Sami Mohammed Salih, Ahmed Amrajaa Abdulrraziq
ABSTRACT. Erythrina lysistemon seeds imported to Libya go through a stage of being unable to germinate; we call this phase “dormancy”. The present study evaluated the efficiency of the following methods in breaking the dormancy of newly collected Erythrina lysistemon seeds from mature pods and stored for 12 months: untreated seeds (control), mechanical scarification with sandpaper, soaking treatments (tap water for 24 h, hydrochloric acid for 60 min, hydrogen peroxide for 48 h, acetone for 72 h, Clorox cleaner for 72 h, cow dung and chicken manure for 24 h, and hot water at 100° C for 30 min), scarification with soaking (distilled water for 24 h, and cow dung and chicken manure for 12 h). All treatments showed a significant increase (p<0.05) in the germination rates of newly collected E. lysistemon seeds from mature pods, except for the treatment in which seeds were soaked in tap water for 48 h, which was ineffective. The different scarification treatments were more efficient than the other treatments, recording the highest germination percentages and lowest mean germination times, while the soaking treatments led to high seed mortality. In contrast, seeds stored for 12 months showed a significant decrease in germination percentage with a delayed mean germination time compared to newly collected seeds under all tested treatments. Soaking all treatments was ineffective in breaking the dormancy of E. lysatetemon seeds stored for 12 months. The results indicate that E. lysistemon seeds have physical dormancy that can be overcome using different scarification.
Keywords: mature seed; mechanical scarification; physical dormancy; stored seed.
Cite
ALSE and ACS Style
Salih, S.M.; Abdulrraziq, A.A. Efficiency of different methods in breaking the dormancy of Erythrina lysistemon Hutch. seeds. Journal of Applied Life Sciences and Environment 2024, 57 (3), 509-518.
https://doi.org/10.46909/alse-573150
AMA Style
Salih SM, Abdulrraziq AA. Efficiency of different methods in breaking the dormancy of Erythrina lysistemon Hutch. seeds. Journal of Applied Life Sciences and Environment. 2024; 57 (3): 509-518.
https://doi.org/10.46909/alse-573150
Chicago/Turabian Style
Salih, Sami Mohammed, and Ahmed Amrajaa Abdulrraziq. 2024. “Efficiency of different methods in breaking the dormancy of Erythrina lysistemon Hutch. Seeds.” Journal of Applied Life Sciences and Environment 57, no. 3: 509-518.
https://doi.org/10.46909/alse-573150
View full article (HTML)
Efficiency of Different Methods in Breaking the Dormancy of Erythrina lysistemon Hutch. Seeds
Sami Mohammed SALIH1,* and Ahmed Amrajaa ABDULRRAZIQ1
1Department of Biology, Faculty of Education, Omar Al-Mukhtar University, Al-Bayda, Libya; email: ahmed.amrajaa@omu.edu.ly
*Correspondence: sami.mohammed@omu.edu.ly
Received: Aug. 28, 2024. Revised: Oct. 14, 2024. Accepted: Oct. 25, 2024. Published online: Nov. 18, 2024
ABSTRACT. Erythrina lysistemon seeds imported to Libya go through a stage of being unable to germinate; we call this phase “dormancy”. The present study evaluated the efficiency of the following methods in breaking the dormancy of newly collected Erythrina lysistemon seeds from mature pods and stored for 12 months: untreated seeds (control), mechanical scarification with sandpaper, soaking treatments (tap water for 24 h, hydrochloric acid for 60 min, hydrogen peroxide for 48 h, acetone for 72 h, Clorox cleaner for 72 h, cow dung and chicken manure for 24 h, and hot water at 100° C for 30 min), scarification with soaking (distilled water for 24 h, and cow dung and chicken manure for 12 h). All treatments showed a significant increase (p<0.05) in the germination rates of newly collected E. lysistemon seeds from mature pods, except for the treatment in which seeds were soaked in tap water for 48 h, which was ineffective. The different scarification treatments were more efficient than the other treatments, recording the highest germination percentages and lowest mean germination times, while the soaking treatments led to high seed mortality. In contrast, seeds stored for 12 months showed a significant decrease in germination percentage with a delayed mean germination time compared to newly collected seeds under all tested treatments. Soaking all treatments was ineffective in breaking the dormancy of E. lysatetemon seeds stored for 12 months. The results indicate that E. lysistemon seeds have physical dormancy that can be overcome using different scarification.
Keywords: mature seed; mechanical scarification; physical dormancy; stored seed.
INTRODUCTION
Erythrina lysistemon Huch. a member of Fabaceae family, known as South African coral, is a deciduous ornamental tree, that grows up to 10 m, and is widely spread in Al-Bayda City, Libya (Salih and Abdulrraziq, 2024). The Erythrina genus comprises roughly 120 species spread across South Africa, the tropics and subtropics, and the southern United States (Krukoff and Barneby 1974). This genus is famous for its many uses, as it is used in agriculture to restore degraded lands, for nitrogen fixation in the soil, as a source for wood production, and in handicraft production (Hardt et al., 2006; Alves Junior et al., 2016). In traditional medicine, it is utilised for the treatment of a range of conditions, including asthma, wounds, abscesses, arthritis, venereal disease, toothache, and nervous system disorders (Sarragiotto et al., 1981; de Lima et al., 2006; Patocka, 2009). Furthermore, Erythrina species contain the most important compounds such as erythroidine alkaloids, isoquinoline alkaloids, isoflavonoids and pterocarpans, flavonoids isoflavonoids, pterocarpanes, flavanones and chalcones (Na et al., 2006; Flausino et al., 2007; Ozawa et al., 2010). According to Majinda et al. (2005), many secondary metabolites derived from tree of the genus Erythrina exhibited antimicrobial, anti-inflammatory, antioxidant, and anticancer effects. In anticipation of the decade of ecosystem restoration (2021–2030), there is a revived emphasis on enhancing wetland restoration, and climate mitigation. The first step in restoring the ecosystem is reforestation through a strategic seed-based approach (Kettenring and Tarsa, 2020).
Seeds are considered the most successful method of sexual reproduction in plants and are responsible for transmitting genetic traits across generations (Bareke, 2018). However, many seeds go through a stage of being unable to germinate, whether under suitable or unsuitable conditions, and this stage is known as dormancy (Otani et al., 2024). Dormancy is linked to internal factors, such as the hardness of the seed coat, immature embryos, and germination inhibitors, and external factors, such as temperature, light, oxygen, and humidity (Prudente and Paiva, 2018). Baskin and Baskin (2004), classified dormancy into five types (physical, morphological, morphophysiological, physiological, and combinational dormancy). Mature seeds of the Erythrina genus exhibit seed coat impermeability (physical dormancy), which must be overcome through the mechanical disruption of the impermeable seed layer to break dormancy and initiate germination (Molizane et al., 2018). This allows the seeds to imbibe water and exchange gases necessary for germination (Salih and Abdulrraziq, 2020). There are many techniques used to break seed dormancy in Erythrina species. For example, the application of mechanical scarification with sandpaper breaks the dormancy of Erythrina falcata Benth seeds (Artur et al., 2023).
Therefore, the main aim of this research was to evaluate the efficiency of different methods in breaking the dormancy of mature E. lysistemon mature seeds and those stored for 12 months.
MATERIALS AND METHODS
Erythrina lysistemon seeds were randomly harvested from the mature pods beginning at dehiscence, of growing trees at the University Campus and Al-Thawra Hospital- Al-Bayda City, Libya, in 2023 and 2024.
The seeds were divided into two groups: newly collected seeds from mature pods in 2024 and seeds harvested in 2023 and stored for 12 months under dry conditions at room temperature.
Seeds vitality was tested by soaking them in distilled water to remove any empty seeds, followed by soaking them in 5% sodium hypochlorite solution for 5 min and then washing them 3 times in distilled water (Luzia Delgado et al., 2015).
Experimental design
The laboratory experiments were conducted at the Department of Biology/Faculty of Education/Omar Al-Mukhtar University. A completely randomised design (CRD) with three replications was used. The experiment 13 treatments as follows:
- Untreated (intact) control (C) seeds.
- Treatment by soaking in tap water for 48 h (SW).
- Treatment with mechanical scarification on the area opposite the hilum with the help of sandpaper (MS).
- Treatment with mechanical scarification + soaking in distilled water for 24 h (MS+SW).
- Treatment with mechanical scarification + soaking in cow dung for 12 h (MS+SCD).
- Treatment of mechanical scarification + soaking in chicken manure for 12 hours (MS+SCM).
- Treatment by soaking in hot water at 100°C for 30 min and cooling to at room temperature (SHW).
- Treatment by soaking in concentrated Hydrochloric acid for 60 min and then washing with distilled water (HCL).
- Treatment by soaking in hydrogen peroxide at a concentration of 6% for 48 h (H2O2).
- Treatment by soaking in acetone for 72 h (C3H6O).
- Treatment by soaking in Clorox cleaner (30966, 121 Oz.) for 72 h (SCC).
- Treatment by soaking in cow dung for 24 h (SCD).
- Treatment by soaking in chicken manure for 24 h (SCM).
The seeds were placed in 9-cm-diameter Petri plates on No. 42 filter paper with 25 seeds/plate, and incubated at room temperature; each treatment was repeated 3 times. Seeds were monitored for 10 days, and as needed, 10 mL of distilled water was added to each of the Petri plates.
Germination was calculated by recording the number of germinated seeds in all treatments starting on the second day of observation when germination frist occurred (Equation 1) (Salih and Abdulrraziq, 2021). The germination criterion was the appearance of the radicle outside the seed cover, and at the end of the experiment took the final results of the following qualities were obtained (Equation 2) (Das et al., 2017):
|
Germination percentage = number of germinated seeds / total number of seeds × 100 |
(1) |
|
Mean germination time = total number of germinated seeds / total number of germinated seeds at end of the experiment |
(2) |
Organic Residue Preparation
A solution of cow dung and chicken manure was prepared separately, in a ratio of 500g/500mL (w/v) of organic matter and water without dilution.
Statistical analysis
The experiments followed a full randomised design (CRD). Statistical analysis including analysis of variance (ANOVA) Tukey’s test at p<0.05, were performed using Minitab 17.
RESULTS AND DISCUSSIONS
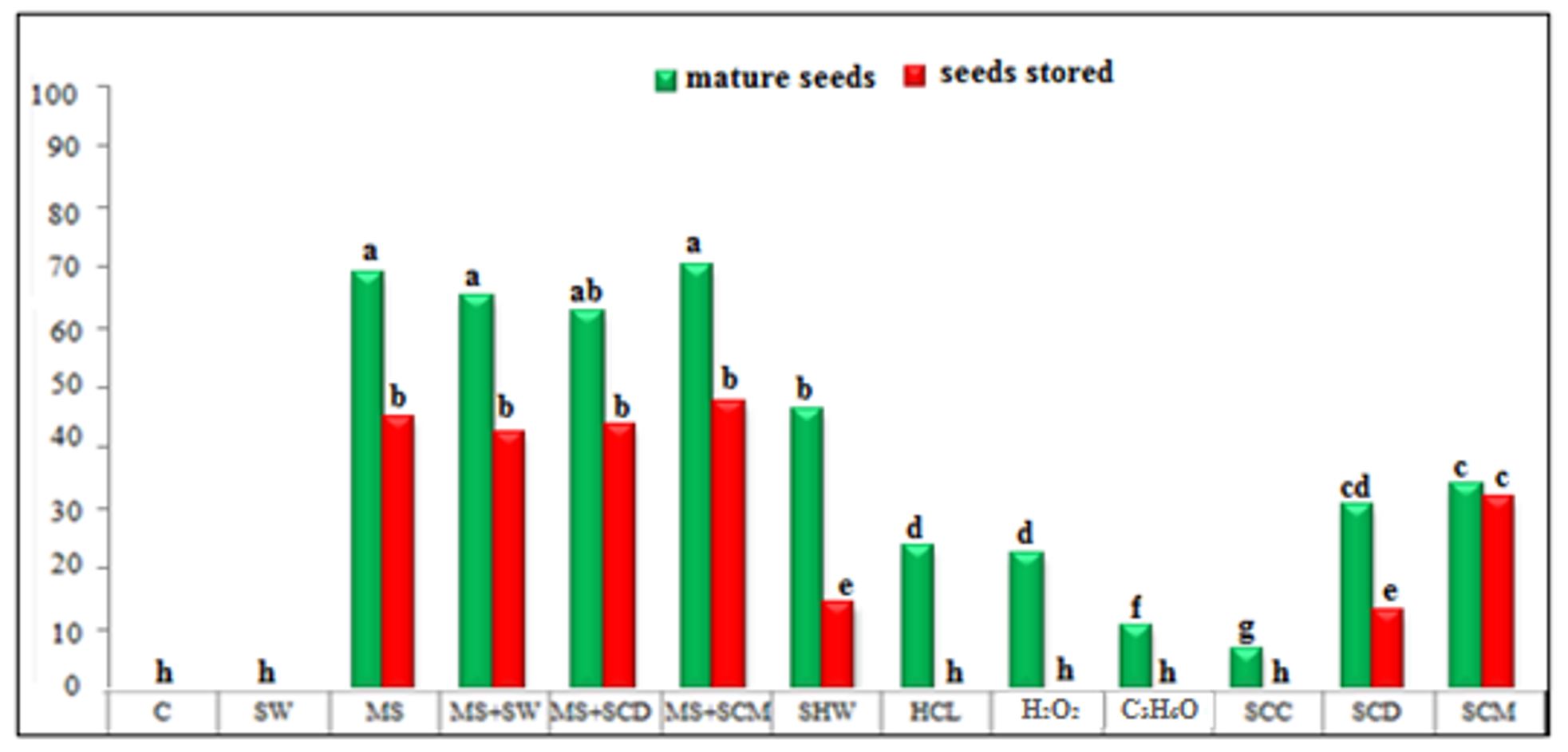
All treatments showed a significant increase (p<0.05) in the germination rate of newly collected E. lysistemon seeds from mature pods, except for the treatment in which seeds were soaked in tap water for 48 h, which did not promote germination (ineffective) compared to non-treated healthy seeds (control) after 10 days of germination (Figure 1, Figure 2 and Figure 6).
The scarification treatments were not significantly different from each other and recorded the highest germination percentages and lowest mean germination times. These methods increased germination percentages to 69.33, 65.33, 62.66, and 70.66% as well as reduced the mean germination time to 3.07, 3.20, 3.39, and 3.24 days with treatments of mechanical scarification, scarification + soaking in water, scarification + soaking in cow dung, and scarification+ soaking chicken manure, respectively.
The obtained results corroborate those reported by Pêgo et al. (2015) and Artur et al. (2023), who suggested that scarification treatment was an efficient method for breaking the physical dormancy of E. verna and E. falcata seeds. The scarification approach offers the highest seedling performance and germination, as shown by Pinheiro et al. (2021). Compared to the control group, the scarification method disrupts the water impermeability of the seed coat due to coat thickness. Manning and Staden (1985) demonstrated that E. lysistemon Hutch seeds had an outer layer rich in phenolic compounds that might enhance the coat thickness. Seeds soaked in hot water for 30 min showed a good germination rate compared to other treatments, as the germination percentage was 46.66% and had the lowest mean germination time (3.75 days).
This result contrasts with that of Artur et al. (2023), who indicated that water treatment at 100°C was most harmful to the germination of E. falcata Benth.
This was followed by treatments of soaking in chicken manure and cow dung for 24 h, which showed germination percentages of 34.00 and 30.66% and mean germination times of 4.00 and 4.42 days, respectively.
These results are consistent with those of Salih and Abdulrraziq (2021), who found that organic residues caused the corrosion of the seed coat by acids present in a cow dung slurry and chicken manure, which break the dormancy of Ceratonia siliqua L.
The treatment in which seeds were soaked in hydrochloric acid and hydrogen peroxide resulted in weak germination percentages (24.00 and 22.66%, respectively), with a clear increase in the mean germination time (4.04 and 4.86 days, respectively).
Finally, the lowest germination percentages (10.66 and 6.66%) and the highest average germination times (6.22 and 5.66 days) were obtained when seed were soaked in acetone and Clorox cleaner, respectively. The decline in germination percentages might be due to the inability of these treatments to break the physical dormancy.
Erythrina lysistemon seeds stored for 12 months showed a significant reduction in their viability 10 days after the start of the experiment. Figure 3 and Figure 4 show that mechanical scarification + soaking in chicken manure for 12 h resulted in a higher germination percentage (48.00%) and a faster germination time (3.09 days). Germination percentages were lower (45.33, 44.00, and 42.66%) and the mean germination time was delayed (4.00, 4.52, and 4.35 days) for seeds pre-treated with mechanical scarification, scarification + soaking in cow dung, and scarification + soaking in water, respectively.
The treatments in which seeds were soaked in chicken manure, hot water, and cow manure resulted in the lowest germination percentages (32.00, 14.66, and 13.33%, respectively) and were associated with the longest mean germination (5.78, 4.91, and 5.36 days, respectively).
This study furthers the conclusions of Pereira et al. (2014), who found that the germination rates of E. mulungu and E. velutina seeds stored for one year were decreased by more than a quarter. However, de Freitas et al. (2020) found that newly collected E. crista-galli L. seeds from mature and immature pods grew naturally without dormancy breaking treatments.
Other than these treatments, exogenous soaking in tap water for 48 h, soaking in concentrated hydrochloric acid for 60 min, soaking in hydrogen peroxide for 48 h, soaking in acetone for 72 h, and soaking in Clorox cleaner for 72 h were ineffective in breaking the dormancy of E. lysistemon seeds stored for 12 months.
Generally, based on the breaking dormancy experiment, seeds stored under dry conditions at laboratory temperature for 12 months showed a clear decrease in germination percentages (24.00. 22,67, 18.66, 22.66, 32.00, 17.33, and 2.00%) for seeds pre-treated with mechanical scarification, scarification + soaking in distilled water for 24 h, scarification + soaking in cow dung for 12 h, scarification + soaking in chicken manure for 12 h, soaking in hot water at 100°C for 30 min, soaking in cow dung for 24 h, and soaking in chicken manure for 24 h, respectively, compared to newly collected seeds from mature pods harvested in 2024 (Figure 5).
The results of our study indicate that the percentage of physical dormancy in E. lysistemon seeds increases with increasing storage time.
Similar findings were reported regarding the storage of E. speciosa seeds (Magalhae and Oliveira, 2020). This may be dormancy because of the accumulation of pectic substances and cutin within the cells of the outer palisade layer, which hinders water absorption (Magalhae and Oliveira, 2020).
Furthermore, drier storage conditions were found to cause the closure of cracks in the mucilaginous stratum for seeds according to Magalhaes et al. (2021). In addition, this dormancy may be caused by environmental conditions experienced by the mother plant, which affects the level of dormancy in Erythrina seeds during the maturation process (Molizane et al., 2018).
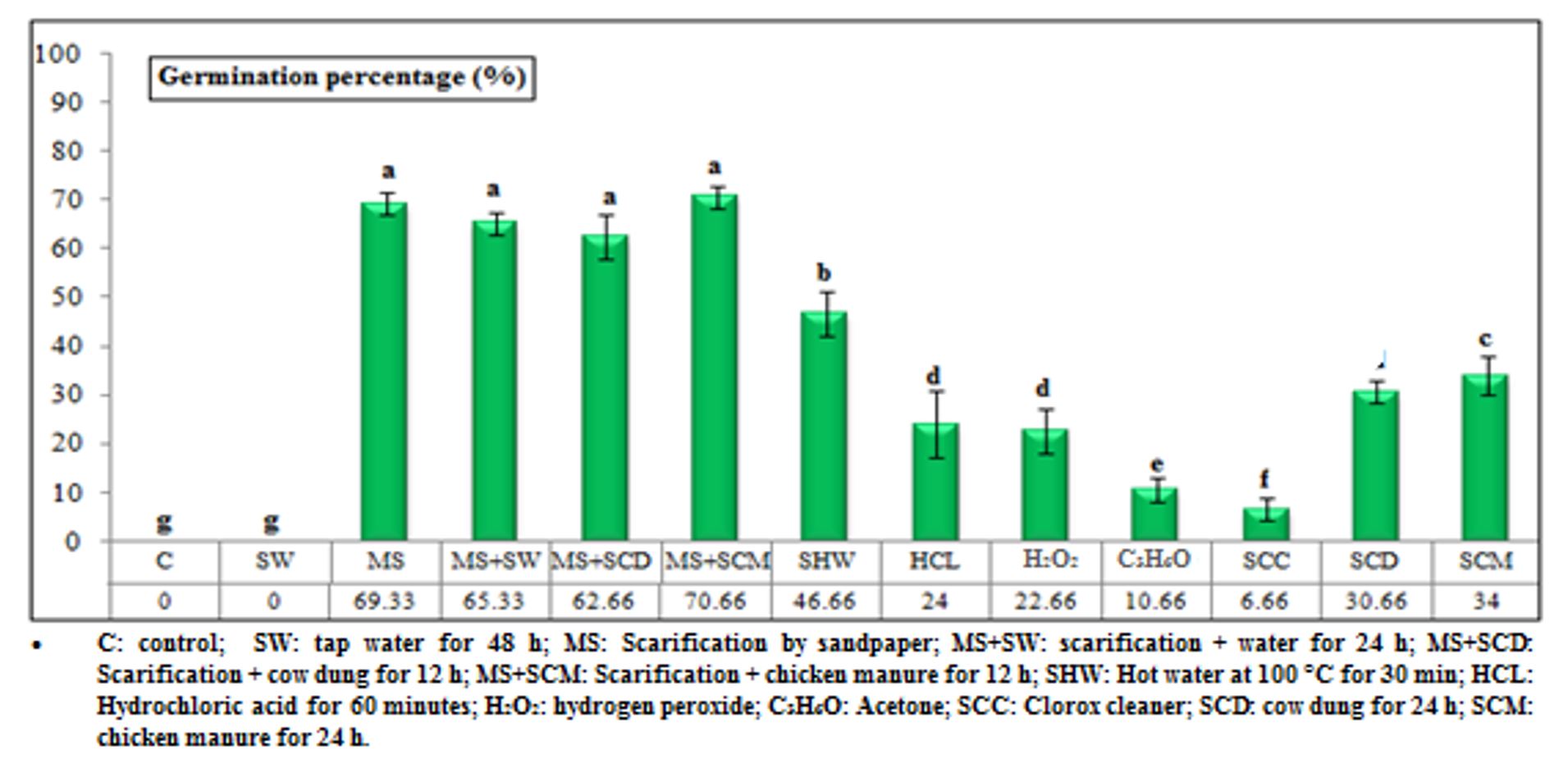
Figure 1 – Germination percentages of Erythrina lysistemon seed (collected from mature pods ) submitted to different treatments. (Mean ± Standard error). Columns with the same letters do not differ from each other according to the Tukey test (5%)
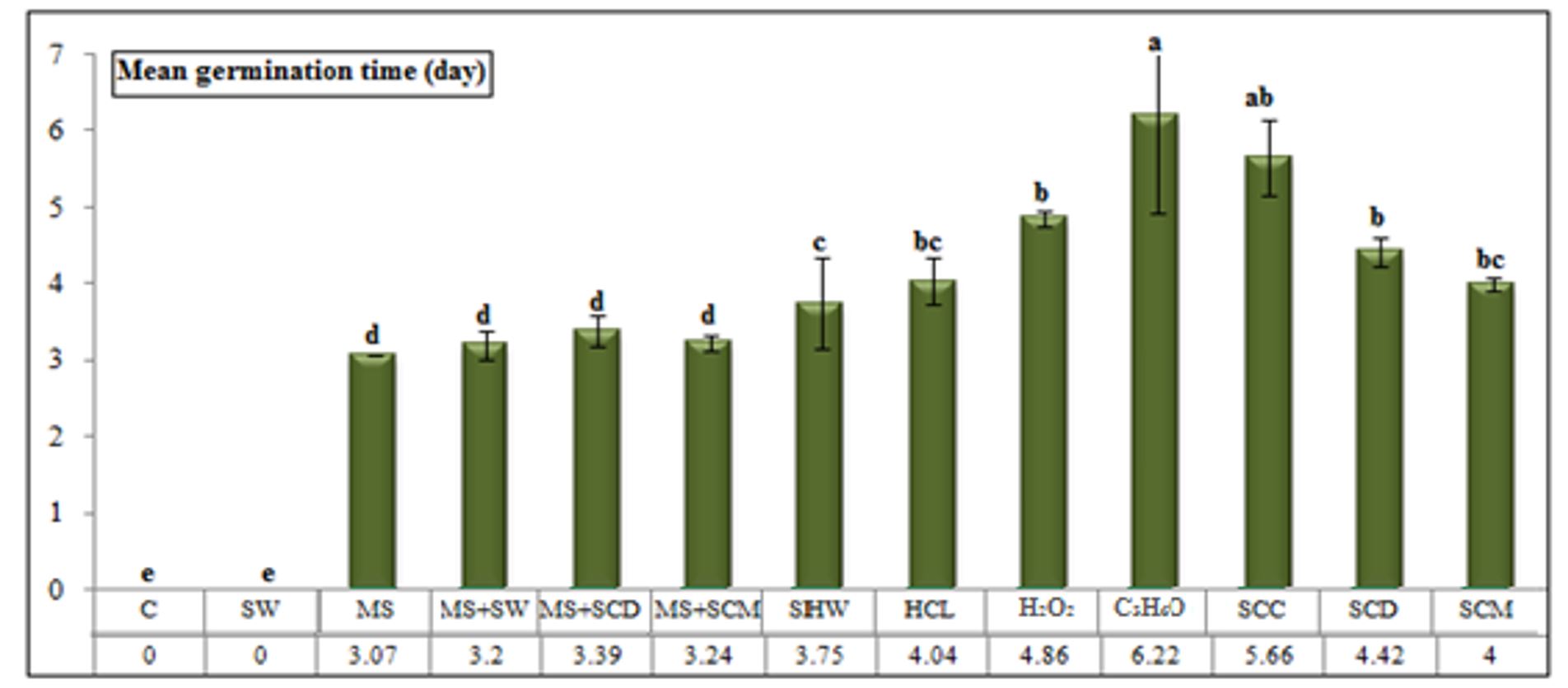
Figure 2 – Mean germination time of Erythrina lysistemon seed (collected from mature pods ) submitted to different treatments
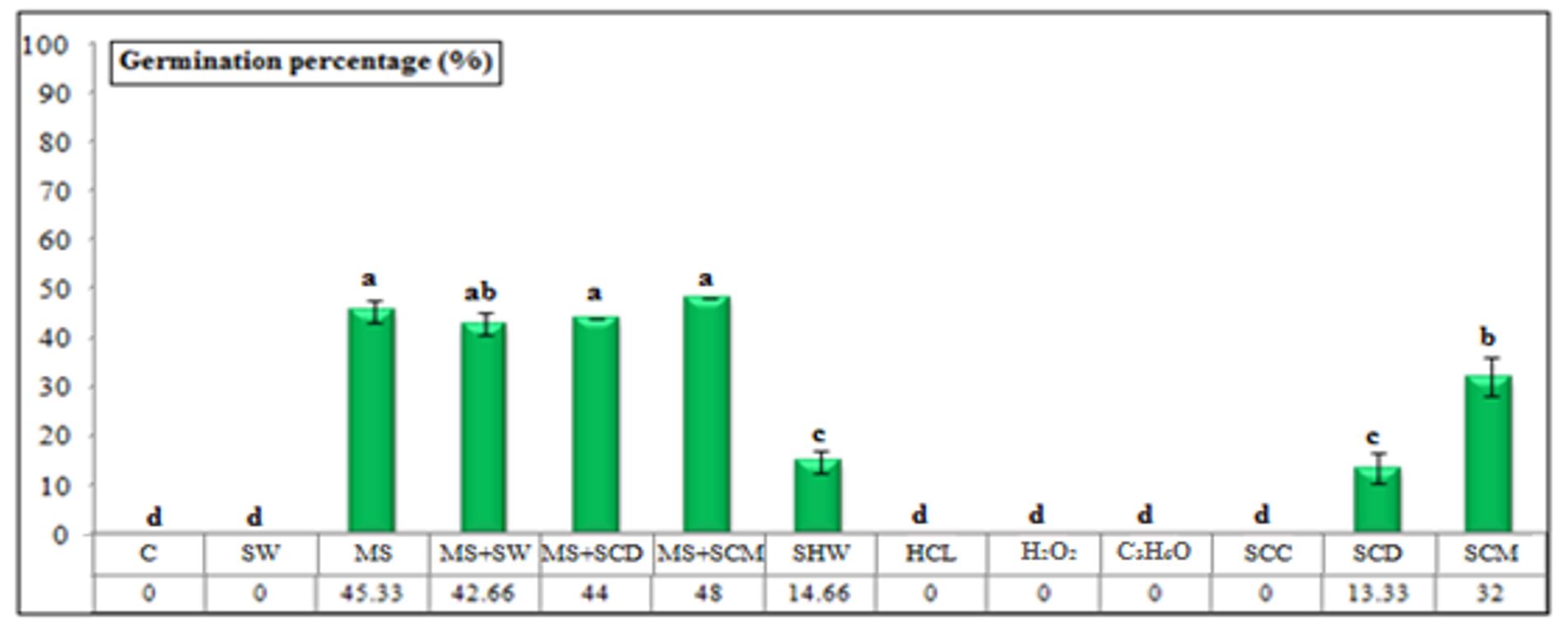
Figure 3 – Germination percentages of Erythrina lysistemon seed (stored for 12 months) submitted to different treatments
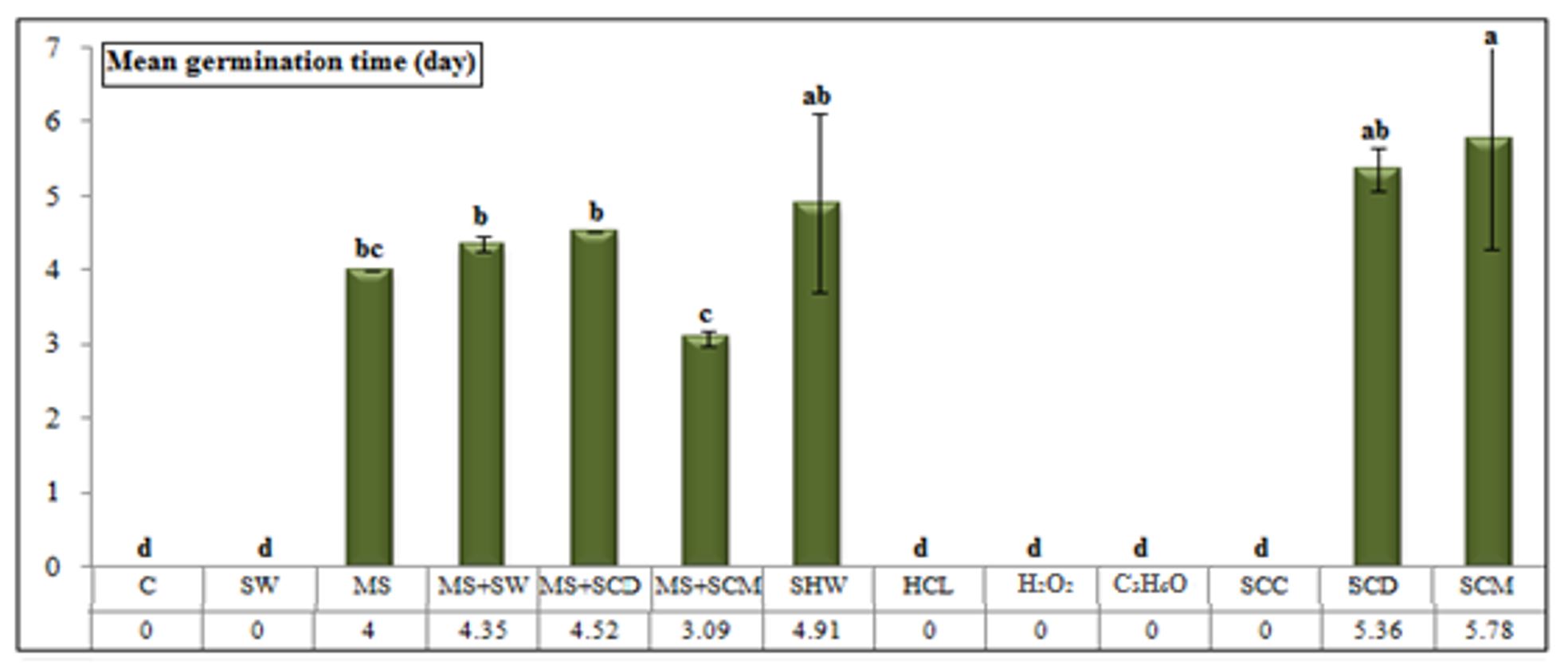
Figure 4 – Mean germination time of Erythrina lysistemon seed (stored for 12 months) submitted to different treatments
CONCLUSIONS
This study discovered that various scarification techniques may be used to break the physical dormancy of both freshly harvested E. lysistemon Huch seeds and seeds that have been kept for a year. However, compared to freshly collected seeds under all studied treatments, seeds held for a year had a significantly lower germination percentage and a delayed mean germination time. As a result, farmers are advised to utilise scarified seeds for roadside and garden decorative trees as soon as they are harvested.
Author Contributions: Conceptualization: SMS, AAA; Methodology: AAA; Analysis: SMS; Investigation: SMS, AAA; Resources: AAA; Data curation: SMS, AAA; Writing, Review, Supervision: SMS, AAA. All authors declare that they have read and approved the publication of the manuscript in the present form.
Funding: There was no external funding for this study.
Acknowledgments: We thank for Faculty of Education/Omar Al-Mukhtar University.
Conflicts of Interest: There are no conflicts of interest.
REFERENCES
Alves Junior, C.; de Oliveira Vitoriano, J.; da Silva, D.L. S.; de Lima Farias, M.; de Lima Dantas, N.B. Water uptake mechanism and germination of Erythrina velutina seeds treated with atmospheric plasma. Scientific reports 2016, 6 (1). https://doi.org/10.1038/srep33722
Artur, M.A.S.; Faria, J.M.R.; José, A.C.; Lira, J.M.D.S.; Souza, K.R.D.D.; Nery, F.C.; Alvarenga, A.A.D. Dormancy breaking and biochemical processes associated with germination of Erythrina falcata Benth. seeds. Cerne 2023, 29. https://doi.org/10.1590/01047760202329013170
Bareke, T.J.A.P.A.R. Biology of seed development and germination physiology. Advances in Plants and Agriculture Research 2018, 8 (4), 336-346.
Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Science Research 2004, 14, 1-16. https://doi.org/10.1079/SSR2003150
Das, M.; Sharma, M.; Sivan, P. Seed germination and seedling vigor index in Bixa orellana and Clitoria ternatea. Indian Journal of Pure & Applied Bioscience 2017, 5 (5), 15-19. http://dx.doi.org/10.18782/2320-7051.2869
de Freitas, T.C.; Gomes, G.C.; Guarino, E.D.S.G.; Molina, A.R.; Medeiros, F.S.; da Silva Souza, L.C.; Cardoso, J.H. Effect of the seed maturation stage and pre-germination treatments on emergence of Erythrina crista-galli L. Iheringia, Série Botânica 2020, 75. https://doi.org/10.21826/2446-82312020v75e2020007
de Lima, M.R.F.; de Souza Luna, J.; dos Santos, A.F.; de Andrade, M.C.C.; Sant’Ana, A.E.G.; Genet, J.P.; Moreau, N. Anti-bacterial activity of some Brazilian medicinal plants. Journal of ethnopharmacology 2006, 105 (1-2), 137-147. https://doi.org/10.1016/j.jep.2005.10.026
Flausino Jr, O.A.; Pereira, A.M.; da Silva Bolzani, V.; Nunes-de-Souza, R.L. Effects of erythrinian alkaloids isolated from Erythrina mulungu (Papilionaceae) in mice submitted to animal models of anxiety. Biological and Pharmaceutical Bulletin 2007, 30 (2), 375-378. https://doi.org/10.1248/bpb.30.375
Hardt, E.; Pereira-Silva, E.F.L.; Zakia, M.J.B.; Lima, W.D.P. Riparian forest restoration in sand minings of the Corumbataí River Basin: efficacy in the recovery of biodiversity. Scientia Forestalis 2006, 70, 107-123.
Kettenring, K.M.; Tarsa, E.E. Need to seed? Ecological, genetic, and evolutionary keys to seed-based wetland restoration. Frontiers in Environmental Science 2020, 8, 109. https://doi.org/10.3389/fenvs.2020.00109
Krukoff, B.A; Barneby, R.C. Conspectus of species of the genus Erythrina. Lloyd Library & Museum, Cincinnati, Ohio, 1974, 37, 332–459.
Luzia Delgado, C.M.; Souza de Paula, A.; Santos, M.; Silveira Paulilo, M.T. Dormancy-breaking requirements of Sophora tomentosa and Erythrina speciosa (Fabaceae) seeds. Revista de Biología Tropical 2015, 63 (1), 285-294.
Magalhães, C.R.; Garcia, Q.S.; Oliveira, D.M. Post-dispersion humidity condition alters the surface of the testa and the proportion of seeds with physical dormancy in Erythrina speciosa. Seed Science Research 2021, 31 (2), 149-156. https://doi.org/10.1017/S0960258520000495
Magalhães, C.R.; Oliveira, D.M.T. Testa structure in Erythrina speciosa (Leguminosae): the role of the mucilaginous stratum in the acquisition of physical dormancy. Acta Botanica Brasilica 2020, 34 (3), 592-598. https://doi.org/10.1590/0102-33062020abb0044
Majinda, R.R.; Wanjala, C.C.; Juma, B.F. Bioactive non-alkaloidal constituents from the genus Erythrina. Studies in natural products chemistry 2005, 32, 821-853. https://doi.org/10.1016/S1572-5995(05)80070-5
Manning, J.C.; Van Staden, J. The development and ultrastructure of the testa and tracheid bar in Erythrina lysistemon Hutch. (Leguminosae: Papilionoideae). Protoplasma 1985, 129, 157-167. https://doi.org/10.1007/BF01279913
Molizane, D.M.; Julio, P.G.D.S.; Carmello-Guerreiro, S.M.; Barbedo, C.J. Physical, physiological and anatomical changes in Erythrina speciosa Andrews seeds from different seasons related to the dormancy degree. Journal of Seed Science 2018, 40, 331-341. https://doi.org/10.1590/2317-1545v40n3199428
Na, M.; Jang, J.; Njamen, D.; Mbafor, J.T.; Fomum, Z.T.; Kim, B.Y.; Ahn, J.S. Protein tyrosine phosphatase-1B inhibitory activity of isoprenylated flavonoids isolated from Erythrina mildbraedii. Journal of natural products 2006, 69 (11), 1572-1576. https://doi.org/10.1021/np0601861
Otani, M., Zheng, L.; Kawakami, N. Genetic, Epigenetic, and Environmental Control of Seed Dormancy and Germination. Seed Dormancy: Methods and Protocols 2024, 3-12. https://doi.org/10.1007/978-1-0716-3965-8_1
Ozawa, M.; Kawamata, S.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kishida, A.; Ohsaki, A. Structures of new erythrinan alkaloids and nitric oxide production inhibitors from Erythrina crista-galli. Chemical and Pharmaceutical Bulletin 2010, 58 (8), 1119-1122. https://doi.org/10.1248/cpb.58.1119
Patocka, J. Mulungu-Anxiolytics from an amazonian rainforest. Psychiatry 2009, 13, 89-91.
Pêgo, R.G.; Grossi, J.A.S.; Queiroz, I.D.D.S.; Vasconcellos, H.C. Physiological responses of Erythrina verna seedlings on seed pre-germinative treatments and sowing depth. Ciência Florestal 2015, 25 (1), 59-66. https://doi.org/10.1590/1980-509820152505059
Pereira, A.M.S.; Souza, V.T.A.; da Silva Coppede, J.; de Castro França, S.; Bertoni, B.W.; de Souza, A.V.V. Seed germination and production of Erythrina mulungu and Erythrina velutina plantlets. American Journal of Plant Sciences 2014, 5, 535-540. https://doi.org/10.4236/ajps.2014.55068
Pinheiro, R.D.M.; Soares, V.N.; Gadotti, G.I.; Silva, E.J.S.D.; Almeida, A.D.S. Germinative performance of Mulungú seeds (Ormosia grossa Rudd) after dormancy overcoming. Revista Árvore 2021, 45. https://doi.org/10.1590/1806-908820210000032
Prudente, D.O.; Paiva, R. Seed dormancy and germination: Physiological considerations. Journal of Cell and Developmental Biology 2018, 2 (1), 2.
Salih, S.M.; Abdulrraziq, A.A. Re-Correcting the Scientific Nomenclature of Species of Erythrina Trees Introduced to Libya. International Journal of Scientific Research in Biological Sciences 2024, 11 (2), 8-12.
Salih, S.M.; Abdulrraziq, A.A. Response germination of Leucaena leucocephala (Lam.) De Wit Trees Seeds to different treatments. Libyan Journal of Basic Sciences 2020, 10 (1), 1-11.
Salih, S.M.; Abdulrraziq, A.A. Role of Organic Residues to Break Seed Dormancy of Ceratonia siliqua L. Libyan Journal of Basic Sciences 2021, 15 (1), 25-33.
Sarragiotto, M.H.; Filho, H.L.; Marsaioli, A.J. Erysotrine-N-oxide and erythrartine-N-oxide, two novel alkaloids from Erythrina mulungu. Canadian Journal of Chemistry 1981, 59 (18), 2771-2775. https://doi.org/10.1139/v81-400
Academic Editor: Dr. Iuliana MOTRESCU
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.