Adams Latif Mohammed, Mariam Iddriss
ABSTRACT. Cowpea (Vigna unguiculata (L.) Walp.), one of the most important leguminous crops, is widely cultivated throughout the tropics, especially in the savanna regions of the world. However, its seed is attacked by several insect pests both in the field and in storage, especially the cowpea weevil. To control weevils, most farmers have adopted the use of chemical insecticides, but these insecticides come with health and environmental problems, and as a result, there is advocacy for the use of bio-pesticides in the form of tree botanicals. This study was conducted to assess the effects of neem leaf powder (NLP), moringa leaf powder (MLP), and camphor against the cowpea weevils (Callosobruchus maculatus) in a completely randomized design. Six treatments were used and allocated as T1 (control), T2 (4g of camphor), T3 (25g of NLP only), T4 (25g of MLP only) T5 (50g of NLP only) and T6 (50g of MLP). The treatments were replicated three times. The parameters studied were the numbers of damaged and undamaged seeds, the numbers of dead and live weevils, and the taste of cowpea seeds. The treatments effectively protected the cowpea seeds during the storage period of eight weeks. The preservation of cowpea seeds given by camphor was significantly different from the other treatments. However, there was no significant difference (p >0.05) between the neem and moringa leaf powders treatments. The use of moringa and neem leaf powders might be adopted by cowpea farmers in sub-Saharan Africa as alternative, cheap, and available sources of biopesticides to use as chemical insecticides. If cowpea seeds are to be stored for consumption purposes, moringa leaf powder should be used since most respondents preferred the taste of cowpea seeds treated with moringa. Additionally, the storage duration of eight weeks was too short for the experiment, so further studies can be carried out for an extended period of more than eight weeks.
Keywords: neem; moringa; cowpea seeds; biopesticide; storage.
Cite
ALSE and ACS Style
Mohammed, A.L.; Iddriss, M. Effect of moringa (Moringa oleifera) leaf powder, neem (Azadirachta indica) leaf powder, and camphor on weevil (Callosobruchus maculatus F.) in stored cowpea (Vigna unguiculata (L.) Walp) seeds. Journal of Applied Life Sciences and Environment 2022, 55 (3), 257-269.
https://doi.org/10.46909/alse-552062
AMA Style
Mohammed AL, Iddriss M. Effect of moringa (Moringa oleifera) leaf powder, neem (Azadirachta indica) leaf powder, and camphor on weevil (Callosobruchus maculatus F.) in stored cowpea (Vigna unguiculata (L.) Walp) seeds. Journal of Applied Life Sciences and Environment. 2022; 55 (3): 257-269.
https://doi.org/10.46909/alse-552062
Chicago/Turabian Style
Mohammed, Adams Latif, and Mariam Iddriss. 2022. “Effect of moringa (Moringa oleifera) leaf powder, neem (Azadirachta indica) leaf powder, and camphor on weevil (Callosobruchus maculatus F.) in stored cowpea (Vigna unguiculata (L.) Walp) seeds” Journal of Applied Life Sciences and Environment 55, no. 3: 257-269.
https://doi.org/10.46909/alse-552062
View full article (HTML)
Effect of Moringa (Moringa Oleifera) Leaf Powder, Neem (Azadirachta Indica) Leaf Powder and Camphor on Weevil (Callosobruchus Maculatus F.) in Stored Cowpea (Vigna Unguiculata (L.) Walp) Seeds
Adams Latif MOHAMMED* and Mariam IDDRISS
Kwame Nkrumah University of Science and Technology, Faculty of Renewable Natural Resources, Department of Agroforestry, Kumasi-Ghana; e-mail: mariamiddriss24@gmail.com
*Correspondence: adamsinho224@gmail.com
Received: Dec. 06, 2022. Revised: Feb. 06, 2023. Accepted: Feb. 10, 2023. Published online: Feb. 23, 2023
ABSTRACT. Cowpea (Vigna unguiculata (L.) Walp.), one of the most important leguminous crops, is widely cultivated throughout the tropics, especially in the savanna regions of the world. However, its seed is attacked by several insect pests both in the field and in storage, especially the cowpea weevil. To control weevils, most farmers have adopted the use of chemical insecticides, but these insecticides come with health and environmental problems, and as a result, there is advocacy for the use of bio-pesticides in the form of tree botanicals. This study was conducted to assess the effects of neem leaf powder (NLP), moringa leaf powder (MLP), and camphor against the cowpea weevils (Callosobruchus maculatus) in a completely randomized design. Six treatments were used and allocated as T1 (control), T2 (4g of camphor), T3 (25g of NLP only), T4 (25g of MLP only) T5 (50g of NLP only) and T6 (50g of MLP). The treatments were replicated three times. The parameters studied were the numbers of damaged and undamaged seeds, the numbers of dead and live weevils, and the taste of cowpea seeds. The treatments effectively protected the cowpea seeds during the storage period of eight weeks. The preservation of cowpea seeds given by camphor was significantly different from the other treatments. However, there was no significant difference (p >0.05) between the neem and moringa leaf powders treatments. The use of moringa and neem leaf powders might be adopted by cowpea farmers in sub-Saharan Africa as alternative, cheap, and available sources of biopesticides to use as chemical insecticides. If cowpea seeds are to be stored for consumption purposes, moringa leaf powder should be used since most respondents preferred the taste of cowpea seeds treated with moringa. Additionally, the storage duration of eight weeks was too short for the experiment, so further studies can be carried out for an extended period of more than eight weeks.
Keywords: neem; moringa; cowpea seeds; biopesticide; storage.
INTRODUCTION
Cowpea (Vigna unguiculata L. Walp) is an essential leguminous crop cultivated throughout all ecological zones of sub-Saharan Africa (Nkhoma et al., 2020). As a major food staple, cowpea accounts for about 23–32% of the plant protein consumed by indigenous people in the tropics (Kpoviessi et al., 2019; Abebe and Alemayehu, 2022). Hiama et al. (2019) reported that, aside from the nutritional benefits of cowpea, it is an important means for soil fertility improvement in tropical soils through nitrogen fixation. In the tropics, infestation of cowpea seeds by weevils is a major constraining issue in the longevity of cowpea seeds in storage (Adebayo and Anjorin, 2018; Mishra et al., 2018; Boukar et al., 2020) due to the failure of smallholder farmers to store seeds using appropriate methods of storage (Mobolade et al., 2019; Omoigui et al., 2020). Additionally, grain damage and losses occur in storage due to inadequate storage structures and a lack of efficient pest management strategies in the tropics (Kiruba et al., 2012). The poor storage of cowpea seeds is a recipe for the cowpea weevil (Callosobruchus maculatus) to infest and destroy seeds during the storage period (Kpoviessi et al., 2019; Seidu, 2019; Ileke et al., 2020). Callosobruchus maculatus is a major destructive pest of stored cowpea seeds in many tropical and subtropical regions around the world, causing qualitative and quantitative damage during storage (Kiruba et al., 2012; Adebayo and Anjorin, 2018; Jehajo and Din, 2020). Most tropical farmers secure low yields of about 200–300 kg/ha on average, which is attributed to insect pest attacks both in the field and in storage (Tiroeselea et al., 2019). According to studies by Sugri et al. (2021), and Bidzakin et al. (2022), about 17–50% of cowpea seed losses can be attributed to poor postharvest handling in developing countries. Infestation starts in the mature crop in the field, at the threshing floor, and is eventually carried into the storehouse, leading to seed deterioration under ambient storage conditions where seeds are severely damaged (Kumar et al., 2020). This results in loss of seed weight, loss of viability and loss of market value of stored seeds—setbacks to tropical food security (Kamara et al., 2014). Baributsa and Njoroge (2020) reported that most smallholder farmers have resorted to using a wide range of pest management strategies to decrease damages and losses caused by the cowpea weevil in storage. Management of cowpea weevils by most smallholder farmers is centred on the usage of chemical insecticides, including camphor (Lengai and Muthomi, 2018; Lengai et al., 2020).
However, the predominant use of these chemical insecticides is not sustainable due to their long-term effects on the environment and, human health and them altering organoleptic properties of stored seeds (Kiruba et al., 2012; DiBartolomeis et al., 2019). Akami et al. (2017) reported that the frequent use of chemical insecticides resulted in these weevils becoming resistant, which can subsequently lead to a qualitative and quantitative reduction in the quality of stored seeds. As a result, the search for botanicals as a cheap and sustainable alternative for controlling stored cowpea pests has evolved and been advocated in sub-Saharan Africa over the years (Obanyi, 2018). To control cowpea weevils in storage, most resource-poor farmers in sub-Saharan Africa have adopted indigenous methods such as ash, dry pepper, and extracts from tree botanicals including Senna/Cassia occidentalis, Vittallaria paradoxa, Piper guineense and Eucalyptus, of which neem and moringa cannot be overlooked (Abdullahi, 2011; Race et al., 2012; Abed, 2020).
Neem is classified under the family Maliaceae, with its various parts used for treating several diseases due to its antibacterial, antifungal, antiviral, and antimalarial properties (Ilesanmi and Gungula, 2013; Islas et al., 2020). Oguh et al. (2019) reported that leaf extracts from plants need to restrain the growth and development of plant pathogens, whereas neem oil with azadirachtin as the major constituent has insect repellent and inhibitory properties. Additionally, moringa is a multipurpose agroforestry tree species in tropical and subtropical regions of the world, and it is categorized in the family Moringaceae (Race et al., 2012; Abiyu et al., 2018; Devkota and Bhusal, 2020). The various parts of moringa contain important constituents essential for antimicrobial activity, analgesic activity, and antihypertensive activity, which makes it ideal to be used as an insecticide (Kamran et al., 2020; Chhikara et al., 2020). Studies have confirmed that camphor has biological properties such as insecticidal, antimicrobial, antiviral, anticoccidial, antinociceptive, anticancer and antitussive activities (Chen et al., 2013, 2018; Zhang et al., 2018).
Despite the potential of moringa and neem leaf powder and camphor to control pests of stored grains, there is little information on their use in the tropics to control cowpea weevils (Race et al., 2012; Blay, 2019). Therefore, this study seeks to assess the potential of neem and moringa leaf powder as alternative methods of cowpea preservation that are cheap, available, and affordable, with lasting preservative effects and that are indigenous to resource-poor farmers, marketers and consumers of cowpea in sub-Saharan Africa.
MATERIALS AND METHODS
Description of the Study Area
The experiment was carried out at the Faculty of Renewable Natural Resources (FRNR) laboratory of Kwame Nkrumah University of Science and Technology, Kumasi-Ghana. The multiannual average values of precipitation are in the range of 1300-1600 mm and the air temperature is between 22 – 31 ºC.
Experimental Approach and Procedure
Cowpea seeds were acquired from the Crop Research Institute of the Council for Scientific and Industrial Research (CSIR) at Fumesua. Neem and moringa leaves were collected from KNUST University. To avoid photochemical breakdown of the active ingredients leaves were dried under shade at 25º C for two weeks. Using a mortar and pestle, the dried leaves were ground into powder, sieved with 0.2-mm mesh, and stored in a cool, dry place until use. Four grams of camphor (Kamara et al., 2014), a chemical insecticide, was acquired at an agrochemical shop. The leaf powders were weighed using a beam balance and divided into 25 g of neem leaf powder (NLP), 25 g of moringa leaf powder (MLP), 50 g of neem leaf powder (NLP) and 50 g of moringa leaf powder (MLP). Jute sacks were purchased from the market, cut into pieces, sewn into 72 mini sacks measuring 20 cm by 30 cm, and sterilized to eliminate any insect pests that may have been present. Two hundred cowpea seeds (Blay, 2019) were placed in each mini sack. The various leaf powders and the camphor were thoroughly mixed with the seeds, tied up with ropes and kept at room temperature (20–23oC) in the laboratory. These were sampled every eight weeks, and observations were done every two weeks (i.e., 2, 4, 6, and 8 weeks).
Experimental Design and Treatment Allocation
The experiment was laid out in a complete randomized design with six treatments replicated three times. The treatments were: T1: Control (No NLP and MLP), T2: 4g of Camphor, T3: 25g of NLP, T4: 25g of MLP, T5: 50g of NLP, and T6: 50g of MLP, where NLP= Neem Leaf Powder and MLP= Moringa Leaf Powder. The sampling period was eight weeks.
Data Collection
Data were collected on the number of live weevils, the number of dead weevils, and the number of damaged and undamaged seeds. The number of weevils was obtained by counting and recording the numbers of live weevils and dead weevils in each-mini sack every two weeks. The number of damaged seeds was calculated by counting all of the seeds in each mini sack that had at least one weevil infestation hole. The number of undamaged seeds was also determined by counting all of the seeds in each mini sack that did not have a perforation or hole caused by weevil infestation. Cowpea seeds were cooked after storage, and sensory analysis was carried out using ten respondents.
Data Analysis
The collected data was analysed for differences using the analysis of variance (ANOVA) technique based on STATISTIX 7 software at a probability level of 5% level of significance. The Least Significant Difference (LSD) was employed to compare the means that were significantly different. Results are presented in tables and in a graph.
RESULTS
Number of damaged cowpea seeds from 2WAS to 8WAS
The effects of the Neem Leaf Powder, Moringa Leaf Powder, and Camphor treatments on the number of damaged cowpea seeds for the duration of storage are shown in Table 1. There was a significant difference (p ≤0.05) in the number of damaged cowpea seeds from the treatments on a weekly basis. Treatment T1 had the greatest number of damaged seeds, while treatment T2 had the lowest number of damaged seeds. At week two, T1 had the greatest number of damaged seeds (34.33), which was significantly different from the remaining treatments (p = 0.0012). Similar results were recorded at weeks 6 and 8. However, at week 4, T1 recorded the greatest number of damaged seeds, with T5 recording the lowest number of damaged seeds, which were significantly different (Table 1).
Number of undamaged cowpea seeds from 2WAS to 8WAS.
Table 2 shows the number of undamaged cowpea seeds from the Neem Seed Powder, Moringa Leaf Powder, and Camphor treatments. The number of undamaged cowpea seeds followed a similar pattern to the number of damaged cowpea seeds, ranging from 2WAS to 8WAS. The analysis of variance showed a significant difference between treatment means with p-values of 0.0000. Treatment T2 had the greatest number of undamaged seeds, while treatment T1 had the lowest number of undamaged seeds. At week 2, T4 had the greatest numbers of undamaged seeds, which was not significantly different from T2, T3, T5, and T6, but was significantly different from T1 (p = 0.0012). Similar observations were made at weeks 6 and 8. On the contrary, T2, T3, T5 and T6 recorded the greatest numbers of undamaged seeds, which were significantly different from T1 and T4 (p = 0.0030) (Table 2).
Number of Live Weevils from 2WAS to 8WAS.
Table 3 shows that there were no significant differences in the numbers of live weevils for the various treatments at 2WAS and 6WAS. On the contrary, there was a significant difference (p≤0.05) between treatment means in terms of the number of weevils that survived at 4 and 8 WAS.
Table 1
The Number of Damaged Cowpea Seeds from 2 to 8 Weeks after Storage
|
Treatment |
2WAS |
4WAS |
6WAS |
8WAS |
Means |
|
T1 (Control) |
34.3±2.85a |
34.7±4.41a |
144.3±18.9a |
195.0±3.6a |
102.1±21.5a |
|
T2 (4 g of C) |
10.3±3.38b |
10.3±2.33c |
11.7±0.88b |
14.0±3.1b |
11.6±1.2b |
|
T3 (25 g NLP) |
14.0±4.51b |
18.7±1.76bc |
41.0±10.0b |
32.3±4.6b |
26.5±4.1b |
|
T4 (25 g MLP) |
9.7±1.76b |
23.7±5.84ab |
43.0±11.7b |
21.3±12.9b |
24.4±5.3b |
|
T5 (50 g NLP) |
13.3±3.33b |
11.0±2.08c |
22.7±4.67b |
27.3±1.5b |
18.6±2.4b |
|
T6 (50 g MLP) |
17.7±2.19b |
12.7±3.28bc |
28.0±4.04b |
15.3±10.8b |
18.4±3.1b |
|
p-VALUE |
0.0012 |
0.0030 |
0.0000 |
0.0000 |
0.0000 |
|
LSD |
9.6533 |
11.054 |
31.707 |
22.855 |
26.420 |
Means accompanied by the same letter(s) are not significantly different at (p ≤ 0.05%) using Least Significance Difference (LSD). WAS=Weeks After Storage
Table 2
The numbers of undamaged cowpea seeds from 2 to 8 weeks after storage
|
Treatment |
2WAS |
4WAS |
6WAS |
8WAS |
Means |
|
|||||
|
T1 (Control) |
165.67±2.9b |
165.3±4.4c |
55.67±18.9b |
5.00±3.6b |
97.92±21.5b |
||||||
|
T2 (4 g of C) |
189.67±3.4a |
189.7±2.3a |
188.3±0.88a |
186.0±3.1a |
188.42±1.2a |
||||||
|
T3 (25 g NLP) |
186.00±4.5a |
181.3±1.8ab |
159.0±10.0a |
167.7±4.6a |
173.50±4.1a |
||||||
|
T4 (25 g MLP) |
190.30±1.8a |
176.3±5.8bc |
157.0±11.7a |
178.7±12.9a |
175.58±5.3a |
||||||
|
T5 (50 g NLP) |
186.67±3.3a |
189.0±2.1a |
176.0±5.0a |
176.0±5.0a |
180.58±2.8a |
||||||
|
T6 (50 g MLP) |
182.30±2.2a |
187.3±3.3ab |
172.0±4.0a |
172.0±4.0a |
181.58±3.1a |
||||||
|
p-VALUE |
0.0012 |
0.0030 |
0.0000 |
0.0000 |
0.0000 |
|
|||||
|
LSD |
9.6522 |
11.1 |
31.796 |
22.055 |
26.469 |
|
|||||
Means accompanied by the same letter(s) are not significantly different at (p ≤ 0.05%) using Least Significance Difference (LSD)
In terms of overall treatments, treatment T1 had the most live weevils (10.67), while treatment T2 had the fewest live weevils (0.00). At weeks 2 and 6, there was no significant difference in the number of live weevils with respect to the various treatments. However, at week 4, T4 had the highest number of live weevils, which was not significantly different from T1 and T6 but was significantly different from T2, T3, and T5 (p=0.0497). At week 8, T3 had the highest number of live weevils, which was significantly different from the remaining treatments (p=0.0206) (Table 3).
Number of Dead Weevils from 2WAS to 8WAS.
In regards to the overall mean number of dead weevils, there was a significant difference (p = 0.0000). On a weekly basis, there was also a significant difference (p≤0.05) between the applied treatment means from 2 WAS to 8 WAS. T1 had the highest number of dead weevils, whilst T2 recorded the lowest number of dead weevils (Table 4). At week 2, T5 and T1 recorded the highest numbers of dead weevils, which were significantly different from other treatments, with T2, T3, and T4 recording the lowest numbers of dead weevils. However, at weeks 4, 6 and 8, T1 had the greatest number of dead weevils, which were significantly different from the remaining treatments (Table 4).
Table 3
Numbers of live weevils from 2 to 8 weeks after storage
|
Treatment |
2WAS |
4WAS |
6WAS |
8WAS |
Means |
|
|
T1 (Control) |
5.00±1.5 |
6.67±2.0ab |
27.00±9.1 |
4.00±2.7b |
10.67±3.5a |
|
|
T2 (4 g of C) |
0.00±0.0 |
0.00±0.0b |
0.00±0.0 |
0.00±0.0b |
0.00±0.0c |
|
|
T3 (25 g NLP) |
2.33±1.5 |
3.67±0.9b |
15.33±7.3 |
12.00±3.6a |
8.33±2.4ab |
|
|
T4 (25 g MLP) |
2.33±0.9 |
12.00±4.2a |
18.67±1.5 |
2.33±1.5b |
8.83±2.3a |
|
|
T5 (50 g NLP) |
2.33±1.9 |
2.67±1.5b |
4.00±2.1 |
1.67±1.2b |
2.67±0.8bc |
|
|
T6 (50 g MLP) |
3.33±0.7 |
7.00±3.1ab |
9.67±7.2 |
3.00±1.5b |
5.75±1.9abc |
|
|
p-VALUE |
0.1987 |
0.0497 |
0.0520 |
0.0206 |
0.0067 |
|
|
LSD |
3.0526NS |
5.0666 |
55.582NS |
40.768 |
38.229 |
|
Means accompanied by the same letter(s) are not significantly different at (p ≤ 0.05%) using Least Significance Difference (LSD)
Table 4
Number of dead weevils from 2 to 8 weeks after storage
|
Treatment |
2WAS |
4WAS |
6WAS |
8WAS |
Means |
|
|||||
|
T1 (Control) |
6.00±1.0ab |
16.33±3.2a |
127.7±31.2a |
248.7±15.9a |
99.67±30.6a |
||||||
|
T2 (4 g of C) |
3.00±1.5bc |
4.33±0.9b |
3.00±1.5b |
5.00±1.15b |
3.83±0.6b |
||||||
|
T3 (25 g NLP) |
2.00±1.0b |
6.67±0.3b |
23.00±9.5b |
29.33±1.20b |
16.08±3.9b |
||||||
|
T4 (25 g MLP) |
2.33±0.9b |
4.00±0.6b |
47.00±26.7b |
30.00±25.5b |
20.83±9.7b |
||||||
|
T5 (50 g NLP) |
7.00±0.6b |
3.33±1.8b |
25.67±10.6b |
31.00±6.35b |
16.75±4.5b |
||||||
|
T6 (50 g MLP) |
3.33±0.7b |
4.67±1.3b |
46.33±7.8b |
12.67±10.2b |
316.75±5.9b |
||||||
|
p-VALUE |
0.0176 |
0.0010 |
0.0059 |
0.0000 |
0.0000 |
|
|||||
|
LSD |
3.0526 |
5.0666 |
55.582 |
40.768 |
38.229 |
|
|||||
Means accompanied by the same letter(s) are not significantly different at (p ≤ 0.05%) using Least Significance Difference (LSD)
Sensory Analysis.
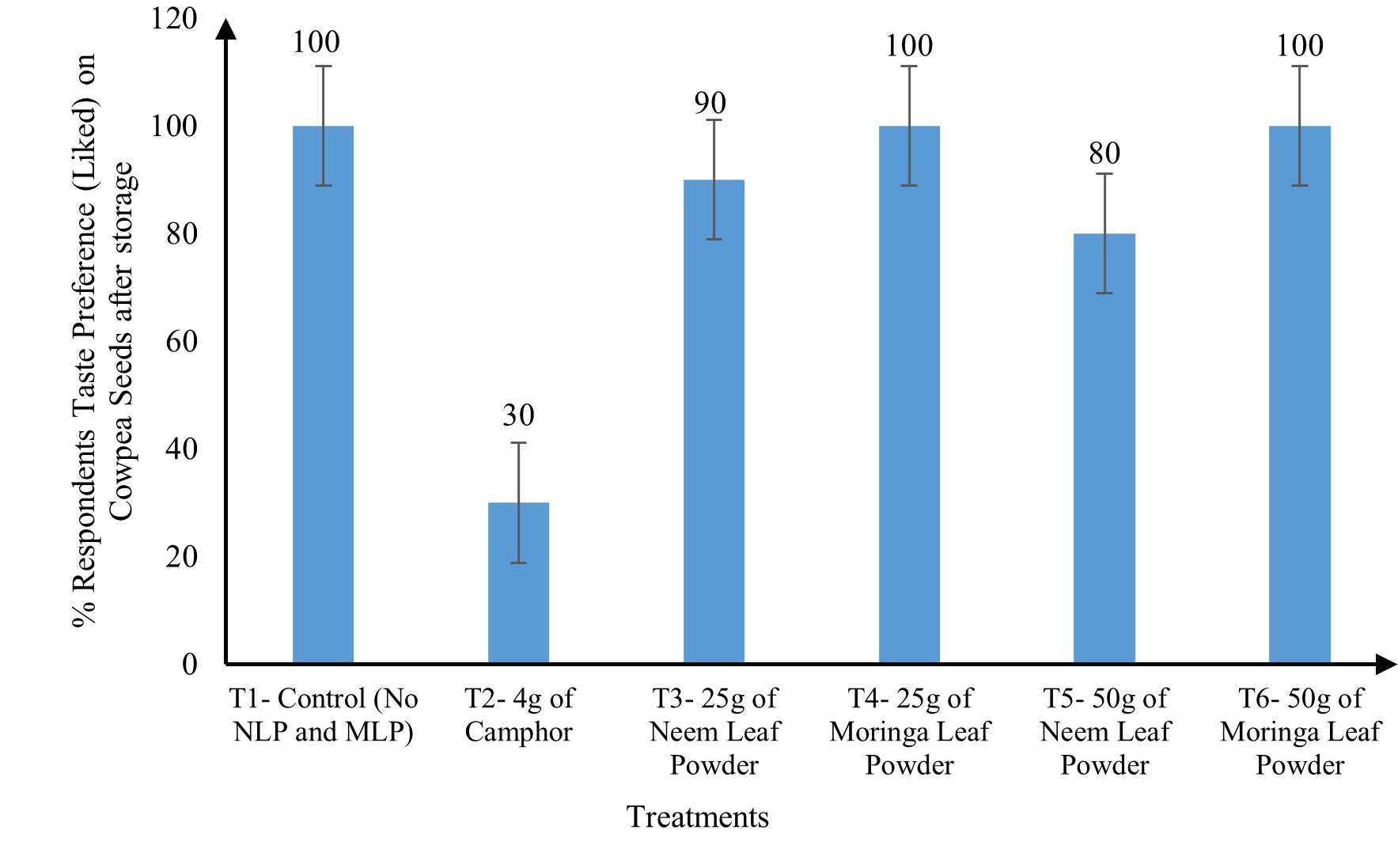
Ten respondents were used for the sensory analysis to assess the effect of the treatments on the taste of cowpea seeds after an eight-week storage period. The cowpea seeds from the various treatments were cooked and served to the respondents to eat and give their taste preferences. Out of the 10 respondents used, all preferred the taste of cowpea seeds from T1 (control), T4 (25 g MLP), and T6 (50 g MLP). Ninety percent of the respondents preferred the taste of cowpea from T3 (25 g NLP), while 80% preferred the taste of seeds from T5 (50 g NLP). On the contrary, a minority of the respondents (30%) liked or preferred seeds from T2 (Camphor) (Figure 1).
DISCUSSION
Effects of Neem Leaf Powder, Moringa Leaf Powder and Camphor on the Number and Quality (Damaged and Undamaged) of Cowpea Seeds and the Population (Live and Dead) of Weevils.
This study shows that neem leaf powder, moringa leaf powder, and camphor (chemical insecticides) have an insecticidal effect on Callosobruchus maculatus and can be used to control the cowpea weevils during storage. With regards to seed quality, little damage from weevils was recorded in the seeds treated with the applied treatments compared to the control. This could be attributed to the fact that both organic and inorganic insecticides containing active constituents have the potential to protect stored seeds of cowpea by either repelling or disrupting the feeding activities of these weevils, either in the field or in storage (David, 2019; Ukoroije and Otayor, 2020; Ileke et al., 2020).
Among all the treatments, camphor appeared to be more effective in controlling the activities of the weevils, which can be attributed to the presence of high amounts of polyphenols. However, there was no significant difference between seeds treated with camphor and the plant botanicals. This is an indication that the tree botanicals provided the required chemicals for controlling the cowpea weevil, as reported by previous studies by Ogunwolu and Odunlami (1996) and David (2019). The findings obtained in this study agree with Paul et al. (2009) and Fotso et al. (2018), who reported that powdered plant parts could adequately protect stored grains against storage insects and pests. The presence of the active ingredient azadirachtin in the leaves may explain the reduction in weevil activity in seeds treated with neem leaf powder. Azadirachtin could be used as an insect repellent, a feeding inhibitor, a growth retardant, and a sterilant. Azadirachtin has both direct and systemic action on the eggs and on insects, thereby reducing their survival (Isman, 2006; Castilhos et al., 2018). Katamssadan et al. (2016) reported that azadirachtin has an antifeedant, sterilizing, and morphogenic effect on target pest species when elaborating on the pesticide properties of neem. It is structurally similar to the insect hormone ecdysone, which controls the moulting process.
The capacity of the insect to emit this hormone appears to be inhibited by azadirachtin, and as a result, the moulting process is hampered, thereby disrupting the insect’s life cycle.
This study has also proved that moringa leaf powder is as effective at controlling the weevils of stored cowpea seeds as neem leaf powder. This could be attributed to the leaf powders’ anti-oviposition, delayed egg hatching, and insect growth-disrupting properties. The leaves of Moringa oleifera have been reported by Loebel (2002) to demonstrate antioxidant activity due to their high amounts of polyphenols. It prevents oxidative damage to major biomolecules and gives significant protein protection against oxidative damage. This is consistent with previous research by Anita (2012), who claimed that the seeds of the moringa contain coagulant lectin and the crushed leaves of the plant can kill insects by disrupting digestion and causing moulting.
Effect of Neem Leaf Powder, Moringa Leaf Powder and Camphor on the Taste of Cowpea Seeds.
Results from the sensory analysis demonstrated that most of the respondents preferred cowpea seeds treated with moringa leaf powder to those treated with either neem leaf powder or camphor. This could be attributed to the fact that the leaves of moringa do not contain chemicals, which can cause a change in taste when used as a biopesticide to store agricultural produce, especially cowpeas. The present finding agrees with Madukwe et al. (2012), who pointed out that M. oleifera can be used as a biopesticide due to the leaves being completely safe for consumption and having no known negative side effects or toxic constituents. The respondents also stated that the cowpea seeds treated with neem leaf powder tasted bitter. This could be due to the presence of an active ingredient known as azadirachtin, causing the seeds to taste bitter. This therefore rendered the seeds unpalatable for consumption by the respondents, as reported by previous findings by Akinkurolele et al. (2006), Oni andIleke (2008) and Blay (2019).
CONCLUSIONS
The application of M. oleifera leaf powder, A. indica leaf powder, and camphor significantly reduced seed damage and weevil populations over the control treatment. Treatment effects on seed quality and weevil populations at different levels were statistically similar, which is an indication that tree botanicals have the potential to reduce seed damage and weevil populations when used as biopesticides similar to chemical insecticides. Also, most respondents preferred the taste of cowpea seeds, the control or those treated with moringa leaf powder.
RECOMMENDATIONS
The use of M. oleifera and A. indica leaf powders might be adopted by cowpea farmers in sub-Saharan Africa as an alternative, cheap, and available source of biopesticide. Farmers can use either neem leaf powder or moringa leaf powder to store cowpea seeds for propagation because their leaves are always available all year and there is no significant difference between the neem and moringa leaf powder treatments. More-so, if cowpea seeds are to be stored and used for food or consumption, then farmers can use moringa leaf powder since it does not negatively affect the taste of the seeds after storage. Additionally, the total storage duration of eight weeks was too short for the experiment, so further studies can be carried out for an extended period of more than eight weeks.
Author Contributions: Conceptualization: ALM, MI; methodology: ALM; analysis ALM; investigation: ALM; MI; writing, review: MI, ALM; supervision MI. All authors declare that they have read and approved the publication of the manuscript in this present form.
Funding: There was no external funding for this study.
Acknowledgement: An extended appreciation to my family, friends for their moral and financial support.
Conflict of interest: All authors declared no conflict of interest.
REFERENCES
Abdullahi, N. Evaluation of the efficacy of different concentrations of mixed leaf powders of Vittallaria paradoxa and Cassia occidentalis against Callosobruchus maculates (F.) (Coleoptera: bruchidae) on stored cowpea seeds. Bayero Journal of Pure and Applied Sciences. 2011, 4, 94-97. https://doi.org/10.4314/bajopas.v4i1.21.
Abebe, B.K.; Alemayehu, M.T. A review of the nutritional use of cowpea (Vigna unguiculata L. Walp) for human and animal diets. Journal of Agriculture and Food Research. 2022, 100383. https://doi.org/10.1016/j.jafr.2022.100383.
Abed, M.S. Study the effect of tree Eucalyptus leaves, shell and fruits on cowpea beetle (Callosobruchus Maculatus), which affects chickpeas in stores. Plant Archives. 2020, 20, 291-297.
Abiyu, A.; Yan, D.; Girma, A.; Song, X.; Wang, H. Wastewater treatment potential of Moringa stenopetala over Moringa olifera as a natural coagulant, antimicrobial agent and heavy metal removals. Cogent Environmental Science. 2018, 4, 1433507. https://doi.org/10.1080/23311843.2018.1433507.
Adebayo, R.A., Anjorin, O.O. Assessment of entomocidal effects of solar radiation for the management of cowpea seed beetle, Callosobruchus Maculatus (F.) (Coleoptera: Chrysomelidae) in stored cowpea. Global Journal of Science Frontier Research. 2018, 18, 21-26.
Akami, M.; Chakira, H.; Andongma, A.A.; Khaeso, K.; Gbaye, O.A.; Nicolas, N.Y.; Nukenine. E.N.; Niu, C.Y. Essential oil optimizes the susceptibility of Callosobruchus maculatus and enhances the nutritional qualities of stored cowpea Vigna unguiculata. Royal Society open science. 2017, 4, 170692. http://dx.doi.org/10.1098/rsos.170692.
Akinkurolele, R.O.; Adedire, C.O.; Odeyemi, O.O. Laboratory evaluation of the toxic properties forest anchomanes, Anhomanes difformis, against pulse beetles, Callosobruchus maculatus (Coleoptera: Bruchidae). Insect Science. 2006, 13, 25-29. https://doi.org/10.1111/j.1744-7917.2006.00064.x.
Anita, S.; Sujatha, P.; Prabhudas, P. Efficacy of pulverized leaves of Annona squamosa (L.), Moringa oleifera (Lam.) and Eucalyptus globules (Labill.) against the stored grain pest, Tribolium castaneum (Herbst.). Recent Research in Science and Technology. 2012, 4, 19-23.
Baributsa, D.; Njoroge, A.W. The use and profitability of hermetic technologies for grain storage among smallholder farmers in eastern Kenya. Journal of stored products research. 2020, 87, 101618. https://doi.org/10.1016/j.jspr.2020.101618.
Bidzakin, J.K.; Yeboah, O.; Sugri, I.; Graves, A.; Awunyo-Vitor, D. Economics of bulk storage techniques: maize and cowpea storage in Ghana. Hindawi Advances in Agriculture. 2022, 8953918. https://doi.org/10.1155/2022/8953918.
Blay, A.P. Effect of Neem Seed and Leaf powders on the storage of cowpea (Vigna unguiculata L. Walp) seeds, BSc. Thesis, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, 2019.
Boukar, O.; Abberton, M.; Oyatomi, O.; Togola, A.; Tripathi, L.; Fatokun, C. Introgression breeding in cowpea (Vigna unguiculata (L.) Walp.). Frontiers in Plant Science. 2020, 11, 567425. https://doi.org/10.3389/fpls.2020.567425.
Castilhos, R.V.; Grützmacher, A.D.; Coats, J.R. Acute toxicity and sublethal effects of terpenoids and essential oils on the predator Chrysoperla externa (Neuroptera: Chrysopidae). Neotropical entomology. 2018, 47, 311-317. https://doi.org/10.1007/s13744-017-0547-6.
Chen, W.Y.; Vermaak, I.; Viljoen, A. Camphor-a fumigant during the black death and a coveted fragrant wood in ancient Egypt and Babylon – A review. Molecules. 2013, 18, 5434-5454. https://doi.org/10.3390/molecules18055434.
Chen, Z.Y.; Guo, S.S.; Cao, J.Q.; Pang, X.; Geng, Z.F.; Wang, Y.; Zhang, Z.; Du, S.S. Insecticidal and repellent activity of essential oil from Amomum villosum Lour. and its main compounds against two stored-product insects. International Journal of Food Properties. 2018, 21, 2265-2275. https://doi.org/10.1080/10942912.2018.1508158.
Chhikara, N.; Kaur, A.; Mann, S.; Garg, M.K.; Sofi, S.A.; Panghal, A. Bioactive compounds, associated health benefits and safety considerations of Moringa oleifera L.: An updated review. Nutrition & Food Science. 2020, 51, 255-277. https://doi.org/10.1108/nfs-03-2020-0087.
David, I.K. The Efficacy of Alstonia boonei Stembark Oil as a Long-term Storage Protectant against Cowpea Bruchid, Callosobruchus maculatus (Fab.) (Coleoptera: Chrysomelidae). Jordan Journal of Biological Sciences. 2019, 12, 329-337.
Devkota, S.; Bhusal, K.K. Moringa oleifera: a miracle multipurpose tree for agroforestry and climate change mitigation from the Himalayas–a review. Cogent Food & Agriculture. 2020, 6, 1805951. https://doi.org/10.1080/23311932.2020.1805951.
DiBartolomeis, M.; Kegley, S.; Mineau, P.; Radford, R.; Klein, K. An assessment of acute insecticide toxicity loading (AITL) of chemical pesticides used on agricultural land in the United States. PLoS ONE. 2019, 14, e0220029. https://doi.org/10.1371/journal.pone.0220029.
Fotso, T.G.; Nukenine, E.N.; Tchameni, R.; Goudoungou, J.W.; Kosini, D.; Tigamba, V.; Adler, C. Use of Cameroonian Hemizygia welwitschii Rolfe Ashby (Lamiaceae) leaf powder against Callosobruchus maculatus and Sitophilus zeamais. Journal of Entomology and Zoology Studies. 2018, 6, 1261-1269.
Hall, J.B.; Swaine, M.D. Distribution and ecology of vascular plants in a tropical rain forest – Forest vegetation in Ghana, Dr. W. Junk Publishers, The Hague, 1981, 355pp. http://dx.doi.org/10.1007/978-94-009-8650-3.
Hiama, P.D.; Ewusi-Mensah, N.; Logah, V. Nutrient uptake and biological nitrogen fixation in cowpea under biochar–phosphorus interaction. Journal of Animal and Plant Sciences. 2019, 29, 1654-1663.
Ileke, K.D.; Adesina, J.M.; Nwosu, L.C.; Olagunju, A. Perforation index assessment of cowpea seeds against cowpea bruchid, Callosobruchus maculatus (Fabricius) (Coleoptera: Chrysomelidae), infestation using Piper guineense. The Journal of Basic and Applied Zoology. 2020, 81, 1-10. https://doi.org/10.1186/s41936-020-00195-7.
Ilesanmi, O.J.; Gungula, D.T. Quality Attributes of Cowpea Seeds Stored with Neem and Moringa Seed Oils. World Journal of Agricultural Sciences. 2013, 9, 155-160. https://doi.org/10.5829/idosi.wjas.2013.9.2.1711.
Islas, J.F.; Acosta, E.; Zuca, G.; Delgado-Gallegos, J.L.; Moreno-Treviño, M.G.; Escalante, B.; Moreno-Cuevas, J.E. An overview of Neem (Azadirachta indica) and its potential impact on health. Journal of Functional Foods. 2020, 74, 104171. https://doi.org/10.1016/j.jff.2020.104171.
Isman, M.B. Botanical insecticides, deterrents and pepellents in mordern agriculture and an increasingly regulated world. Annual Review on Entomology. 2006, 51, 45-66. https://doi.org/10.1146/annurev.ento.51.110104.151146.
Jehajo, N.; Din, N.M. Evaluation of the host preference and life history of Callosobruchus maculatus (F) (cowpea weevil) (Coleoptera: Chrysomelidae) on stored pulses. Pure and Applied Biology (PAB). 2020, 9, 2167-2174. http://dx.doi.org/10.19045/bspab.2020.90231.
Kamara, E.G.; Massaquoi, F.B.; James, M.S.; George, A. Effects of packaging material and seed treatment on Weevil (Callosobruchus maculatus (F) Coleoptera: Bruchidae) infestation and quality of cowpea seeds. African Journal of Agricultural Resources. 2014, 9, 3313-331. https://doi.org/10.5897/AJAR2014.8821
Kamran, M.; Hussain, S.; Abid, M.A.; Syed, S.K.; Suleman, M.; Riaz, M.; Qadir, R. Phytochemical composition of Moringa oleifera its nutritional and pharmacological importance. Postepy Biologii Komorki. 2020, 47, 321-334.
Katamssadan, H.T.; Elias, N.N.; Matthias, S.; Cornel, A. Degradation of azadirachtin A on treated maize and cowpea and the persistence of Azadirachta indica seed oil on Callosobruchus maculatus and Sitophilus zea mais. Journal of Stored Products Research. 2016, 69, 207-212.
Kiruba, S.; Mohan, S.; Manohar, D.S.; Papadopoulou, S. Experimental confirmation of the Bruchidae natural parasitism efficacy using an innovative device friendly to the environment. Biotechnology & Biotechnological Equipment. 2012, 26, 2722-2725. https://doi.org/10.5504/BBEQ.2011.0100.
Kpoviessi, A.D.; Agbahoungba, S.; Agoyi, E.E.; Chougourou, D.C.; Assogbadjo, A.E. Resistance of cowpea to Cowpea bruchid (Callosobruchus maculatus Fab.): Knowledge level on the genetic advances. Journal of Plant Breeding and Crop Science. 2019, 11, 185-195. http://dx.doi.org/10.5897/JPBCS2019.0818.
Kumar, A.; Kadam, S.S.; Arif, M.; Meena, R.K.; Verma, T.P. Legumes an alternative land use options for sustaining soil health. Agriculture & Food e-newsletter. 2020, 6.
Lengai, G.M.; Muthomi, J.W. Biopesticides and their role in sustainable agricultural production. Journal of Biosciences and Medicines. 2018, 6, 7-41. https://doi.org/10.4236/jbm.2018.66002
Lengai, G.M.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Scientific African. 2020, 7, e00239. https://doi.org/10.1016/j.sciaf.2019.e00239.
Loebel, F. Studies of the nutritive value of Moringa oleifera (Drumstick tree). Agriculture and Food Chemistry. 2002, 28, 1163-1166.
Madukwe, D.K.; Onuh, M.O.; Christo, I.E. Effects of Moringa oleifera leaf extract on the growth and yield of soybean (Glucine max L. Merri and sweet pepper Capsicum annum L.). International Journal of Applied Resource Technology. 2012, 201, 90-97.
Mishra, S.K.; Macedo, M.L.R.; Panda, S.K.; Panigrahi, J. Bruchid pest management in pulses: past practices, present status and use of modern breeding tools for development of resistant varieties. Annals of Applied Biology. 2018, 172, 4-19. https://doi.org/10.1111/aab.12401.
Mobolade, A.J.; Bunindro, N.; Sahoo, D.; Rajashekar, Y. Traditional methods of food grains preservation and storage in Nigeria and India. Annals of Agricultural Sciences. 2019, 64, 196-205. https://doi.org/10.1016/j.aoas.2019.12.003.
Nkhoma, N.; Shimelis, H.; Laing, M.D.; Shayanowako, A.; Mathew, I. Assessing the genetic diversity of cowpea (Vigna unguiculata (L.) Walp) germplasm collections using phenotypic traits and SNP markers. BMC genetics. 2020, 21, 1-16. https://doi.org/10.1186/s12863-020-00914-7.
Obanyi, J.N. Effect of legume diversity intercrop and varieties on population and severity of damage by foliage beetles (Ootheca spp. and Medythia sp.) on yields of common beans (Phaseolus vulgaris l.) in western Kenya. MSc Thesis, Egerton University, Njoro. 2018. http://41.89.96.81:8080/xmlui/handle/123456789/1671.
Oguh, C.E.; Okpaka, C.O.; Ubani, C.S.; Okekeaji, U.; Joseph, P.S.; Amadi, E.U. Natural pesticides (biopesticides) and uses in pest management-a critical review. Asian Journal of Biotechnology and Genetic Engineering. 2019, 2, 1-18.
Ogunwolu, E.O.; Odunlami, A.T. Suppression of seed bruchid (Callosobruchus maculatus (F.) development and damage on cowpea (Vigna unguiculata (L.) Walp.) with Zanthoxylum xanthoxyloides (Lam.) Waterm. (Rutaceae) root bark powder when compared to neem seed powder and pirimiphos-methyl. Crop Protection. 1996, 15, 603-607. https://doi.org/10.1016/0261-2194(95)00088-7.
Omoigui, L.O.; Kamara, A.Y.; Kamai, N.; Ekeleme, F.; Aliyu, K.T. Guide to cowpea production in Northern Nigeria. IITA, Ibadan, Nigeria, 2020, 48.
Oni, M.O.; Ileke, K.D. Fumigant toxicity of four botanical plant oils on survival, egg laying and progeny development of the dried yam beetle, Dinoderus porcellus (Coleoptera: Bostrichidae). Ibadan Journal on Agricultural Resources. 2008, 4, 31-36.
Paul, U.V.; Lossini, J.S.; Edwards, P.J.; Hilbeck, A. Effectiveness of products from four locally grown plants for the management of Acanthoscelides obtectus (Say) and Zabrotes subfasciatus (Boheman) (both Coleoptera: Bruchidae) in stored beans under laboratory and farm conditions in Northern Tanzania. Journal of Stored Products Research. 2009, 45, 97-107. https://doi.org/10.1016/j.jspr.2008.09.006.
Race, M.; Karabo, O.; Obopile, M.; Tiroesle, B.; Mmolotsi, R.; Rampart, M.; Tshegofatso, A.B.N. Effects of Moringa oleifera root and leaf powder on reproductive capacity and damage caused on stored cowpea seed by Callosobruchus maculatus (F). Journal of Agricultural Technology. 2012, 8, 2319-2329.
Seidu, A. Susceptibility of Cowpea (Vigna Unguiculata (L.) Walpers.) Varieties to The Pulse Beetle (Callosobruchus Maculatus Fab.). MSc Thesis, University for Development Studies, Tamale, 2019.
Sugri, I.; Abubakari, M.; Owusu, R.K.; Bidzakin, J.K. Postharvest losses and mitigating technologies: evidence from upper East Region of Ghana. Sustainable Futures. 2021, 3, 100048. https://doi.org/10.1016/j.sftr.2021.100048.
Tiroeselea, B.; Ranthoakgalea, G.; Ullah, M.I.; Mehmood, N.; Zahid, S.M.A.; Abid, B. Tamboti wood ash and burnt goat dropping ash, safe alternatives to control cowpea weevils, Callosobruchus maculatus (Fabr.) (Coleoptera: Bruchidae) during storage for subsistence farming. Environmental monitoring and assessment. 2019, 191, 1-9. https://doi.org/10.1007/s10661-019-7632-8.
Ukoroije, R.B.; Otayor, R.A. Review on the bio-insecticidal properties of some plant secondary metabolites: types, formulations, modes of action, advantages and limitations. Asian Journal of Research in Zoology. 2020, 3, 27-60. http://dx.doi.org/10.9734/ajriz/2020/v3i430099.
Zhang, Z.; Pang, X.; Guo, S.; Cao, J.; Wang, Y.; Chen, Z.; Feng, Y.; Lei, N.; Du, S. Insecticidal activity of Artemisia frigida Willd. Essential oil and its constituents against three stored product insects. Records of Natural Products. 2018, 13, 176-181. http://dx.doi.org/10.25135/rnp.91.18.06.114.
Academic Editor: Dr. Isabela Maria Simion
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.