Joyce Kwakye, Sydney Stanley Blankson
ABSTRACT. Albizia lebbeck is a multipurpose tree that has many benefits for the environment and the end user. The extent to which this tree species is cultivated is very low due to seed coat dormancy, which causes a longer germination period and late seedling growth. This research was done at Kwame Nkrumah University of Science and Technology, located in Kumasi, Ghana, at the Department of Horticulture, to ascertain the effect of seed pretreatment on germination and early seedling growth. Germination was observed in seeds pretreated with hot water (100º) for 1 minute (T1), dry heat (90º) for 2 minutes (T2), in 6% hydrogen peroxide solution for 30 minutes (T3), cold water at room temperature for 24 hours (T4) and untreated seed (T5) as the control. The experiment was conducted using a randomised complete block design (RCBD) with three replications. The data obtained for seed germination percentage were statistically analysed using one-way analysis of variance in Statistix 7.0 software, and the least significant difference at 5% probability level was used to separate the means of height, collar diameter and number of leaves. A comparison between the pretreated and control seeds showed no significant difference (P > 0.05) on germination percentage, germination rate, and root and shoot dry weights of Albizia lebbeck. However, it had a positive influence (P < 0.05) on early growth characteristics. Seeds soaked in cold water (T4) had maximum early seedling growth. Hydrogen peroxide treatment (T3) yielded the highest collar diameter growth. Hot water treatment (T1) had the lowest height, collar diameter and number of leaves. Cold water at room temperature should be encouraged as a pretreatment method for the early growth of Albizia lebbeck seedlings.
Keywords: Albizia lebbeck, Pre-sowing treatments, Germination, Early growth.
Cite
ALSE and ACS Style
Kwakye, J.; Blankson, SS. Effect of seed pre-sowing treatments on the germination and early growth of Albizia lebbeck seeds. Journal of Applied Life Sciences and Environment 2021, 54(3), 342-353.
https://doi.org/10.46909/journalalse-2021-030
AMA Style
Kwakye J, Blankson SS. Effect of seed pre-sowing treatments on the germination and early growth of Albizia lebbeck seeds. Journal of Applied Life Sciences and Environment. 2021; 54(3): 342-353.
https://doi.org/10.46909/journalalse-2021-030
Chicago/Turabian Style
Kwakye, Joyce, and Sydney Stanley Blankson. 2021. “Effect of seed pre-sowing treatments on the germination and early growth of Albizia lebbeck seeds” Journal of Applied Life Sciences and Environment 54, no. 3: 342-353.
https://doi.org/10.46909/journalalse-2021-030
View full article (HTML)
Effect of Seed Pre-Sowing Treatments on the Germination and Early Growth of Albizia Lebbeck Seeds
Joyce Kwakye1, Sydney Stanley Blankson1
1Kwame Nkrumah University of Science and Technology, Department of Agroforestry, Kumasi, Ghana
*E-mail: efyajoyce98@gmail.com
Received: Feb. 01, 2022. Revised: Mar. 16, 2022. Accepted: Mar. 17, 2022. Published online: Mar. 30, 2022
ABSTRACT. Albizia lebbeck is a multipurpose tree that has many benefits for the environment and the end user. The extent to which this tree species is cultivated is very low due to seed coat dormancy, which causes a longer germination period and late seedling growth. This research was done at Kwame Nkrumah University of Science and Technology, located in Kumasi, Ghana, at the Department of Horticulture, to ascertain the effect of seed pretreatment on germination and early seedling growth. Germination was observed in seeds pretreated with hot water (100º) for 1 minute (T1), dry heat (90º) for 2 minutes (T2), in 6% hydrogen peroxide solution for 30 minutes (T3), cold water at room temperature for 24 hours (T4) and untreated seed (T5) as the control. The experiment was conducted using a randomised complete block design (RCBD) with three replications. The data obtained for seed germination percentage were statistically analysed using one-way analysis of variance in Statistix 7.0 software, and the least significant difference at 5% probability level was used to separate the means of height, collar diameter and number of leaves. A comparison between the pretreated and control seeds showed no significant difference (P > 0.05) on germination percentage, germination rate, and root and shoot dry weights of Albizia lebbeck. However, it had a positive influence (P < 0.05) on early growth characteristics. Seeds soaked in cold water (T4) had maximum early seedling growth. Hydrogen peroxide treatment (T3) yielded the highest collar diameter growth. Hot water treatment (T1) had the lowest height, collar diameter and number of leaves. Cold water at room temperature should be encouraged as a pretreatment method for the early growth of Albizia lebbeck seedlings.
Keywords: Albizia lebbeck, Pre-sowing treatments, Germination, Early growth.
INTRODUCTION
Trees have been cultivated for hundreds of years, but their role in improving quality of life has never been recognised (Ahmad et al., 2015). Today, however, we are facing alarming population growth and food shortages, as well as environmental problems, such as global warming; thus, there is a need to plant more trees and ensure that they benefit the environment and the end user (Ahmad et al., 2015).
Seed has been considered the basis and simplest form of plant regeneration, conservation and dissemination. The seed phase is the most important stage in the life cycle of most species for survival, dormancy and germination, but to achieve these purposes, the seed must germinate when conditions are optimal for seedling development and subsequent plant growth. However, during this period, germination is inhibited by various dormancy mechanisms (Depali et al., 2007); therefore, seeds are treated. Germination in some species growing in semi-arid and arid areas of the tropics is very poor due to seed coat dormancy. Seeds are treated to ensure that they germinate quickly and evenly. Pretreatment techniques have been devised and described for several species; however, dormancy is still a problem for many tropical species due to low germination rates, a lack of general knowledge of their seed physiology and partly to dormancy variability (Azad et al., 2006). Pretreatment techniques often need to be adapted to individual species and seed lots based on experience and experimentation (Azad et al., 2006) to resolve the low germination rate for several tropical species, including Albizia lebbeck.
Albizia lebbeck (L.) Benth, belonging to the family Fabaceae, is a medium-sized, multi-purpose, deciduous tree species characterised by vigorous growth, nitrogen fixation and soil structure improvement (Kumar et al., 2018). Albizia lebbeck is native to Myanmar, Bangladesh, Australia, India, Indonesia, Pakistan, Nepal and Thailand (Kumar et al., 2018). It reaches 3-15 m in a plantation and up to 30 m in the open. Leaves are bipinnate. The fruit contains reddish brown pods that have flat rounded free-moving seeds (Sauvant et al., 2015). Albizia lebbeck is also known as ‘woman’s tongue’ and ‘rattle pod’, derived from the sounds of its pods swaying in the wind. Albizia lebbeck is a promising fodder and green manure tree. It is cultivated for shelter belts and serves as a shade tree in coffee and tea plantations (Kumar et al., 2018). It is most suitable for reforestation of degraded sites, erosion control, a fuel wood plantation and forage crop, and the source of hardwood in the agroforestry system. Albizia lebbeck is an economically important plant for industrial and medicinal uses (Gothecha et al., 2010). It can adapt to diverse types of soils, from acidic to alkaline and saline (Krishnakumar et al., 2010). It develops best on moist, well-drained loamy soils and undergoes epigeal germination. The seeds of Albizia lebbeck have been noted to exhibit physical dormancy due to the hardiness of its seed coat, resulting in poor germination (Kumar et al., 2018). The cause of its dormancy is its permeability to water. The greater the dormancy of the seeds, the fewer trees grow (Ahmad et al., 2015).
The role of hot water in seed pretreatment is to soften the seedcoat and make it permeable to permit germination through imbibition and gaseous exchange and to control coat-borne pathogens (Keshavkant et al., 2006). However, hydrogen peroxide breaks down the seed coat, thus allowing the seed to take in more oxygen, which is a necessary factor for germination.
The effects of different pre-sowing treatments on the seed germination of some species were published by Alamgir and Hossain (2005). Therefore, this study sought to assess the impact of different pre-treatment techniques on Albizia lebbeck seeds to break their dormancy for germination and early growth.
The aim of this study was to assess the effects of different pretreatment methods on the germination and early growth of Albizia lebbeck.
The scope of the study was to (1) determine the effect of different pretreatment methods on germination percentage and rate of Albizia lebbeck and (2) determine the early growth rate (height, collar diameter and number of leaves) of Albizia lebbeck after pretreatment.
MATERIALS AND METHODS
Study Site Description
This study was conducted at the Department of Horticulture, Kwame Nkrumah University of Science and Technology, Kumasi. The site is located in the moist semi-deciduous forest zone of Ghana with a bimodal rainfall distribution, where the highest rainfall occurs from March to July. The area receives between 1250 and 1500 mm of rainfall per year. The average annual temperature is 26º (Bakpa et al., 2018).
Seed Collection and Processing
Seeds were obtained from the Forestry Research Institute of Ghana (FORIG), Fumesua. First, a viability test was conducted using the floatation test method for 60 minutes. Using this method, seeds were put in a bowl of undisturbed water. After 60 minutes, the suspended seeds were considered dead, empty, unviable or insect damaged and they were discarded. Seeds that sank were gathered and used for the experiment because they were regarded as viable.
Experimental Design and Treatment
The experiment was designed using a randomised complete block design (RCBD) with 5 treatments, arranged in three blocks. The treatments were selected based on cost, availability and gentleness. Each of the five treatments contained 42 seeds. In total, 210 seeds were sown directly in polybags (10 cm × 15 cm), and two seeds were planted per bag. Fourteen seeds were allocated to each treatment in each block.
The treatments were allocated as follows (Table 1):
T1: Immersion in hot water (100ºC). The seeds were soaked for 1 minute, after which the water was drained, and the seeds were allowed to cool to room temperature overnight. Sowing was performed immediately.
T2: Dry heat. The seeds were placed in an oven at 90ºC for 2 minutes. The seeds were allowed to cool to room temperature overnight and then sown immediately.
T3: Immersion in 6% hydrogen peroxide solution. The seeds were soaked in a hydrogen peroxide solution for 30 minutes. The seeds were then rinsed thoroughly with tap water to remove the acid residue and were sown immediately. Hydrogen peroxide at a 6% concentration was used based on its positive influence on seed germination in a study conducted by Szopińska, (2014).
T4: Immersion in cold water at room temperature (25ºC). Seeds were soaked in tap water for 24 hours, after which the seeds were sown.
T5: Untreated seeds (as control).
Table 1 shows the treatments and design used for the experiment.
Table 1
Experimental Layout
|
BLOCK 1 |
BLOCK 2 |
BLOCK 3 |
|
T1 |
T5 |
T2 |
|
T3 |
T1 |
T4 |
|
T2 |
T4 |
T5 |
|
T4 |
T3 |
T1 |
|
T5 |
T2 |
T3 |
Field Experiment
The poly-bags were filled with loamy soil and perforated to allow excess water to drain. Pretreated seeds were randomly allocated to the bed. Seeds were sown at a depth of 3–4 cm and covered with a thin layer of soil. Poly-bags were arranged in a position of partial sunlight to create the warmth needed for seed germination. Labels were made on poly-bags to differentiate treatments. Watering was performed every day in the morning to keep the soil surface from getting dry, except on rainy days, by sprinkling the water using a rose can. Weeds that grew in the poly-bags were removed by hand. Bags were monitored for any signs of germination.
After germination, seedlings were monitored for two months to ascertain the effects of the treatments on growth. Measurements were made weekly on plant growth parameters (height, collar diameter, number of leaves).
DATA COLLECTION
Germination
Data were recorded on the number of seeds that germinated in each treatment, starting the day after sowing (1 December 2019), until no more seed germinated. The criterion for germination was visible protrusion on the surface of the soil, at least 0.5 cm from the cotyledon and hypocotyl of the seedlings. The germination counts for each treatment were added to obtain cumulative germination for the different treatments. At the end of the germination period, the germination percentage and rate for each treatment were calculated from the data using the following equation from Maguire (1962):
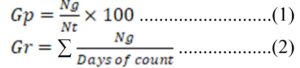
Where Gp is the germination percentage, Ng is the number of germinated seeds, Nt is the total number of seeds planted and Gr is the rate of germination.
Early seedling growth
Data were collected on early seedling growth. Measurements of the seedling height were made weekly (from the shoot tip of the plant to the base) using a measuring ruler. The collar diameter was measured at the base (2 cm above the ground) using a Vernier calliper. The number of leaves was counted directly. At the end of the experiment, seedlings were uprooted and separated into roots and shoots. They were then placed into respective envelopes and dried in an oven for 72 hours at 60ºC to a constant weight. Then, after cooling to room temperature, the roots and shoots were weighed on an electronic scale.
DATA ANALYSIS
Germination and Early Seedling Growth
Data were analysed for germination and seedling growth. The collected data were subjected to a one-way analysis of variance using Statistix 7.0 software to test the effects of the various treatments on seed and seedling. The least significant difference was used for mean separation at the p-value ≤ 0.05 level of significance. The results obtained on germination and the various growth parameters under the different treatments were presented in graphs using Microsoft Excel.
RESULTS AND DISCUSSION
Germination percentage of Albizia lebbeck seeds
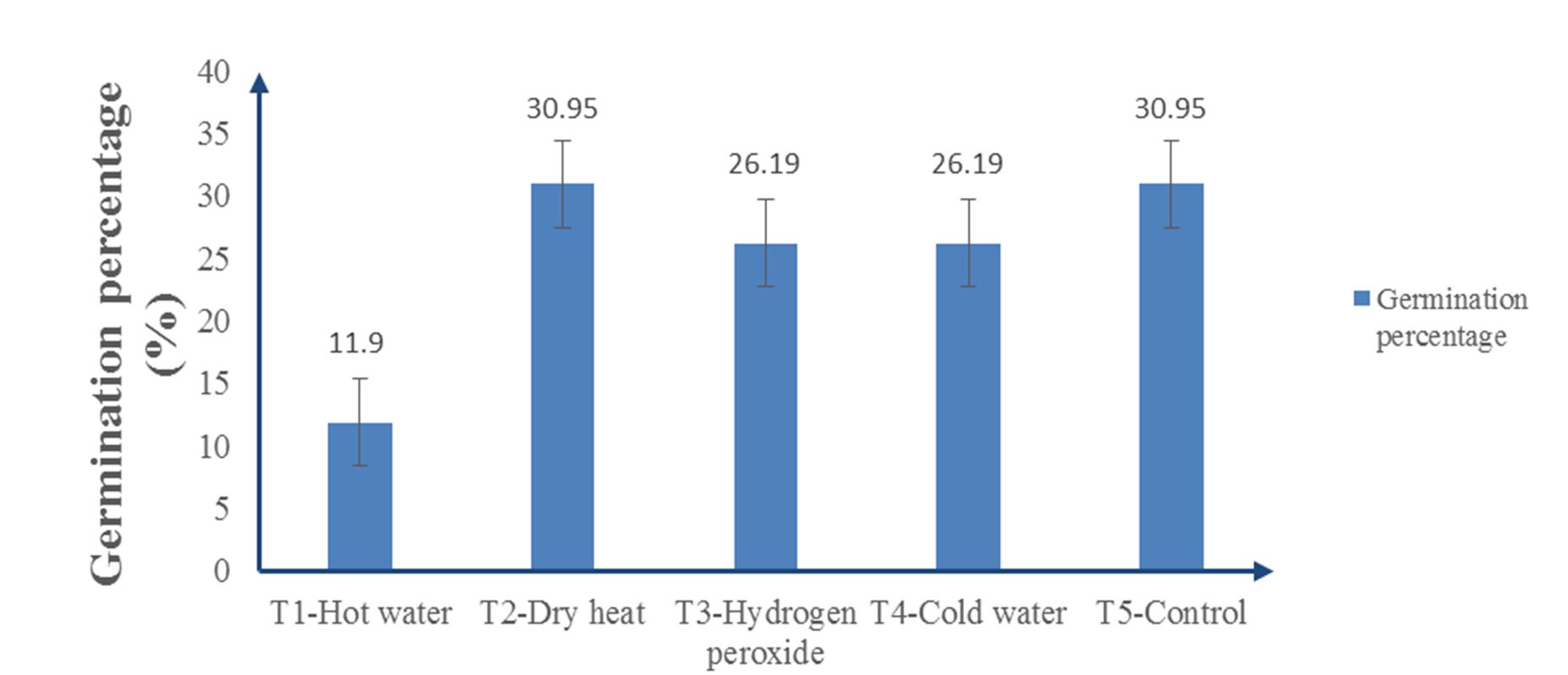
Fig. 1 shows the highest germination percentage of 30.95% in both dry heat (T2) and control (T5), while 26.19% was recorded for both the hydrogen peroxide (T3) and cold water (T4) treatments. Hot water (T1) had the lowest germination percentage of 11.9%. The treatments did not have any significant influence on the germination percentage of Albizia lebbeck seeds (P ≥ 0.05).
Germination rate of Albizia lebbeck seeds
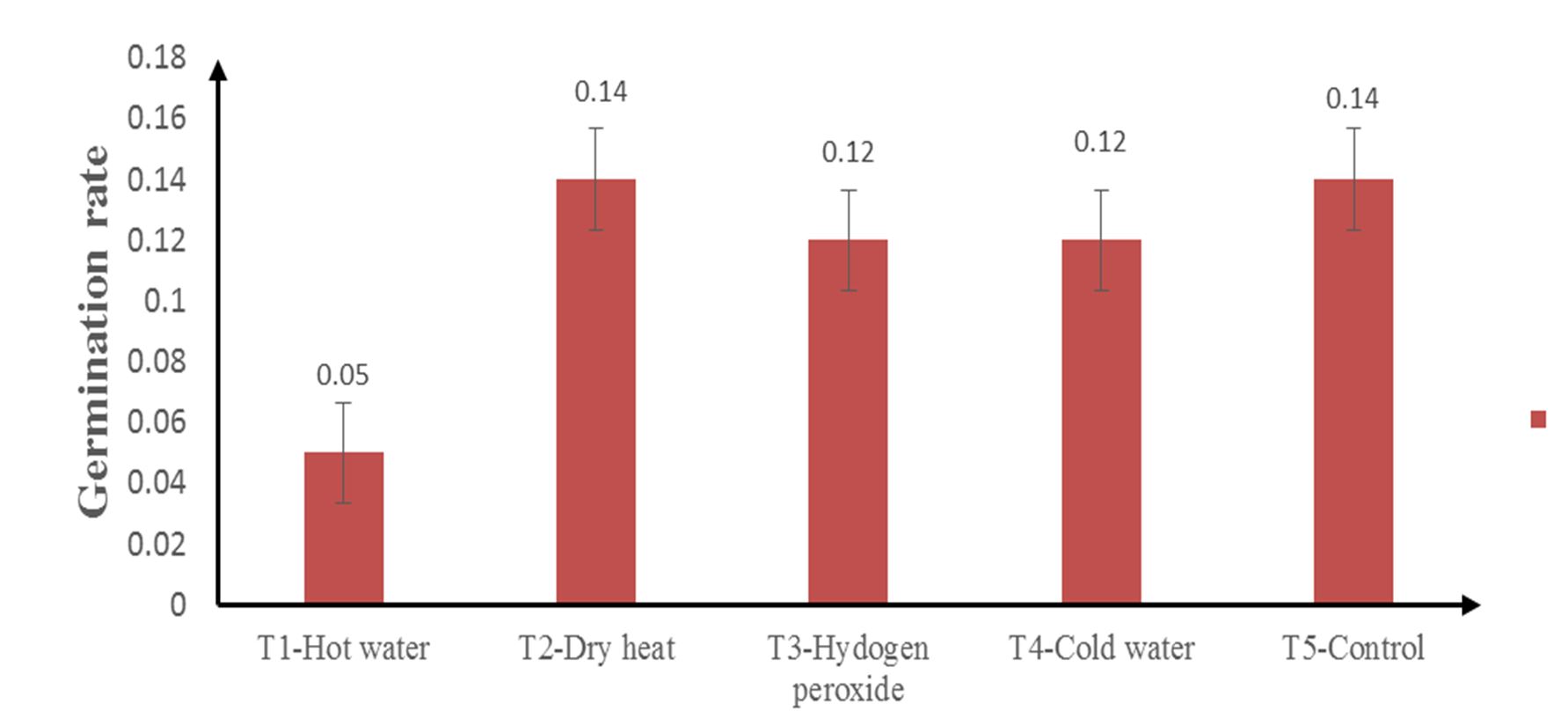
As seen in Fig. 2, the control (T5) and dry heat (T2) had the highest germination rate of 0.14, while 0.12 was recorded for both hydrogen peroxide (T3) and cold water (T4) treatments. Hot water (T1) had the lowest germination rate (0.05). The treatments had no significant influence on the germination rate (P ≥ 0.05).
GROWTH PARAMETERS OF ALBIZIA LEBBECK SEEDLINGS
Height growth of Albizia lebbeck seedlings
Fig. 3 is a graphical representation of the weekly height growth of Albizia lebbeck seedlings subjected to different seed pretreatment methods. Generally, height growth increased from week 1 to week 6 for all treatments. On a weekly basis, cold water (T4) treatment yielded the highest plants, followed by the control (T5), hydrogen peroxide (T3), dry heat (T2) and hot water (T1). The application of different treatments had a significant effect on plant height (P ≤ 0.05).
Collar diameter of Albizia lebbeck seedlings
Generally, the collar diameter increased in all treatments from week 1 to week 6. Hydrogen peroxide (T3) showed the highest growth, followed by cold water (T4), dry heat (T2), control (T5) and hot water (T1) (Fig. 4; P ≤ 0.05).
Leaf count of Albizia lebbeck seedlings
There was a weekly variation in the number of leaves. An increase in leaf count was observed from week 1 to week 6. The application of the different pretreatments had a significant effect on leaf growth (P ≤ 0.05). Cold water (T4) had the highest number of leaves, followed by hydrogen peroxide (T3), control (T5), dry heat (T2) and hot water treatment (T1) (Fig. 5).
Root and shoot dry weight of Albizia lebbeck seedlings
Pretreatment had no effect on root and shoot dry weight (P ≥ 0.05). The control (T5) had the highest root and shoot dry weights. This was followed by dry heat (T2), cold water (T4), hydrogen peroxide (T3) and hot water (T1) (Fig. 6).
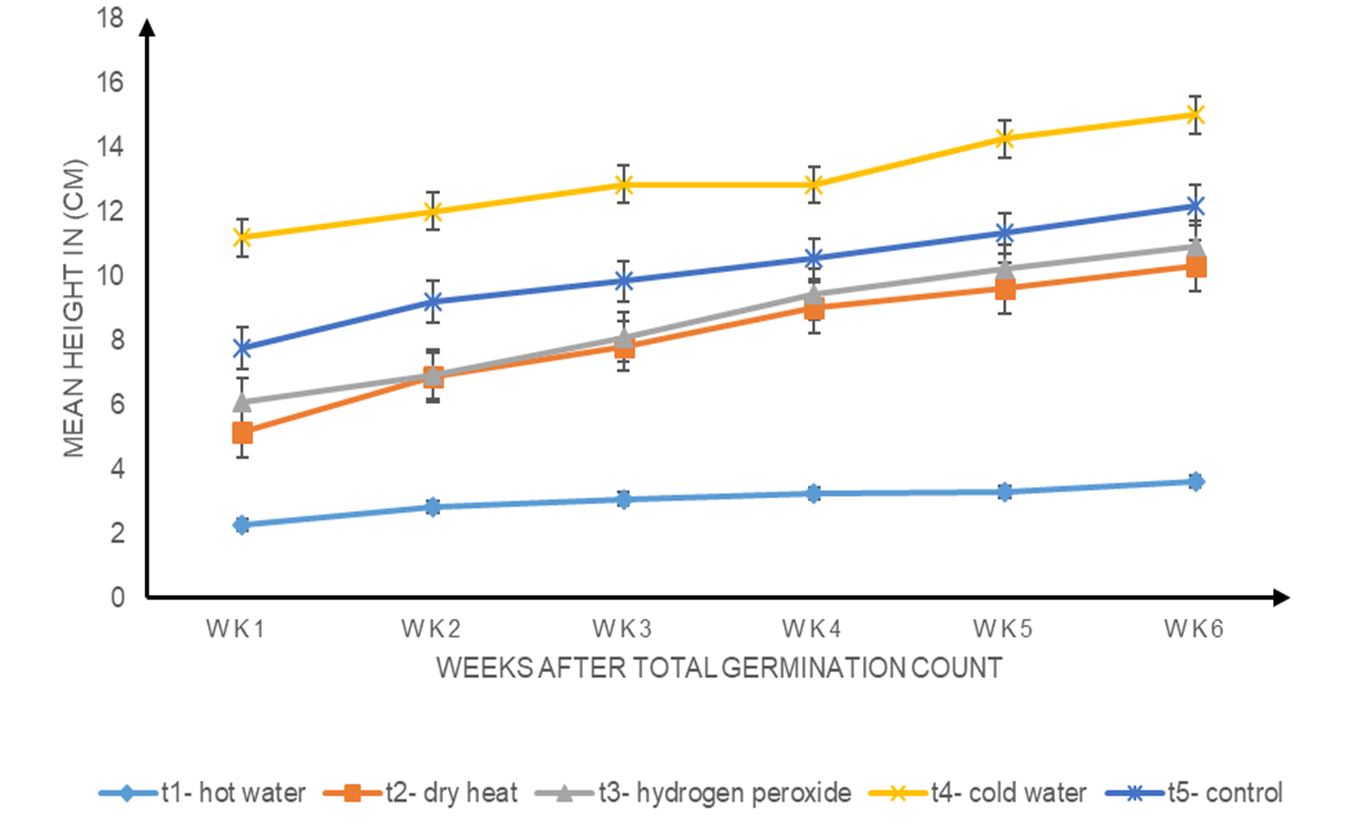
Figure 3. Effects of different seed pre-sowing treatments on the weekly mean height growth of Albizia lebbeck seedlings.
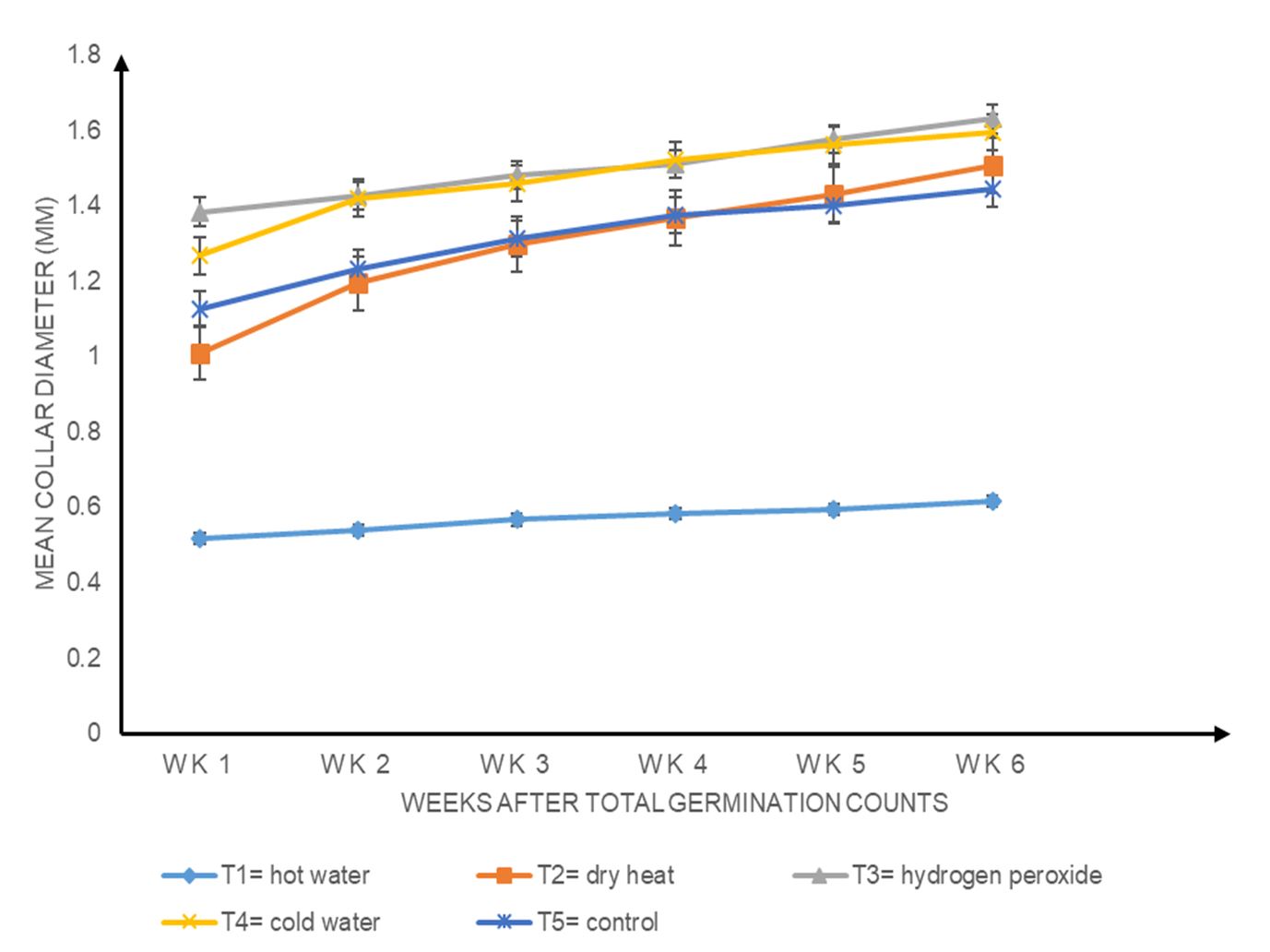
Figure 4. Effects of different seed pre-sowing treatments on the weekly mean collar diameter of Albizia lebbeck seedlings.
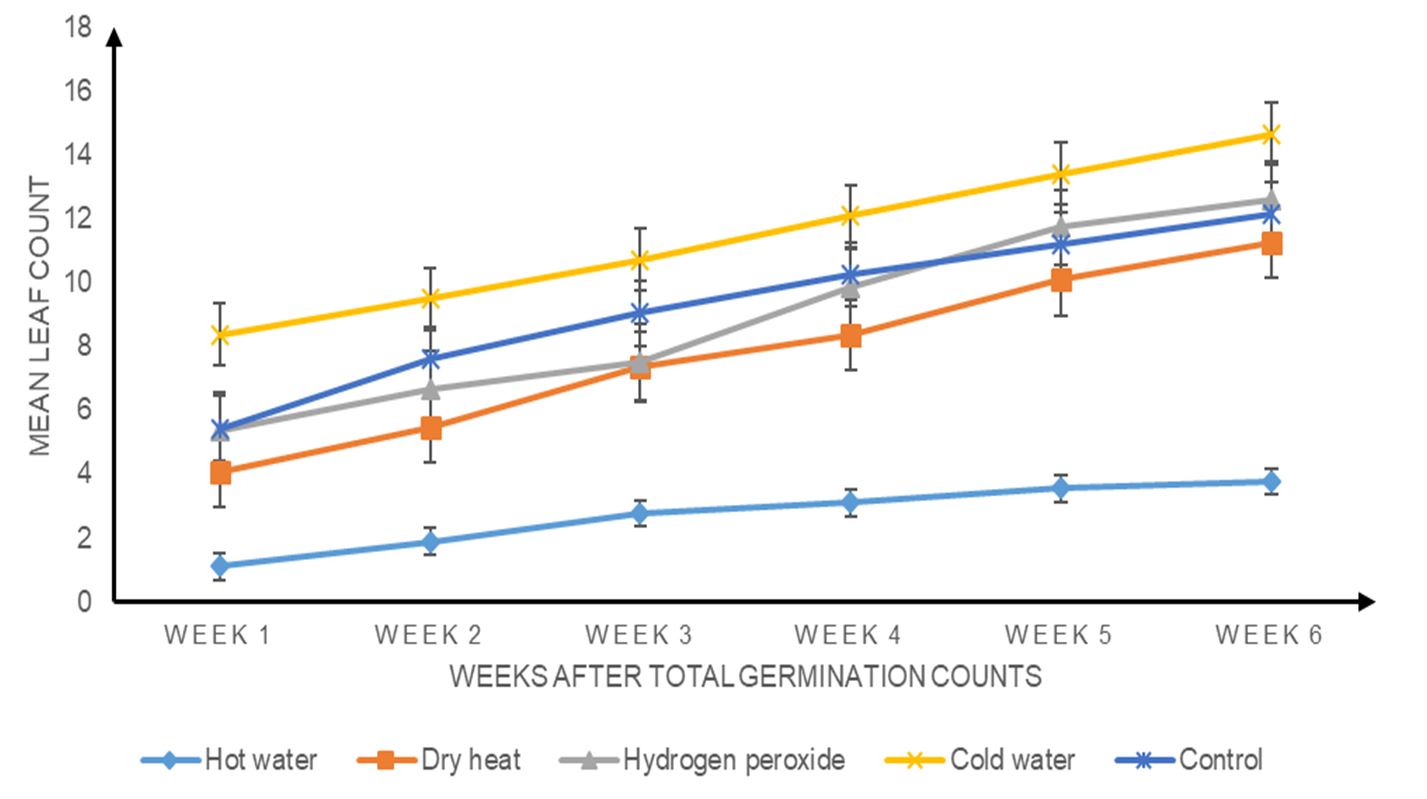
Figure 5. Effects of different seed pre-sowing treatments on the weekly mean leaf counts of Albizia lebbeck seedlings.
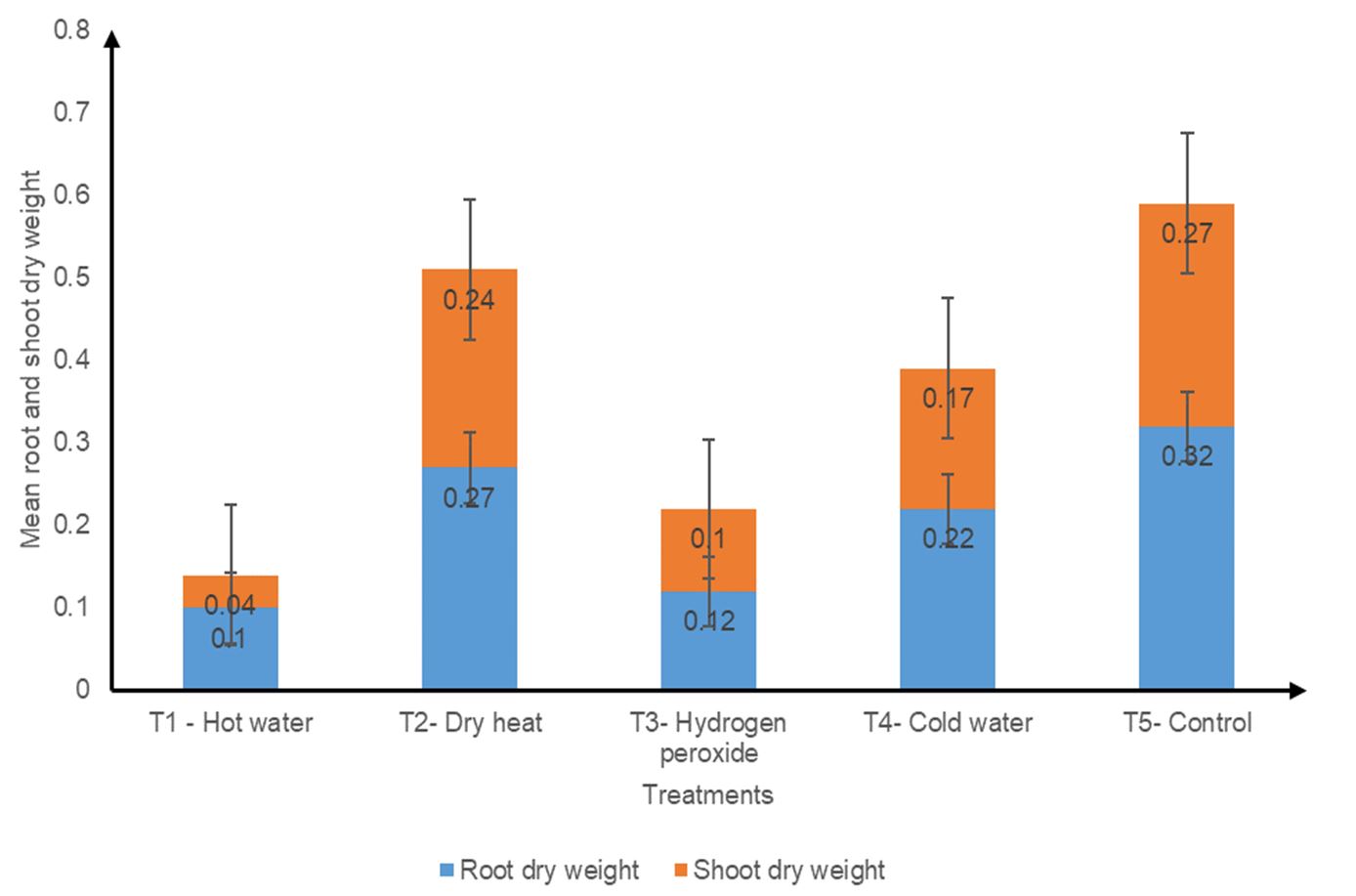
Figure 6. Effect of different seed pre-sowing treatments on the mean root and shoot dry weights of Albizia lebbeck seedlings.
DISCUSSION
Germination percentage and rate of Albizia lebbeck seeds
Germination of the seeds began differently in each pre-sowing treatment, causing differences in the seedlings’ germination percentage and rate.
Azad et al. (2011) argued that to break seed dormancy for germination, germination enhancement and rate can be increased by different seed treatments before sowing. According to Alamgir and Hossain (2005), hot water treatment (T1) can conquer physical dormancy in leguminous species. Azad et al. (2011) recorded the highest germination in a hot water treatment (T1) in Albizia lebbeck, and they stated that this may be caused by differences in seed coat thickness. However, in this study (Fig. 1 and Fig. 2), heat damage was observed in the seed. This resulted in hot water (T1) having the lowest germination percentage and rate, making it ineffective for pretreatment. This agrees with the findings of Alamgir and Hossain (2005), in which hot water was lethal to some degree.
Dry heat (T2) and the control (T5) increased both the germination rate and percentage and had the highest values (Fig. 1 and Fig. 2). Generally, the control (T5) germinates slowly and irregularly or later and unevenly (Azad et al., 2011). However, this contradicts the present experiment, in which the control had the highest germination rate and percentage. This may have to do with the fact that the outer coat of the seeds were more or less equally thinned, which allowed easy imbibition of water for germination. Dry heat treatment (T2) also decreases the dormancy of forest tree seeds, and there have been cases where high-temperature treatments have been used to eliminate dormancy (Ajiboye, 2010), changing the structure of the seed coat and becoming permeable to water and air, which are necessary for germination. Observations from Gupta et al. (2017) showed that there was a drastic reduction in the germination of Gymnocladus assamicus as a result of prolonged treatments under dry heat, causing lethal effects to the embryo. In contrast to the present experiment, the duration was shorter, increasing the germination percentage and rate.
When the seeds were soaked in cold water (T4) for 24 hours, germination was high, which is in contrast to El-Bakkosh (2013), who reported that excessive immersion of seeds in water reduced germination due to lower submergence and a lack of oxygen. Studies by Ibrahim and Otegbeye (2004) showed that seed immersion in cold water improved the germination of some tropical trees. Owonubi et al. (2005) observed that soaking Azadirachta indica seeds in cold water for 1, 12 and 24 hours increased seed germination, implying that the seed coats of different species are differently permeable to water and gas (Owonubi et al., 2005).
T3 (hydrogen peroxide) had a similar pattern in germination percentage and rate to T4 (cold water) (Fig. 1 and Fig. 2). According to Kannan et al. (1996), soaking seeds in a hydrogen peroxide solution completely inhibited the germination of some Albizia species. Nevertheless, in this experiment, the opposite was true. These differences may be due to the acid breaking exogenous dormancy and disintegrating the seed coat, increasing imbibition and subsequent germination. This experiment agrees with Luna et al. (2012), who showed that tropical species, such as Albizia lebbeck, benefit from hydrogen peroxide as an effective cleaner, thereby enhancing germination.
Mean height, leaf count and collar diameter of Albizia lebbeck seedlings
After pretreatment, Albizia lebbeck seedlings tended to have a rapid growth rate, although the treatments had no effect on germination. Early growth rate showed that all treatments (T1, T2, T3, T4 and T5) had a significant influence on the height, collar diameter and leaf count of the seedlings (P < 0.05). Although the treatments were tested for significance on these growth parameters, there were variations in the growth rate. This could be attributed to the fact that the seeds had the advantage of germinating early and had early exposure to environmental conditions, such as temperature, humidity, light and water; hence, some started the photosynthesis process faster than others. This agrees with the present study because seeds in the cold water treatment (T4) emerged first and were thus exposed to the environmental conditions first. Therefore, T4 seedlings took the lead in height and number of leaves from week 1 to week 6. The lowest seedling growth (height, collar diameter and leaf count) observed in seedlings treated with hot water (T1) indicated that it did not enhance growth in Albizia lebbeck. In addition to changes in growth patterns, light direction, quality and quantity of light, sensed by a photo sensor, worked together to regulate plant growth and development, probably to maintain the efficiency of photosynthesis because (Ologundudu et al., 2013) light has been reported to have a key role in plant growth and development. Hartmann et al. (1997) also found that environmental factors regulate plant species diversity and germination timing, and sun orientation may have had an effect on the randomness of the samples examined.
Root and shoot dry weight of Albizia lebbeck seedlings
The dry matter of Albizia lebbeck seedlings was not influenced by the different pre-sowing treatments (P ≥ 0.05). As shown in Fig. 6, the higher the root biomass produced by a treatment, the higher the corresponding shoot biomass. This may be because there is an interdependence of roots and shoots for growth and development. The shoot depends on the roots for nutrients and water, while the roots depend on the shoot for carbohydrates (Fageria and Moreira, 2011). This may have contributed to this trend. The control (T5) had the highest root and shoot dry weight (Fig. 6), which disagrees with the findings of Alamgir and Hossain (2005), whose control was the third highest in relation to root and shoot dry weights in Albizia saman. Hot water (T1) had the lowest root and shoot dry weights (Fig. 6)
CONCLUSIONS
Albizia lebbeck is an important tree species in agroforestry because of its multipurpose uses, such as its ability to fix nitrogen and improve soil structure. Albizia lebbeck seeds did not exhibit dormancy, but to grow this species in a minimum amount of time and to ensure quick, homogenous germination and good growth, it is appropriate to pretreat the seeds before sowing. Among the pre-sowing treatments for the production of desirable seed germination percentage and rate, seeds originating from dry heat and control performed significantly well, although the performance of cold water and hydrogen peroxide treatments was not poor. Hot water treatment was not encouraging. Therefore, for small-scale nursery owners who are interested in establishing Albizia lebbeck, a 24-hour soak in cold water should be adopted because the treatment can be performed without measuring temperature and concentration and will be the best strategy for early seed germination and growth.
Acknowledgements. We would like to thank Dr. Twum-Ampofo for his support and contributions towards this research and the Kwakye family for their prayers and support.
REFERENCES
Ahmad, Z.R., Shaheen, M.N.U.K., Afzal, J., Siddique, S.U., Qamar, I.A., Ahmed, J. (2015). Improvement of seed germinatiom in some important multi-purpose leguminous trees of Islamabad Area: An experimental study. Basic Research Journals, 4 (7): 217 – 224.
Ajiboye, A.A. (2010). Dormancy and Seed Germination in Tamarindus indica (L). The Pacific Journal of Science and Technology, 11 (2): 463 – 470.
Alamgir, M. and Hossain, M.K. (2005). Effect of pre-sowing treatments on germination and initial seedling development of Albizia saman in the nursery. Journal of Forestry Research, 16 (3): 200 – 204.
Azad, M.S., Islam, M.W., Matin, M.A., Bari, M.A. (2006). Effect of pre-sowing treatment on seed germination of Albizia lebbeck (L.) Benth. South Asian Journal Agriculture, 1 (2) : 32 – 34.
Azad, S., Manik, M. R., Hasan, S., & Matin, A. (2011). Effect of different pre-sowing treatments on seed germination percentage and growth performance of Acacia auriculiformis. Journal of Forestry Research, 22(2), 183-188.
Bakpa, E. P., Maalekuu, B. K., Tandoh, P. K., & Aculey, P. (2018). Effect of Ash-Based Storage Media on the Physical Quality Characteristics and Shelf Life of Three Cultivars of Tomato (Lycopersicon esculentum, Mill) Grown in the Greenhouse. Asian Journal of Agricultural and Horticultural Research, 2(4), 1-10.
Chakayla, A. (2019). The effects of 3% hydrogen peroxide on peas seeds. https://www.coursehero.com/file/4539239
Depali, D.N., Rahman, M.M., Rahman, G.M.M., Islam, K.K., Mondal, M.A. (2007). Effect of pre-treatment of seeds of Kalo Koroi [Albizia lebbeck (L.) Benth.] on germination and seedling growth. Journal of Agroforestry and Environment, 1(2): 43 – 46.
El–Bakkosh, M.A. (2013). Breaking seed dormancy of some ornamental trees by different chemical and physical treatments. African Journal of Biological Science, 9 (1): 221 – 228.
Fageria, N.K. and Moreira, A. (2011). The Role of Mineral Nutrition on Root Growth of Crop plants. Advances in Agronomy Journal, 110, pp 251- 331.
Gupta, S., Mao, A.A., Sarma, S., Satish, T. (2017). Responses of pretreatment and nutrient media on seed germination in Gymnocladus assamicus, a critically endangered legume tree species from North–East India. Journal of Tree Sciences, 36 (1): 86 – 92.
Heuze, V., Tran, G., Sauvant, D., (2015). Lebbeck (Albizia lebbeck). Feedipedia, a programme by INRAE, CIRAD, AFZ and FAO. https://www.feedipedia.org/ node/334.
Hartmann, H.T., Kester, D.E., Davies, F.T.Jr., and Geneve, R.L. (1997). Plant Propagation, Principles and Practices. Sixth Edition. Prentice-Hall, Inc. Upper Saddle River, New Jersey, USA. 770pp.
Ibrahim A., and Otegbeye, G.O. (2004). Methods of achieving optimum germination in Adansonia digitata. Bowen Journal of Agriculture. 1 (1): 53 – 58.
Krishnakumar, N., Palanisamy, K., Hedge, M., K., Warrier, K.C.S., Krishnamoorthy, M. (2010). Manual of Economically Important Forestry Species in South India, Coimbatore – 641002, Tamil Nadu, India, Institute of Forest Genetics and Tree Breeding.
Kumar, N., Handa, A.K., Dev, I., Ram, A., Uthappa, A.R., Shukla, A., Chand, L. (2018). Effect of pre-sowing treatments and growing media on seed germination and seedling growth of Albizia lebbeck (L.) Benth. Journal of Applied and Natural Science, 10 (3): 860 – 863.
Luna, T., Dumroese, R. K., Wilkinson, K.M. (2012). Seed germination and sowing options. In Wilkinson, K.M., Landis, T.D., Haase, D. L., Daley, B.F. (eds). Tropical Nursery Manual, volume 1, U.S. Department of Agriculture, Forest Service, pp 163- 183.
Kannan, C.S., Sudhakara, K., Augustine, A., Ashokan, P.K. (1996). Seed dormancy and pre-treatments to enhance germination in selected Albizia species. Journal of Tropical Forest Science, 8 (3): 369 – 380.
Maguire, J. D. (1962). Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci., 2, 176-177.
Ologundudu, A.F., Adelusi, A.A., Adekoya, K.P. (2013). Effects of light stress on germination and growth parameters of Corchorus olitorius, Celosia argentea, Amaranthus cruentus, Abelmoschus esculentus and Delonix regia. Journal of Notulae Scientia Biologicae, 5 (4): 468 – 475.
Owonubi, J.J., Otegbeye, G.O and Nwokedi, C. (2005). Development of pre-germination technique for Azadirachta indica: preliminary investigation. In: Sustainable Forest Management in Nigeria: Lessons and Prospects. Proceedings of the 30th Annual Conference of the Forestry Association of Nigeria, held in Kaduna State. 7-11th November, 2005. p: 29 – 38.
Sharma, A., Gothecha, VK., Mishra, SS. (2010). Albizia lebbeck: a short review. Journal of herbal medicine and toxicology, 4 (2), 9 – 15.
Sharma, S ., Ranjana Naithani, B., Varghese, B., Keshavkant, S., Naithani, S.C. (2006). Effects of hot water treatment on seed germination of some fast growing tropical tree species. Journal of Tropical Forestry, 24: 3-4
Szopińska, D. (2014). Effects of hydrogen peroxide treatment on the germination, vigour and health of seeds. Folia Horticulturae, 26(1), 19-29.