://doi.org/10.1016/j.agwat.2016.06.001
Kumar, J.; Kumar, M.; Kumar, A.; Singh, S.K.; Singh, L. Estimation of genetic variability and heritability in bread wheat under abiotic stress. International Journal of Pure and Applied Bioscience. 2017, 5, 156-163. http://dx.doi.org/10.18782/2320-7051.2475
Kwon S.H.; Torrie, J.H. Heritability and inter-relationship among traits of two Soybean Populations. Crop Science. 1964, 4, 196-198. https://doi.org/10.2135/cropsci1964.0011183X000400020023x
Malbhage, A.B.; Talpada, M.M.; Shekhawat, V.S.; Mehta, D.R. Genetic variability, heritability and genetic advance in durum wheat (Triticum durum L.). Journal of Pharmacognosy and Phytochemistry. 2020, 9, 3233-3236.
Mecha, B.; Alamerew, S.; Assefa, A.; Dutamo, D.; Assefa, E. Correlation and path coefficient studies of yield and yield associated traits in bread wheat (Triticum aestivum L.) genotypes. Advance Plants Agriculture Research. 2017, 6, 128-136. https://doi.org/10.15406/apar.2017.06.00226
Mecha, B.; Almerew, S.; Assefa, A.; Assefa, E.; Dutamo, D. Genetic variability heritability and genetic advance for yield and yield related traits in bread wheat (Triticum Aestivum L.) genotypes. Global Journal of Science Frontier Research. 2016, 16, 12-15.
PBS. Pakistan Statistical Year Book 2020, Government of Pakistan, Ministry of Planning Development &Special Initiatives,Pakistan Bureau of Statictics, Islamabad, Pakistan, 2022.
Preeti, S.; Kamboj, M.C.; Singh, N.; Chand, M.; Yadava, R.K. Path coefficient and correlation studies of yield and yield associated traits in advance homozygous lines of bread wheat germplasm. International Journal of Current. Microbiology and Applied Science. 2018, 7, 51-63. https://doi.org/10.20546/ijcmas.2018.702.008
Rajput, S.R. Correlation, path analysis, heritability and genetic advance for morpho-physiological character in bread wheat (Triticum aestivum L.). Journal of Pharmacognosy and Phytochemistry. 2018, 7, 107-112.
Rao, S.D.; Raghavendra, M.; Gill, P.; Madan, S.; Munjal, R. Effect of drought stress on phenological and yield attributes in Wheat (Triticum aestivum L). Journal of Eco-friend and Agriculture. 2022, 17, 65-71. https://doi.org/10.5958/2582-2683.2022.00014.4
Reynolds, M.; Bonnett, D.; Chapman, S.C.; Furbank, R.T.; Mane, Y.; Mather, D.E.; Parry, M.A.J. Raising yield potential of wheat. I. Overview of a consortium approach and breeding strategies. Journal of Experimental Botany. 2011, 62, 439-452. https://doi.org/10.1093/jxb/erq311
Savadi, S.; Prasad, P.; Kashyap, P.L.; Bhardwaj, S.C. Molecular breeding technologies and strategies for rust resistance in wheat (Triticum aestivum) for sustained food security. Plant pathology. 2017, 67, 771-791. https://doi.org/10.1111/ppa.12802
Singh, R.K.; Chaudhary, B.D. Path analysis and biometrical methods in quantitative genetic analysis, 1985, pp. 70-79.
Singh, R.K.; Yadav, H.C.; Kumar, M.; Lal, K.; Amir, M. Genetic variability, heritability and genetic advance analysis for seed yield and its physiological quality parameters in rice (Oryza sativa L.). Journal of. Pharmacognosy and Phytochemistry. 2019, 8, 511-513.
Slafer, G.A.; Elia, M.; Savin, R.; García, G.A.; Terrile, I.I.; Ferrante, A.; González, F.G. Fruiting efficiency: an alternative trait to further rise wheat yield. Food and Energy Security. 2015, 4, 92-109. https://doi.org/10.1002/fes3.59
Talebi, R.; Fayyaz, F. Estimation of heritability and genetic parameters associated with agronomic traits of bread wheat (Triticum aestivum L.) under two constructing water regimes. Journal of Applied Biological Science. 2012, 6, 35-39.
Tilahun, B.; Habtamu, T.; Tesfaye, L. Genetic variability, heritability and genetic advance among bread wheat genotypes at southeastern Ethiopia. Agriculture, Forestry and Fisheries. 2020, 9, 128-134. https://doi.org/10.11648/j.aff.20200904.15
Ullah, N.; Ullah, H.; Afridi, K.; Alam, M.; Jadoon., S.A.; Khan, W.U.; Masood, A.; Uddin, H. Genetic variability, heritability and correlation analysis among morphological and yield traits in wheat advanced lines. Biyolojik Çeşitlilik ve Koruma. 2018, 11, 166-180.
Wang, X.; Xu, Y.; Hu, Z.; Xu, C. Genomic selection methods for crop improvement: Current status and prospects. The Crop Journal. 2018, 6, 330-340. https://doi.org/10.1016/j.cj.2018.03.001
Yang, L.; Zhao, D.; Meng, Z.; Xu, K.; Yan, J.; Xia, X.; Zhang, Y. QTL mapping for grain yield-related traits in bread wheat via SNP-based selective genotyping. Theoretical and Applied Genetics. 2020, 133, 857-872. https://doi.org/10.1007/s00122-019-03511-0
Academic Editor: Dr. Isabela Maria Simion
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Alam Ayesha, Ali Akbar, Ali Muhammad, Javed Mansoor, Kashif Muhammad, Ullah Saif