Oluropo Ayotunde Apalowo, Nkechi Betsy Izuogu, Halimat Shola Baba, Isaiah Olusesan Adepoju, C.M. Olajide, Muhammed Adewole Adeyemi, Olusegun Samuel Balogun
ABSTRACT. Watermelon production in Kwara State, Nigeria, is affected by root-knot nematode (RKN), as all varieties of the crop are susceptible. The aim of this study was to identify readily available and environmentally safe nematicides for the management of RKN. The field was located at the Teaching and Research Farm of the University of Ilorin and set out in a randomised complete block design, with four replications. Aqueous and powdered extracts of Chromolaena odorata (L) King and Robinson and Ficus mucuso Welw. ex Ficalho were applied alone and in combination. There were seven total treatments: C. odorata aqueous, F. mucuso aqueous, C. odorata powder, F. mucuso powder, C. odorata and F. mucuso aqueous, C. odorata and F. mucuso powder, and the control. Data were collected on growth, yield and nematode populations. All data collected were subjected to analysis of variance, and treatments were compared using Duncan’s multiple range test at a 5% level of significance. The essential oil of each botanical was determined using gas chromatography-mass spectrometry. The vine length (185.61) and yield (2401.05) of plants treated with botanicals were significantly higher than those of the control at P<0.005. The nematode population was also significantly lower in plants treated with botanicals than in the control (318.30 and 230.00, first and second year, respectively) at P<0.005. Among the treatments, the combination of C. odorata and F. mucuso powders was the most effective, with higher growth and yield performance. The experiment showed that aqueous and powdered extracts of C. odorata and F. mucuso were effective in managing RKN in the field.
Keywords: Botanicals; C. odorata; extracts; F. mucuso; watermelon.
Cite
ALSE and ACS Style
Apalowo, O.A.; Izuogu, N.B.; Baba, H.S.; Adepoju, I.O.; Olajide, C.M.; Adeyemi, M.A.; Balogun, O.S. Efficacy of aqueous and powdered leaf extracts of Chromolaena odorata (Asterales: Asteraceae) and Ficus mucuso (Rosales: Moraceae) botanicals on root-knot nematode infecting watermelon in Kwara state, Nigeria. Journal of Applied Life Sciences and Environment 2023, 56 (4), 527-539.
https://doi.org/10.46909/alse-564114
AMA Style
Apalowo OA, Izuogu NB, Baba HS, Adepoju IO, Olajide CM, Adeyemi MA, Balogun OS. Efficacy of aqueous and powdered leaf extracts of Chromolaena odorata (Asterales: Asteraceae) and Ficus mucuso (Rosales: Moraceae) botanicals on root-knot nematode infecting watermelon in Kwara state, Nigeria. Journal of Applied Life Sciences and Environment. 2023; 56 (4), 527-539.
https://doi.org/10.46909/alse-564114
Chicago/Turabian Style
Apalowo, Oluropo Ayotunde, Nkechi Betsy Izuogu, Halimat Shola Baba, Isaiah Olusesan Adepoju, C.M. Olajide, Muhammed Adewole Adeyemi, and Olusegun Samuel Balogun. 2023. “Efficacy of aqueous and powdered leaf extracts of Chromolaena odorata (Asterales: Asteraceae) and Ficus mucuso (Rosales: Moraceae) botanicals on root-knot nematode infecting watermelon in Kwara state, Nigeria” Journal of Applied Life Sciences and Environment 56, no. 4: 527-539.
https://doi.org/10.46909/alse-564114
View full article (HTML)
Efficacy of Aqueous and Powdered Leaf Extracts Of Chromolaena odorata (Asterales: Asteraceae) and Ficus mucuso (Rosales: Moraceae) Botanicals on Root-Knot Nematode Infecting Watermelon in Kwara State, Nigeria
Oluropo Ayotunde APALOWO1,2*, Nkechi Betsy IZUOGU2, Halimat Shola BABA2, Isaiah Olusesan ADEPOJU3, C. M. OLAJIDE4, Muhammed Adewole ADEYEMI5 and Olusegun Samuel BALOGUN2
1Department of Crop Science and Horticulture, Nigeria Nnamdi Azikiwe University, Awka
2Department of Crop Protection, University of Ilorin, Ilorin; e-mail: nkbetsyizuogu@gmail.com; bhalimatshola@gmail.com; sambalo@unilorin.edu.ng
3Department of Crop Production and Protection, Federal University Wukari, Taraba; e-mail: adepoju@fuwukari.edu.ng
4College of Agriculture and Natural Resources, Iguroriaghi, Nigeria; e-mail: ma.adeyemi@unizik.edu.ng
5Department of Forestry and Wildlife, Nnamdi Azikiwe University, Awka; e-mail: ma.adeyemi@unizik.edu.ng
*Correspondence: oa.apalowo@unizik.edu.ng
Received: Apr. 08, 2023. Revised: Nov. 14, 2023. Accepted: Nov. 24, 2023. Published online: Jan. 10, 2023
ABSTRACT. Watermelon production in Kwara State, Nigeria, is affected by root-knot nematode (RKN), as all varieties of the crop are susceptible. The aim of this study was to identify readily available and environmentally safe nematicides for the management of RKN. The field was located at the Teaching and Research Farm of the University of Ilorin and set out in a randomised complete block design, with four replications. Aqueous and powdered extracts of Chromolaena odorata (L) King and Robinson and Ficus mucuso Welw. ex Ficalho were applied alone and in combination. There were seven total treatments: C. odorata aqueous, F. mucuso aqueous, C. odorata powder, F. mucuso powder, C. odorata and F. mucuso aqueous, C. odorata and F. mucuso powder, and the control. Data were collected on growth, yield and nematode populations. All data collected were subjected to analysis of variance, and treatments were compared using Duncan’s multiple range test at a 5% level of significance. The essential oil of each botanical was determined using gas chromatography-mass spectrometry. The vine length (185.61) and yield (2401.05) of plants treated with botanicals were significantly higher than those of the control at P<0.005. The nematode population was also significantly lower in plants treated with botanicals than in the control (318.30 and 230.00, first and second year, respectively) at P<0.005. Among the treatments, the combination of C. odorata and F. mucuso powders was the most effective, with higher growth and yield performance. The experiment showed that aqueous and powdered extracts of C. odorata and F. mucuso were effective in managing RKN in the field.
Keywords: Botanicals; C. odorata; extracts; F. mucuso; watermelon.
INTRODUCTION
Citrullus lanatus (Thunb.) Matsum. and Nakai (watermelon) belongs to the Cucurbitaceae family. It is unique for its fleshy and tasty fruit with a high water content. Watermelon is also used for nutritional, medicinal, economic and cultural purposes (Sabo et al., 2013). It is cultivated worldwide, both in the sub-tropic and tropics. In Sub-Saharan Africa, it is a source of income.
The largest producers of watermelon in the world are China, Iran and Turkiye (Atlasbig, 2022). Watermelon production in developing nations is affected by factors such as insufficient farm inputs, soil infertility, poor transportation, inadequate storage facilities and the prevalence of pests and other disease-causing organisms (Adojutelegan et al., 2015).
Of these factors, plant disease is one of the biggest constraints. Diseases are usually caused by insect pests and/or pathogens, such as bacteria, fungi, viruses and nematodes. Globally, diseases account for about 20-40% of the annual loss incrop yield, with an economic loss of around $220 billion (FAO, 2019). In watermelon, root-knot nematodes are among the most problematic pathogens, causing yield loss (Thies et al., 2016).
Root-knot nematodes (Meloidogyne spp.) are the most economically important plant parasitic nematodes (Shakeel et al., 2020). They are also the most damaging plant nematode, having the broadest host range and infecting over 1200 plant species, including watermelon (Bello et al., 2020). All cultivated varieties of watermelon are susceptible to root-knot nematode (Thies et al., 2016). Most plants in the cucurbit family are also very susceptible to nematodes (Noling, 2019). Root-knot nematode threats are compounded by numerous species adapted to different climatic conditions. Over 100 nematode species have been reported throughout the world, with 22 found in Africa (Onkendi et al., 2014). Of these, 4 nematode species (Meloidogyne incognita, Meloidogyne javanica, Meloidogyne enterolobii and Meloidogyne arenaria) have been associated with watermelon in Africa (Bello et al., 2020). On cultivated land, the root-knot nematode can cause a yield loss of 25-50% (Feyisa, 2021).
To combat the scourge of root-knot nematode, different management practices have been adopted. Some of them include synthetic pesticides, biological control and solarisation (Aioub et al., 2022; Ali et al., 2022; Bhat et al., 2023; Feyisa, 2021; Forghani and Hajihassani, 2020). While they may be effective in controlling nematodes, they also have limitations that arise from a low rate of acquisition, overuse and regulation bottlenecks.
For instance, the use of synthetic pesticides has been associated with abuse in Sub-Saharan Africa, which leaves pesticidal residue on crop produce, causes soil contamination and pollutes underground water (Fuhrimann et al., 2022).
Similarly, the adoption of biological controls is generally low in the region. This is because of high-cost purchases and inadequate awareness of the new technology among local farmers. Chromolaena odorata (L) King (Figure 1) and Robinsonand Ficus mucuso Welw. ex Ficalho (Figure 2) were selected for this study because of their availability and easy accessibility to farmers in the state. Some earlier reports have also indicated that they contain phytochemicals, such as tannins, alkaloids, saponins, tannins, terpenoids and phenols, that could have nematocidal properties (Usunomena and Efosa, 2016). This may serve as a practical alternative to the use of synthetic pesticides.
Therefore, the purpose of this study wasto examine the effects of botanicals as a practical alternative in the management of root-knot nematode. Specifically, this study aimed to determine the efficacy of C. odorata and F. mucuso botanicals on the growth and yield attributes of watermelon infected by root-knot nematode in Kwara state, Nigeria. Botanicals are environmentally friendly, accessible and readily available for use by farmers (El-Ashry et al., 2023; Eldeeb et al., 2022; Olabiyi, 2004).
MATERIALS AND METHODS
Description of the experimental site
The experiment was carried out at the Teaching and Research Farm of the University of Ilorin, Ilorin, during the 2018 and 2019 cropping seasons, starting in September. The site was located at 8”29N, 43”5E of the equator. It is characterised by two rainfall patterns, which are highest between July and September, with a short dry spell in August.

Figure 1 – Chromolaena odorata plant
Source: Wikipedia

Figure 2 – Ficus mucuso leaf
Credit: Photo by N.J. Cordeiro, Post-Production by J. Quicho. Licenced under CC BY 2.0
The soil type of the area issandy-loam, and rainfall ranges annually from 1000 to 1200 mm (Ajala et al., 2021). The average length of daylight is 12 hours, which does not vary substantially throughout the year. The topography of the study area is plain, and the land is typically used for rain-fed agriculture.
The site was cultivated with vegetables and maize in previous years. Some cucurbits that have recently been cultivated in the field include cucumber and fluted pumpkin. Other crops cultivated around the field include vegetables, sugarcane, tuber, cucumber and spices. The soil has a history of root-knot nematodes, such as M. incognita and M. javanica (Sossou et al., 2021).
Collection of planting materials and botanicals
The watermelon variety used for this experiment was kaolak. This variety was selected because it is the most widely cultivated watermelon variety in the state. The botanicals C. odorata and F. mucuso were collected on the premises of the University of Ilorin, Ilorin. The identification of both plants was done at the Department of Plant Biology, University of Ilorin.
Field preparation and experimental layout
Weeds and debris were cleared manually from the field. A land area measuring 15 m × 40 m was ploughed and arrowed into ridges. A pre-emergence herbicide, Paraquat, was sprayed into the soil to prevent weeds atthe site. The experiment was set out in a randomised complete block design (RCBD) and each treatment was applied in four replicate plots. The land was divided into 20 plots with each plot measuring 6.3 m × 5.2 m. A 1-m alley was left between plots and a 2-m alley between blocks. Before planting, composite samples were collected from each plot at a depth of 15 cm to determine the initial nematode population and the physiochemical properties of the soil.
Sowing of watermelon seeds
Four seeds of the kaolak variety were sown at a depth of 2.5 cm, with a space of 90 cm × 75 cm between plants (Enujeke, 2013). The seeds were watered after sowing. Germinated seedlings were reduced to two per stand to prevent overcrowding.
Preparation of aqueous and powdered leaf extracts of botanicals
Preparation of the aqueous extract
Leaf samples of C. odorata and F. mucuso were collected separately and airdried at room temperature (27°C) for 14 days. The leaves were then pulverised using a pestle and mortar. Precisely 500 g of the pulverised leaf powder was soaked in 5000 mL of warm distilled water for 24 hours (Olajide et al., 2018). The resulting suspension was filtered through a muslin cloth, and the sieved extract was diluted to a 95% concentration. The aqueous extract from each of the botanicals was kept inside a sealed plastic bottle until it was ready for use. The remaining samples were used for gas chromatography-mass spectrometry (GC-MS) analysis. GC-MS analysis was performe during the standard procedure described by Vimalavady and Kadavul (2013).
Preparation of the leaf powder
The leaves of both botanicals were separately air-dried for 14 days and finely pulverised to powder using a pestle and mortar. About 10 kg of the powder was prepared from each botanical and applied at a concentration of 100 g per watermelon plant (Moosavi, 2012).
Treatment application
The prepared treatments are described in Table 1. The treatments were applied twice, 1 week after planting and 1 month after germination.
Round holes were made around the base of each plant, and 200 ml of aqueous crude extract was applied to the holes. For the powder extract, 100g was incorporated into the holes and covered with soil. The dosage used for the treatments was based on the historyof RKN in the field (Sossou et al., 2021). Untreated plots were used as a control.
Determination of the nematode population
The numberof nematodes per 100 g of soil was determined using the modified extraction tray method by Whitehead and Hemming (1965). The root-knot nematodes present in the soil were identified under a compound microscope using the CABI Crop Protection Compendium (CABI, 2007) and interactive diagnostic keys (Coyne et al., 2007).
Cultural practices
Manual weeding was performed with hoes every 14 days. Seedlings were thinned to 2 per stand by hand. Water was supplied to the roots of the plants using watering cans during dry spells throughout the experiment.
Data collection and analysis
Data were collected on vine length, fruit weight and shoot weight. The length of the highest vine was taken as the vine length, and the average weights of the fruit and shoots of the plants were taken as the fruit and shoot weights, respectively. The vine length was determined using a measuring tape and a measuring scale was used to determine the weights of the fruit and shoots. Data on vine length werecollected between 2 and 11 weeks after planting (WAP), while the shoot and fruit weights were determined at 11 WAP.
Other data collected include the initial nematode population, population after 1 month, final nematode population, reproduction index, gall index and egg mass/root. The reproduction index was determined using the formula, R=Pf/Pi, where Pf represents the final nematode population and Pi represents the initial nematode population.
The gall index was rated using the Bridge and Page (1980) method, as described below:
0 – No knots on roots;
1 – Few small knots, difficult to find;
2 – Small knots only, but clearly visible, main roots clean;
3 – Some larger knots visible, main root clean;
4 – Large, knots predominant but main root clean;
5 – 50% of roots affected knotting on some main roots;
6 – Knotting on main roots;
7 – Majority of main roots knotted;
8 – All main roots, including tap roots, knotted. Few clean roots visible;
9 – All roots severely knotted;
10 – All roots severely knotted; no root system. Plant usually dead.
All numerical data were analysed by an analysis of variance (ANOVA) using SPSS software, and significant means were separated using Duncan’s Multiple Range Test (DMRT) at alpha = 0.05.
Table 1
The botanical treatments applied in the management of root-knot nematode and their full description
|
S/N |
Treatments |
Treatment description |
|
1. |
Chromo aq. |
Chromolaenaodorata Aqueous |
|
2. |
Ficus aq. |
Ficusmucuso Aqueous |
|
3. |
Chromo pw. |
Chromolaenaodorata Powder |
|
4. |
Ficus pw. |
Ficusmucuso Powder |
|
5. |
Chromo+ Ficus aq. |
Chromolaenaodorata and Ficusmucuso Aqueous |
|
6. |
Chromo+Ficus pw |
Chromolaenaodorata and Ficusmucuso Powder |
|
7. |
Control |
Untreated |
S/N= serial number
RESULTS
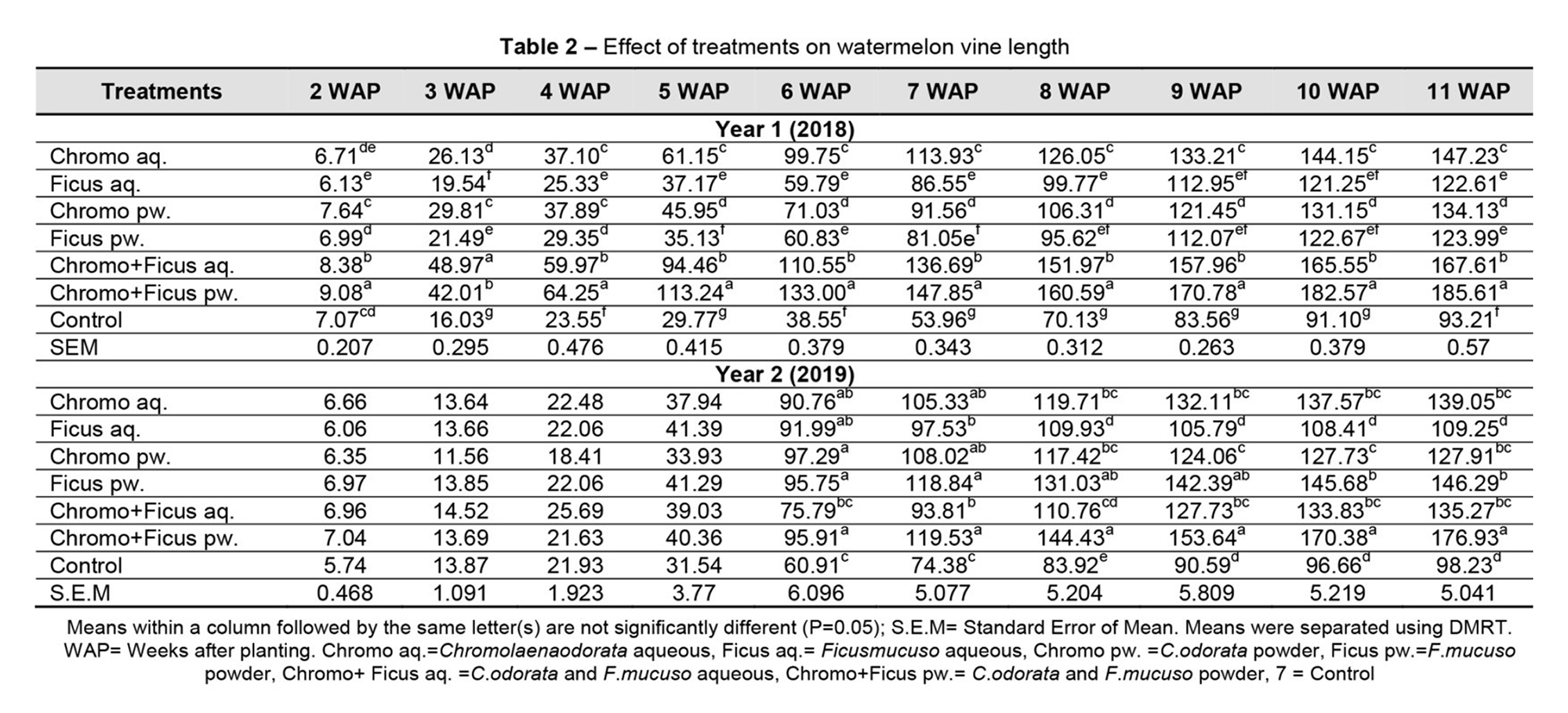
Table 2 shows the effect of treatments on the vine length of watermelon. The results revealed an increase in vine length among all treatments, from 2 to 11 WAP. At 2 WAP, the plants treated with C. odorata and F. mucuso powder had a vine length of 9.08 cm and performed significantly better than the other treatments. From 3 WAP until the end of the experiment, all plants treated with botanicals performed significantly better than the controls. Among the botanicals, Chromo and Ficus pw-treated plants had a significantly longer vine length than the other treatments (4-11 WAP). A similar trend was observed in 2019.
The effects of the treatments on yield (fruit and shoot weight) are shown in Table 3. The weight of the watermelon fruit treated with botanicals was significantly higher than that of the control at P < 0.05. The performance among the botanical treatments revealed that C. odorata and F. mucuso pw. had the highest fruit weight, which was significantly higher than treatment with C. odorata aq., F. mucuso aq., C. odorata pw., F. mucuso pw., and C. odorata and F. mucuso aq. However, in 2019, the shoot weight of the treated plants was not significantly different from that of the control.
Table 4 shows the effects of treatments on the root-knot nematode population initially, one month after planting, and at harvest, the gall index, the reproduction index and egg mass/root during the 2018 and 2019 planting seasons. In 2018, the root-knot nematode population in plants treated with botanicals was significantly lower than in the control. This reduction in the nematode population was observed among all botanical treatments. The reproduction index, gall index and egg mass/root was also higher in the control plantsthan botanicals. Similarly, the root-knot nemato depopulation, reproduction index, gall index and egg mass/root were higher in the control during the 2019 planting season. Table 5 shows the essential oils identified from C. odorata and F. mucuso using GC-MS. Nine essential oils were identified in C. odorata, while eight were discovered on F. mucuso. Four of the compounds, namely Bicyclo[3.1.1] heptane, 2,6,6-trimethyl-(1.alpha., 2.beta., 5.alpha.), n-Hexadecanoic acid, Phytol and Isophytol, were found in both C. odorata and F. mucuso. However, the remaining essential oils were discovered separately in each botanical.
DISCUSSION
The use of botanicals in the management of plant pathogens has been widely viewed as one of the most practical alternatives to the abuse of synthetic pesticides, especially in developing nations. Botanicals are cheaper, readily available and can be easily adopted by local farmers (Ngegba et al., 2022). This study demonstrated the pesticidal potential of C. odorata and F. mucuso in the management of root-knot nematodes infecting watermelon. All applied botanicals (alone and in combination) showed toxicity to the nematode, leading to a reduction in the reproduction rate, gall index and population density. This reduction in the nematode population could be attributed to the presence of pesticidal compounds in C. odorata and F. mucuso extracts. Some of the essential oils found in extracts have been reported to have anti-microbial properties that inhibit the function of pathogens in plants (Murgananthan and Pabbithi, 2012). For instance, findings by Vimalavady and Kadavul (2013) showed that compounds such asn-Hexadecanoic acid, which is associated with both C. odorata and F. mucuso, have antimicrobial and antioxidant activities against many pathogens.
A similar investigation by Kamatchi et al. (2019) revealed that an aqueous extract of C. odorata led to the suppression of egg hatching and an increase in juvenile mortality of nematodes. Murgananthan and Pabbithi (2012) made a similar observation of the bioactive compounds of F. mucuso against some pathogens.
Among the botanical treatments, the combination of C. odorata and F. mucuso powder was the most effective in suppressing the nematode population and enhancing the growth and yield attributes of watermelon (Table 2 and Table 3). This suggests that there was a higher concentration of essential oils in extract combinations than in single extracts, thereby increasing their nematocidal properties. The presence of n-Hexadecanoic acid and Bicyclo[3.1.1]heptane, 2,6,6-trimethyl- (1.alpha., 2.beta., 5.alpha.) in both C. odorata and F. mucuso could have increased the concentration of the compounds and also increased their toxicity to the root-knot nematode.
The type of extract may also determine its effectiveness on nematodesin the field. Unlike aqueous extracts, which can be taken up within hours or a few days, powder takes a longer time to decompose, making it more effective over a longer period.
The powders may also act as composts to provide a form of organic supplement, thereby adding nutrients to the plants. The nutrients released can be taken up by plants and used as part of the photosynthates. Chromolaena odorata are known to provide useful hormones to standing crops, enhance soil organic carbon and steadily release nutrients during crop growth, thereby contributing to better growth and yield (Kumari et al., 2013).
These factors can enhance plant growth under field conditions. All botanical-treated plants had higher growth and yield than the control.
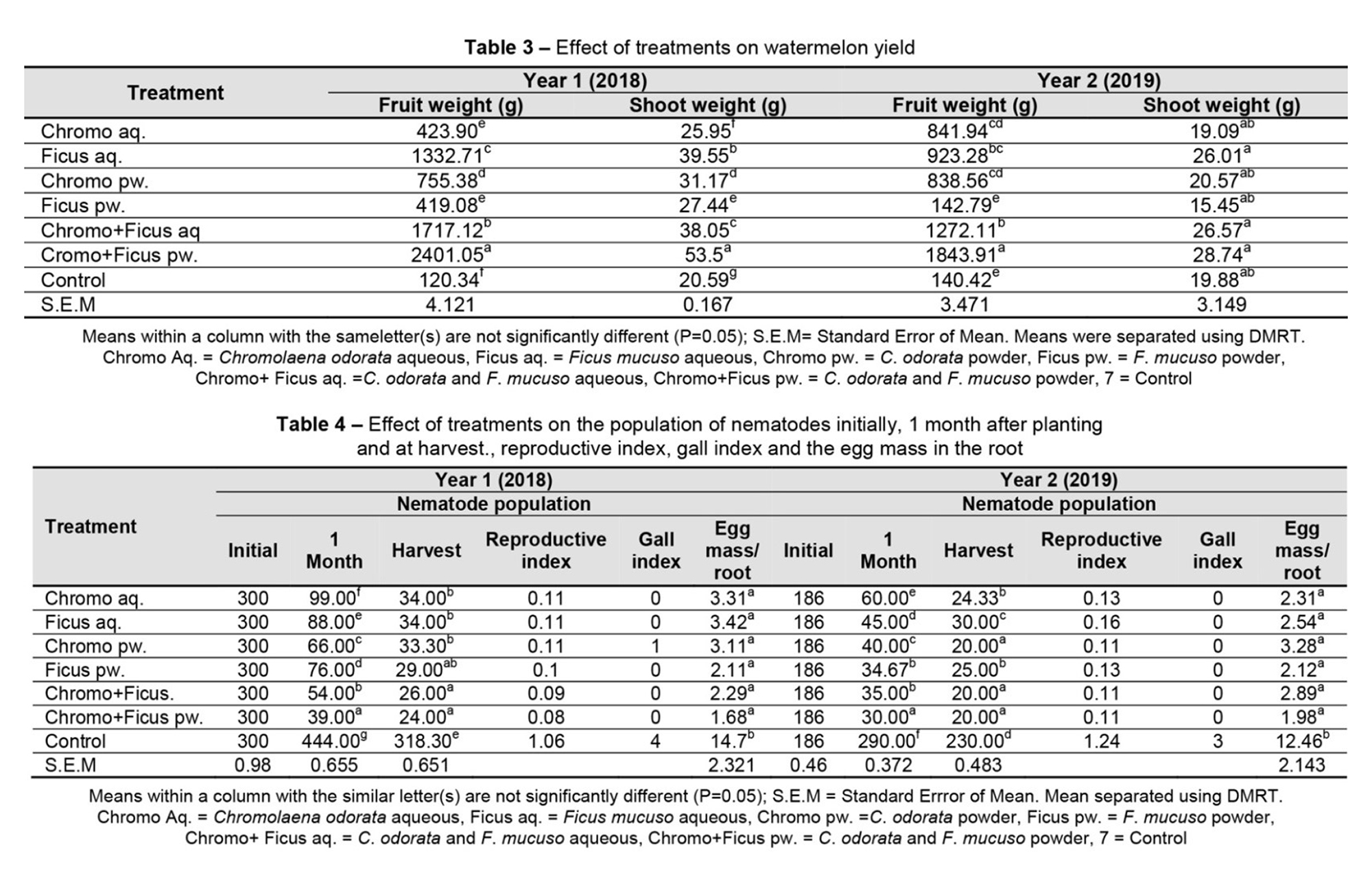
Table 5
List of essential oils found in Chromolaena odorata and Ficus mucuso using gas chromatography-mass spectrometry (GC-MS)
|
Botanical |
S/n |
Essential oil |
Molecular formula |
Quality |
Peak area (%) |
|
Chromolaena odorata |
1 |
1,9-Nonanediol, dimethanesulfonate |
C11H24O6S2 |
64 |
16.92 |
|
2 |
Bicyclo[3.1.1]heptane, 2,6,6-trimethyl- (1.alpha.,2.beta.,5.alpha.) |
C10H18 |
58 |
16.92 |
|
|
3 |
9-Octadecyne |
C18H34 |
64 |
6.13 |
|
|
4 |
1-Methoxy-3-(2-hydroxyethyl)nonane |
C12H26O2 |
46 |
6.13 |
|
|
5 |
n-Hexadecanoic acid |
C16H32O2 |
99 |
38.59 |
|
|
6 |
Tetradecanoic acid |
C14H28O2 |
83 |
38.59 |
|
|
7 |
Phytol |
C20H40O |
91 |
12.19 |
|
|
8 |
Isophytol |
C20H40O |
53 |
12.19 |
|
|
9 |
(Z)6,(Z)9-Pentadecadien-1-ol |
C15H28O |
90 |
23.01 |
|
|
Ficus mucuso |
1 |
Bicyclo[3.1.1]heptane, 2,6,6-trimethyl- (1.alpha.,2.beta.,5.alpha.) |
C10H18 |
70 |
5.73 |
|
2 |
Z-12-Tetradecen-1-ol |
C16H30O2 |
49 |
5.73 |
|
|
3 |
Camphor |
C10H16O |
38 |
2 |
|
|
4 |
n-Hexadecanoic acid |
C16H32O2 |
99 |
34.12 |
|
|
5 |
Tridecanoic acid |
C13H26O2 |
83 |
34.12 |
|
|
6 |
Phytol |
C20H40O |
86 |
12.75 |
|
|
7 |
Isophytol |
C20H40O |
80 |
12.75 |
|
|
8 |
Oleic Acid |
C18H34O2 |
92 |
45.4 |
% = percentage
This is understandable because a reduction in the pathogen population is expected to lead to better crop growth and yield (Shakeel et al., 2022a,b).
This finding agrees with the study of Izuogu et al. (2016) on the effectiveness of botanicals in suppressing the population of root-knot nematodes infecting cucurbits. Similar to their report, this investigation revealed that botanicals could be a cheaper alternative to synthetic pesticides in the management of RKN, especially in fields with a history of this nematode.
CONCLUSIONS
This study has established the importance of botanicals in the management of root-knot nematodes. Both C. odorata and F. mucuso were identified as major biopesticides that could effectively reduce the nematode population, leading to improved crop growth and yield. Therefore, we recommend the use of botanicals. We also suggest that more studies should be done to identify and optimise the essential oils present in botanicals to formulate them into effective pesticides.
Author Contributions: Conceptualisation (AOA, INB), methodology (AOA), analysis AOA, INB, BOS), investigation (AOA, AIO, OCM), resources (AOA, BHS, AIO, AMA), data curation (AOA, AMA, OCM), writing (AOA), editing (AOA, INB, BOS); review (AOA, INB), supervision (INB, BOS). All authors declare that they have read and approved the publication of the manuscript in the present form.
Funding: No special funding or resources were provided for this research.
Conflicts of Interest: Authors declare no conflict of interest.
REFERENCES
Adojutelegan, O.T.; Adereti, F.O.; Makanju, T.S.; Olorunfemi, O.D. Analysis of Factors Affecting Watermelon Production in Ekiti State, Nigeria. Science, Technology and Arts Research Journal. 2015, 4, 324-329. http://dx.doi.org/10.4314/star.v4i2.45.
Ajala, O.N.; Adjadeh, T.A.; Olaniyan, J.O.; Isimikalu, T.O.; Nartey, E.K.; James, F.O. Characterization, classification and suitability evaluation of soils under sugarcane (Saccharum officinarum L.) cultivation at the Sugar Research Farm, University of Ilorin, Nigeria. Agro-Science. 2021, 20, 14-23. https://doi.org/10.4314/as.v20i3.3.
Aioub, A.A.; Elesawy, A.E.; Ammar, E.E. Plant growth promoting rhizobacteria (PGPR) and their role in plant-parasitic nematodes control: a fresh look at an old issue. Journal of Plant Diseases and Protection. 2022, 129, 1305-1321. https://doi.org/10.1007/s41348-022-00642-3.
Ali, A.A.; El-Ashry, R.M.; Aioub, A.A. Animal manure rhizobacteria co-fertilization suppresses phytonematodes and enhances plant production: evidence from field and greenhouse. Journal of Plant Diseases and Protection. 2022, 129, 155-169. https://doi.org/10.1007/s41348-021-00529-9.
Atlasbig. World watermelon production by country. https://www.atlasbig.com/en-gb/countries-by-watermelon-production (accesed on 23 October 2022).
Bhat, A.A.; Shakeel, A.; Waqar, S.; Handoo, Z.A.; Khan, A.A. Microbes vs. Nematodes: Insights into Biocontrol through Antagonistic Organisms to Control Root-Knot Nematodes. Plants. 2023, 12, 451. https://doi.org/10.3390/plants12030451
Bello, T.T.; Coyne, D.L.; Rashidifard, M.; Fourie, H. Abundance and diversity of plant-parasitic nematodes associated with watermelon in Nigeria, with focus on Meloidogyne spp. Nematology. 2020, 22, 781-797. https://doi.org/10.1163/15685411-00003340.
Coyne, D.L.; Nicol, J.M.; Claudius-Cole, B. Practical plant nematology: a field and laboratory guide. 3rd edition, International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria, 2018.
CABI (Crop Protection Compendium). Citrullus lanatus (watermelon), 2007. Available at: http://www.cabi.org/cpc/datasheet/13678.
El-Ashry, R.M.; Aioub, A.A.; Awad, S.E. Suppression of Meloiodogyne incognita (Tylenchida: Heteroderidae) and Tylenchulus semipenterans (Tylenchida: Tylenchulidae) using Tilapia fish powder and plant growth promoting rhizobacteria in vivo and in vitro. European Journal of Plant Pathology. 2023, 165, 665-676. https://doi.org/10.1007/s10658-023-02637-8.
Eldeeb, A.M.; Farag, A.A.G.; Al-Harbi, M.S.; Kesba, H.; Sayed, S.; Elesawy, A.E.; et al. Controlling of Meloidgyne incognita (Tylenchida: Heteroderidae) using nematicides, Linum usitatissimum extract and certain organic acids on four peppers cultivars under greenhouse conditions. Saudi Journal of Biological Sciences. 2022, 29, 3107-3113. https://doi.org/10.1016/j.sjbs.2022.03.018.
Enujeke, E.C. An assessment of some growth and yield indices of six varieties of watermelon (Citrulus lanatus Thumb) in Asaba area of Delta State, Nigeria. International Research Journal of Agricultural Science and Soil Science. 2013, 3, 376-382.
FAO. New standards to curb the global spread of plant pests and diseases. https://www.fao.org/news/story/en/item/1187738/icode (accesed on 23 October 2019).
Feyisa, B. A Review on Root Knot Nematodes (RKNs): Impact and Methods for Control. Journal of Plant Pathology & Microbiology. 2021, 12, 547.
Forghani, F.; Hajihassani, A. Recent Advances in the Development of Environmentally Benign Treatments to Control Root-Knot Nematodes. Frontiers in Plant Science. 2020, 11, 1125. https://doi.org/10.3389/fpls.2020.01125.
Fuhrimann, S.; Wan, C.; Blouzard, E.; Veludo, A.; Holtman, Z.; Chetty-Mhlanga, S.; Dalvie, M.A.; Atuhaire, A.; Kromhout, H.; Röösli, M.; Rother, H.A. Pesticide Research on Environmental and Human Exposure and Risks in Sub-Saharan Africa: A Systematic Literature Review. International Journal of Environmental Research and Public Health. 2021, 19, 259. https://doi.org/10.3390/ijerph19010259
Izuogu, N.B.; Abolusoro, S.A.; Yakub, L.B. Nematicidal potential of aqueous extract of Hyptis suaveolensin the management of root-knot nematode, Meloidogyne incognita of some cowpea cultivars. Croation Journal of Food Science and Technology. 2016, 8, 15-19. http://dx.doi.org/10.17508/CJFST.2016.8.1.05.
Kamatchi, K.; Nattuthurai, N.; Krishnamoorthy, S. Evaluation of egg hatchability and larval mortality of methanolic extracts of Chromolaena odorata and Annona squamosa on Meloidogyne incognita. International Journal of Life Sciences Research. 2019, 7, 240248.
Kumari, P.; Yadav, P.; Verma, P.R.; Kumar, S.; Arya, A. A review on wound healing properties of Indian medicinal plants. Indian Journal of Fundamental and Applied Life Sciences. 2013, 3, 220-232.
Moosavi, M.R. Nematicidal effect of some herbal powders and their aqueous extracts against Meloidogyne javanica. Nematropica. 2012, 42, 48-56.
Murgananthan, G.; Pabbithi, S.C. Antimicrobial constituents from plants. International Research Journal of Pharmacy. 2012, 3, 5-9.
Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of Botanical Pesticides in Agriculture as an Alternative to Synthetic Pesticides. Agriculture. 2022, 12, 600. https://doi.org/10.3390/agriculture12050600.
Noling, J.W. Nematode Management in Cucurbits (Cucumber, Melons, Squash). University of Florida Cooperative extension service, 2019. https://edis.ifas.ufl.edu/publication/NG025.
Onkendi, E.M.; Kariuki, G.M.; Marais, M.; Moleleki, L.N. The threat of root-knot nematodes ( Meloidogyne spp.) in Africa: A review. Plant Pathology. 2014, 63, 727-737. https://doi.org/10.1111/ppa.12202.
Olabiyi, T.I. Assessment of the Nematicidal Properties of Extracta Tagetes erecta, Ocimum gratissimum, Hyptis suaveolens and Crotaloria retusa. PhD Thesis, Department of Crop Protection, University of Ilorin, Ilorin. Nigeria, 2004, pp. 177.
Sabo, M.U.; Wailare, M.A.; Aliyu, M.; Jari, S.; Shuaibu Y.M. Effect of NPK fertilizer and spacing on growth and yield of watermelon (Citrillus lanatus L.) in Kaltungo Local Government area of Gombe State, Nigeria. Scholarly Journal of Agricultural Science. 2013, 3, 325-330.
Shakeel, A.; Bhat, A.H.; Bhat, A.A.; Khan, A.A. Interactive effect of Meloidogyne incognita and fly ash on the growth, physiology, and antioxidant properties of carrot (Daucus carota L.). Environmental Science and Pollution Research. 2022a, 29, 7661-7677. https://doi.org/10.1007/s11356-021-16160-y.
Shakeel, A.; Khan, A.A.; Upadhyay, S.K. Eco-friendly dual-edged management of fly ash and its antagonistic interplay with Meloidogyne incognita on beetroot (Beta vulgaris L.). Environmental Research. 2022b, 209, 112767. https://doi.org/10.1016/j.envres.2022.112767.
Shakeel, A.; Khan, A.; Haris, M. Multifaceted Strategies Used by Root-Knot Nematodes to Parasitize Plants-A Review. Phyton-International Journal of Experimental Botany. 2020, 89. https://doi.org/10.32604/phyton.2020.08922.
Sossou, B.K.; Izuogu, N.B.; Anifowose, A.O.; Ahamefule, H.E. Controlling root-knot nematode Meloidogyne incognita in tomatoes using modified effective microorganisms-fermented plant extract and compost manure. International Journal of Recycling of Organic Waste in Agriculture. 2022, 11, 427-436. https://doi.org/10.30486/IJROWA.2021.1937252.1307.
Thies, J.A.; Ariss, J.J.; Kousik, C.S.; Hassell, R.L.; Levi, A. Resistance to Southern Root-knot Nematode (Meloidogyne incognita) in Wild Watermelon (Citrullus lanatus var. Citroides). Journal of Nematology. 2016, 48, 14-19. https://doi.org/10.21307/jofnem-2017-004.
Usunomena, U.; Efosa, E.G. Phytochemical analysis, mineral composition and in vitro antioxidant activities of Chromolaena Odorata leaves. Journal of Pharmaceutical Sciences. 2016, 2, 16-20. http://dx.doi.org/10.20431/2455-1538.0202003.
Vimalavady, A.; Kadavul, K. Phytocomponents identified on the various extracts of stem of Hugonia mystax L. (Linaceae). European Journal of Experimental Biology. 2013, 3, 73-80.
Whitehead, A.G.; Hemming, J.R. A comparison of some quantitative methods of extracting vermiform nematodes from soil. Annals of Applied Biology. 1965, 52, 25-28. http://dx.doi.org/10.1111/j.1744-7348.1965.tb07864.x.
Academic Editor: Dr. Iuliana Motrescu
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Adepoju Isaiah Olusesan, Adeyemi Muhammed Adewole, Apalowo Oluropo Ayotunde, Baba Halimat Shola, Balogun Olusegun Samuel, Izuogu Nkechi Betsy, Olajide C.M.



