Alina Elena Trofin, Elena Ungureanu, Iuliana Motrescu, Lucia Carmen Trincă, Denis Constantin Țopa, Diana Beatrice Eperjessy
ABSTRACT. The retention of nitrite ions in solutions of different concentrations by three cornhusks-based powders was analyzed. Natural cornhusk powder (NCHP), as waste obtained from local market, the alkalized cornhusk powder (ACHP) and the biochar from the original material (CHBC) have been characterized through scanning electron microscopy (SEM) and elemental composition EDAX – TEAM analysis (Energy dispersive analysis X-ray – Texture and ele-mental analytical microscopy) and tested for the removal of nitrite ions. The influence of initial nitrite concentration and contact time was studied under slow stirring rate conditions (150 rpm). For all three adsorbents both Freundlich and Langmuir isotherm equations described the process with R2 > 0.95, denoting physical adsorption and chemisorption on the surface. The estimated retained quantities (mg·g-1) determined from isotherms were 4.4783 (NCHP), 8.3542 (ACHP) and 8.7413 (CHBC). The Ho&McKay model was better adjusted to the adsorption data with R2 > 0.985, while the Lagergren model produced regression factors between 0.61 and 0.88. Considering the biggest concentration of nitrite solution of 50 mg·L-1 and the longest contact time of 150 minutes, the equilibrium capacity qe (mg·g-1) predicted by the Ho&McKay model for the considered adsorbents were: 4.5065 (NCHP), 8.5179 (ACHP) and 8.9445 (CHBC) compared to the obtained qt (mg·g-1) of 4.4384 (NCHP), 8.0685 (ACHP) and 8.5753 (CHBC). The nitrite uptake in the experiments reached a maximum of 2.2192 mg·g-1 on NCHP, 4.0342 mg·g-1 on ACHP and 4.2877 mg·g-1 on CHBC. Considering the cost-effective treatment steps, there is the possibility of valorising an important amount of waste as adsorbent materials.
Keywords: cornhusks powder; nitrite removal; waste valorisation.
Cite
ALSE and ACS Style
Trofin, A.E.; Ungureanu, E.; Motrescu, I.; Trincă, L.C.; Țopa, D.C.; Eperjessy, D.B. Cornhusk powders as adsorbents for nitrites in solution: a thermodynamic and kinetic approach. Journal of Applied Life Sciences and Environment 2023, 56 (3), 321-344.
https://doi.org/10.46909/alse-563103
AMA Style
Trofin AE, Ungureanu E, Motrescu I, Trincă LC, Țopa DC, Eperjessy DB. Cornhusk powders as adsorbents for nitrites in solution: a thermodynamic and kinetic approach. Journal of Applied Life Sciences and Environment. 2023; 56 (3): 321-344.
https://doi.org/10.46909/alse-563103
Chicago/Turabian Style
Trofin, Alina Elena, Elena Ungureanu, Iuliana Motrescu, Lucia Carmen Trincă, Denis Constantin Țopa, and Diana Beatrice Eperjessy. 2023. “Cornhusk powders as adsorbents for nitrites in solution: a thermodynamic and kinetic approach” Journal of Applied Life Sciences and Environment 56, no. 3: 321-344.
https://doi.org/10.46909/alse-563103
View full article (HTML)
Cornhusk Powders as Adsorbents for Nitrites in Solution: A Thermodynamic and Kinetic Approach
Alina Elena TROFIN1*, Elena UNGUREANU1, Iuliana MOTRESCU1, Lucia Carmen TRINCĂ1, Denis Constantin ȚOPA2 and Diana Beatrice EPERJESSY3
1Department of Exact Sciences, Faculty of Horticulture, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 3, Mihail Sadoveanu Alley, 700489, Iasi, Romania; email: eungureanu@uaiasi.ro; imotrescu@uaiasi.ro; lctrinca@uaiasi.ro
2Department of Pedotechnics, Faculty of Agriculture, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 3, Mihail Sadoveanu Alley, 700489, Iasi, Romania; email: topadennis@uaiasi.ro
3”Saint Mary” Emergency Hospital for Children Iași; e-mail: eperjessydiana@gmail.com
*Correspondence: alina.trofin@gmail.com
Received: Jun. 15, 2023. Revised: Aug. 17, 2023. Accepted: Sep. 07, 2023. Published online: Oct. 04, 2023
ABSTRACT. The retention of nitrite ions in solutions of different concentrations by three cornhusks-based powders was analyzed. Natural cornhusk powder (NCHP), as waste obtained from local market, the alkalized cornhusk powder (ACHP) and the biochar from the original material (CHBC) have been characterized through scanning electron microscopy (SEM) and elemental composition EDAX – TEAM analysis (Energy dispersive analysis X-ray – Texture and ele-mental analytical microscopy) and tested for the removal of nitrite ions. The influence of initial nitrite concentration and contact time was studied under slow stirring rate conditions (150 rpm). For all three adsorbents both Freundlich and Langmuir isotherm equations described the process with R2 > 0.95, denoting physical adsorption and chemisorption on the surface. The estimated retained quantities (mg·g-1) determined from isotherms were 4.4783 (NCHP), 8.3542 (ACHP) and 8.7413 (CHBC). The Ho&McKay model was better adjusted to the adsorption data with R2 > 0.985, while the Lagergren model produced regression factors between 0.61 and 0.88. Considering the biggest concentration of nitrite solution of 50 mg·L-1 and the longest contact time of 150 minutes, the equilibrium capacity qe (mg·g-1) predicted by the Ho&McKay model for the considered adsorbents were: 4.5065 (NCHP), 8.5179 (ACHP) and 8.9445 (CHBC) compared to the obtained qt (mg·g-1) of 4.4384 (NCHP), 8.0685 (ACHP) and 8.5753 (CHBC). The nitrite uptake in the experiments reached a maximum of 2.2192 mg·g-1 on NCHP, 4.0342 mg·g-1 on ACHP and 4.2877 mg·g-1 on CHBC. Considering the cost-effective treatment steps, there is the possibility of valorising an important amount of waste as adsorbent materials.
Keywords: cornhusks powder; nitrite removal; waste valorisation.
INTRODUCTION
The global production of corn exceeds that of any other grain every year. Approximately 850 million tons of corn grains are produced on an area of 162 million hectares, the main producers being the USA, China, Argentina and Brazil (Yara, 2023). Apart from grains, cobs can be used in animal feed and as an ingredient in obtaining compost, but large quantities remain unused.
A series of materials with a porous structure and a large number of superficial functional groups can be used effectively for the removal of pollutants from aqueous solutions. Thus, natural materials and agricultural or industrial waste can remove heavy metal ions and some organic substances from wastewater.
In order to protect the environment, research has been intensified to obtain innovative ecological materials, easily degradable, with minimal processing costs compared to classic plastic materials (Omran et al., 2021).
Recently, solutions have been sought for the problem of large amounts of urban or agricultural waste, one of them being their use in the preparation of biodegradable and renewable materials.
The availability of these wastes offers the possibility to prepare cheap biocomposites. It was found that the materials produced from mixtures of natural polymers can present superior properties to polymers processed separately.
Composites based on cellulose, such as that extracted from corn husks, are fragile, brittle and rigid if they are processed without the addition of plasticizers (Abe et al., 2021; Sari et al., 2020a).
In order for the processing of these wastes to be profitable, it is preferable to use all the compounds extracted from them, such as lignin that can be used in the production of activated carbon, resins or as an adsorbent for toxic metals from wastewater.
Modified lignin Protobind 1000, lignin obtained from Sarkanda grass and sawdust were tested by authors in experiments regarding the removal of certain ions from aqueous solution: Pb2+ – 27.23 mg·g-1 on Protobind 1000 and 27.18 mg·g-1 on Sarkanda grass lignin, Zn2+ – 8.28 mg·g-1 on Protobind 1000 and 8.24 mg·g-1 on Sarkanda grass lignin;, As3+ – 13.33 mg·g-1 on Sarkanda grass lignin , Cu2+ – 27.15 mg·g-1 on Sarkanda grass lignin) (Trofin et al., 2021; Ungureanu et al., 2021, 2022). Both lignin and cornhusks are considered waste and the possibility to valorise them as adsorbents in water remediation is taken into account.
These materials are available in large quantities, require a small number of operations for their preparation, can retain various pollutants through ion exchange or complexation, can be regenerated and reused or incinerated (Rusu, 2015).
Bio composite products can substitute plastic materials, being currently used as alternatives in housing material components or as engine or electronic and circuit board parts, etc. Corn fibers, among others, are constantly subjected to experiments regarding enhancing their physical and chemical properties (Mohamed et al., 2022).
Cellulose extracted from different parts of corn plants proved chemical and mechanical properties suited for application in composite materials (Ibrahim et al., 2019). Tan et al. (2022) stated that the composite polyamide 6 coated with cellulose nanocrystals membranes demonstrated improved mechanical strength and lower porosity, thus proving useful for packaging and infiltration. Cornhusks were compared to piassava, coir, caroa and olive husks and proved to have almost same levels of hemicellulose and cellulose and lower lignin percentage. Their surface’s structure presented a high number of micro fibrils (De Carvalho Mendes et al., 2015).
Cornhusks are subjected to surface treatment mainly to increase the roughness, to allow a better adhesion of other materials in the preparation of composites and to release carboxyl and hydroxyl groups on the surface. The treatment generates improved fibres regarding the morphology and mechanical properties (Hashim et al., 2017; Sari et al., 2022).
A comparison between alkali and acid treated cornhusk fibres showed that 7.5 g·L-1 sulfuric acid application reduces fiber length, but results in more thermally stable fibers (Wubneh et al., 2022). When treated with NaOH, cornhusk fibers loose moisture, hemicellulose and lignin, thus increasing the roughness of the surface and the appearance of tunnels between the fibres. Used in composites with polyester resin, when cornhusks content is higher and immersion time in water prolonged, they tend to have lower mechanical properties, an increased impact strength but lower compressive strenght (Sari et al., 2018, 2020b, 2022). In composites with a higher content of fibers extracted from corn huskss (20-30%), the increase in flexural and tensile strength is observed, due to the strong interaction between them and other components in the composite (Sari et al., 2021). Also, the type of cornhusk varies in terms of coarseness; the fibers obtained from lower parts of outer husks hold better mechanical properties than the alkali treated ones, while the upper parts and the interior husks have finer fibrils that retain moist easier (Yilmaz et al., 2016).
Corn stalks bleached with 5 g·L-1 NaOH for 60 minutes at boiling point using 50 mL solution for 1 g material, produce fibers with increased crystallinity that can be used in bio composites or as materials in the textile industry (Devi et al., 2022).
Fibers extracted from cornhusks were compared with cotton and jute fibers in terms of morphology and resistance properties. The application of banana stem sap as a flame retardant also improved the thermal stability of cornhusk fibers (Nishant et al., 2018). Cornhusks were also used by Dahliyanti et al. (2022) to synthesize and characterize a silica dry gel in order to use it as adsorbent for two cationic dyes, with removal efficiencies over 98 % and 96 %.
Cornhusks and other agricultural waste were mineralized, KOH activated and tested as potential adsorbents for metribuzin removal from polluted water, with promising results (Cara et al., 2015).
Nitrate contamination in natural water bodies has become a growing environmental concern around the world, owing mostly to the widespread use of chemical fertilizers, inefficient wastewater treatment from industrial and municipal facilities, and animal wastes (Ogata et al., 2018).
Nitrates are not harmful, but their potential endogenous conversion to nitrites and N-nitroso compounds has been linked to various acute and chronic ailments such as methemoglobinemia, thyroid problems, and carcinogenesis (Eperjessy et al., 2020). The permissible limit of nitrites in natural waters is 0.5 mg·L-1, according to European regulations. Water used for consumption that exceeds this limit is especially harmful for extreme ages, babies under 1 year old and elderly people (EFSA, 2008; WHO, 2023).
Vegetables and drinking water are primary sources of exogenous exposure, whereas processed meat and animal food items are major nitrite-containing foods; vegetables, particularly green leafy vegetables, account for more 80-95 percent of dietary nitrate intake (Hord et al., 2009; Reinik et al., 2009).
The presence of excess nitrogen ions in various forms has a negative impact on the ecosystem and, as a result, can harm human health. It produces inorganic chemicals in water, primarily nitrate ions, nitrite, and ammonium. It can be found in drinking water as well as surface or subsurface water in these forms (Gierak and Łazarska, 2017).
Therefore, a number of techniques for the retention of nitrate from polluted waters have been applied. These techniques include reverse osmosis, electro dialysis and biologically based denitrification. However, they are expensive to be used on a large scale. Therefore, many low-cost materials (industrial and agricultural wastes) have been used as adsorbents for nitrate removal (Xing et al., 2013).
The mechanism of nitrate removal on activated carbon is mostly a chemical adsorption that follows pseudo-second order kinetic model (Ho) and the Lagergren isotherm, which proves a multilayer nitrate adsorption on clinoptilolite adsorbent (Asl et al., 2016).
A study on NO3– adsorption on clay showed a proper development at acid pH, following Freundlich isotherm and pseudo-second-order kinetics, with a retained amount of 5.1 mg·g-1 (Battas et al., 2019).
The retention of NO3– on Amberlite IRA 400 ion exchange resin also followed Freundlich adsorption isotherm and a reversible first-order model kinetics, with a maximum capacity of 769.2 mg·g-1 at room temperature (Chabani et al., 2006).
Gierak and Lazarka (2017) tested the retention of NO3– and NO2– ions from low concentrated solutions on columns filled with commercial carbon and carbon oxidized with 30% H2O2 and found a maximum of 0.3665 mg·g-1 nitrite for commercial carbon and 0.9053 mg·g-1 nitrite for the oxidized product. Another study used Fe – Mg type hydrotalcites for NO2– and NO3– adsorption and concluded that both the Langmuir and Freundlich equations fit the data and the kinetic studies followed the Ho&McKay equation and the experimental pH ranged widely between 4 and 12 with no effect on the process (Ogata et al., 2018).
Asl et al. (2016) also experimented with activated carbon and clinoptilolite to remove NO3– considering the variation of contact time, nitrate concentration, pH, adsorbent dosage and temperature. They found a better adsorption efficiency (over 62%) for activated carbon, whose data complied to Freundlich isotherm and second order kinetics, with 60 minutes contact time at pH of 5.5 and room temperature, while the data obtained for clinoptilolite followed Langmuir isotherm and first order kinetics and gave an efficiency of 8.7% in the same conditions. On powdered activated carbon, Neşe and Ennil (2008) found that only 1.2 mg·g-1 nitrite ions are retained from a solution of 25 mg·L-1 concentration, using 0.5 g of adsorbent for a sample of 50 mL solution.
Another adsorbent used for NO2– ions is a hyper-branched polyaminoester, with a large number of hydroxyl groups, used to form H bonds with the NO2– ions. The hydrophilicity of the initial material was diminished by end capping in thrichloroctadecylsilan which allowed the separation after the adsorption took place. Kinetics followed the pseudo-first order model and the equilibrium data corresponded better to the Freundlich isotherm (Bai et al., 2012).
Resins produced from wheat stalk and cotton stalk were employed in static adsorption tests, and results showed that nitrate removal was almost constant in 4–10 pH range. The qmax for NO3– were between 33.35 and 50.24 mg·g-1. Also, when HCl and NaCl were used in the desorption process in columns the efficiencies reached 95-98%. Pseudo- second-order kinetic model fit the obtained data with regression factors of 0.999, implying that chemical sorption or ion exchange were mainly occurring. The analysis provided by the intraparticle diffusion model proved that other kinetic processes were also involved during adsorption (Xu et al., 2013). The results obtained by Ouakouak et al. (2013) indicated that active carbon fairly removes NO3– after 1.5 hours at optimal pH of 4 using 1 g·L-1 for concentrations of 5-100 mg ·L-1 NO3– fitting both Langmuir and Freundlich equations. The removal of NO3– and NO2– from wastewater was also tested on activated carbon obtained from rice straws. Chemisorption proved to be involved as the adsorption data was better predicted by second-order kinetics (Hanafi et al., 2016).
Nanomaterials are lately tested to remediate pollutants’ concentrations in the environment, but as some of them are used as pigments or additives, can rapidly become contaminants (Saleh, 2020). Nitrate adsorption on activated carbon covered with magnetite nanoparticles tested by Kalantary et al. (2014) followed the Langmuir and second-order kinetic models. The equilibrium parameters were found to be 60 min and 57.1 mg·g-1 at pH equal 3.
Activated carbon, clay minerals or zeolite and resins have been often used in water treatment methods (Saleh, 2021). Results obtained by Zhang et al. (2014) regarding the retention of NO3– from concentrated solution on alkalized activated carbon obtained from rice husks also showed that the second order kinetics explained the adsorption process with a maximum efficiency of 70.6%.
The present study aims to evaluate a possible use of cornhusks powder, treated with alkali or transformed into char, to increase the contact surface, as adsorbent materials for nitrites (NO2–) from aqueous solutions of different concentration, at neutral pH and room temperature. Considering that nitrites concentrations above admissible limits are found in polluted water, but also in wells, and cornhusks are available, we tested these powders for nitrite retention, aiming at using them as an affordable material to decrease nitrite concentration in water. The possibility of remediation of water with minimal costs for materials and reagents and also decreasing the number of processing steps required to obtain adsorbent materials is aimed at, which can make them more attractive from an economic point of view.
MATERIALS AND METHODS
Considered as waste, the cornhusks used as the basic material for obtaining the three powders taken in study were acquired from local markets. They were washed with distilled water, air-dried for two weeks, then ground and sifted three times through a sieve ROTH Rotilabo, ø 50 mm, mesh size 0.45 mm. The first adsorbent considered was natural cornhusk powder (NCHP), prepared as described above, without any other processing step. For the second adsorbent, alkalized cornhusk powder (ACHP), the initial powder was mixed in a ratio of 1:50 with a KOH solution of 5.6 g·L-1 and kept at the boiling temperature of the mixture for 30 minutes on an electric hot plate with magnetic stirring D-Lab-ECO-STIR 300-2000 rotation·minute-1. After cooling and vacuum filtration on filter paper 113A ROTH Rotilabo, the powder was brought to neutral pH on the filter by repeated washings with distilled water then dried for 4 hours at 105ºC in the hot air oven BIOBASE model BOV-T30C. For the preparation of the activation solution, potassium hydroxide (pellets of analytical grade) supplied by Chemicalls Lab Supplier Romania was used. The third adsorbent, cornhusk biochar (CHBC), was prepared by calcining NCHP for six hours in a porcelain crucible ISOLAB, 50*32 mm, in a NABERTHERM oven, model L3/11/B170, at 550ºC, when 3.19 % of initial material remained.
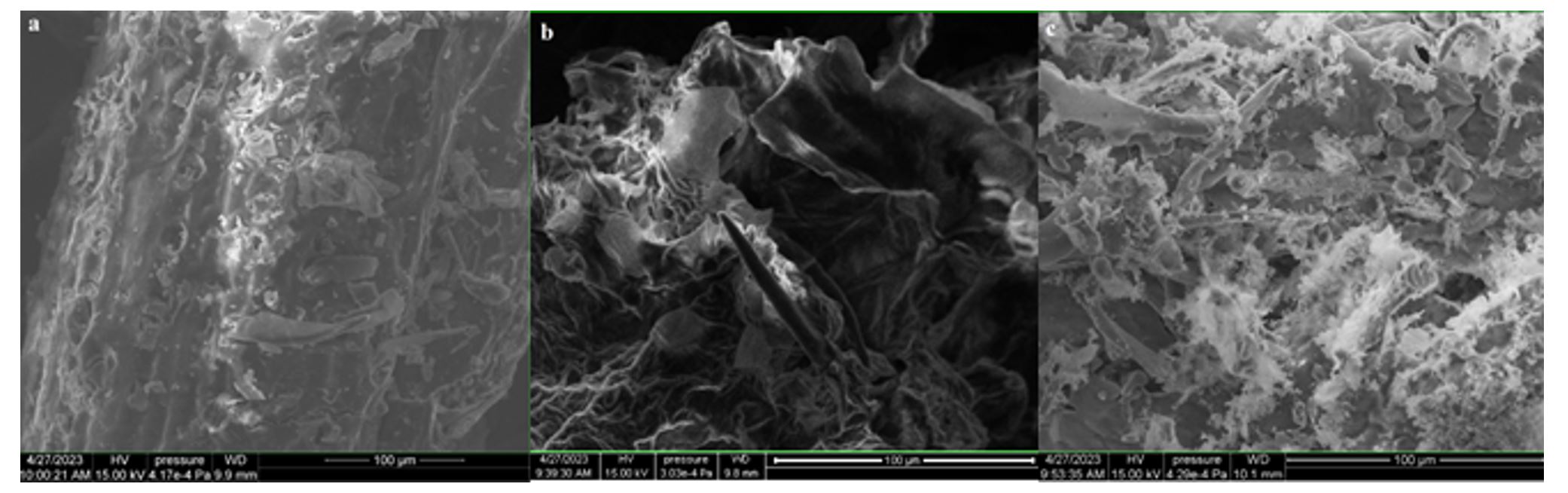
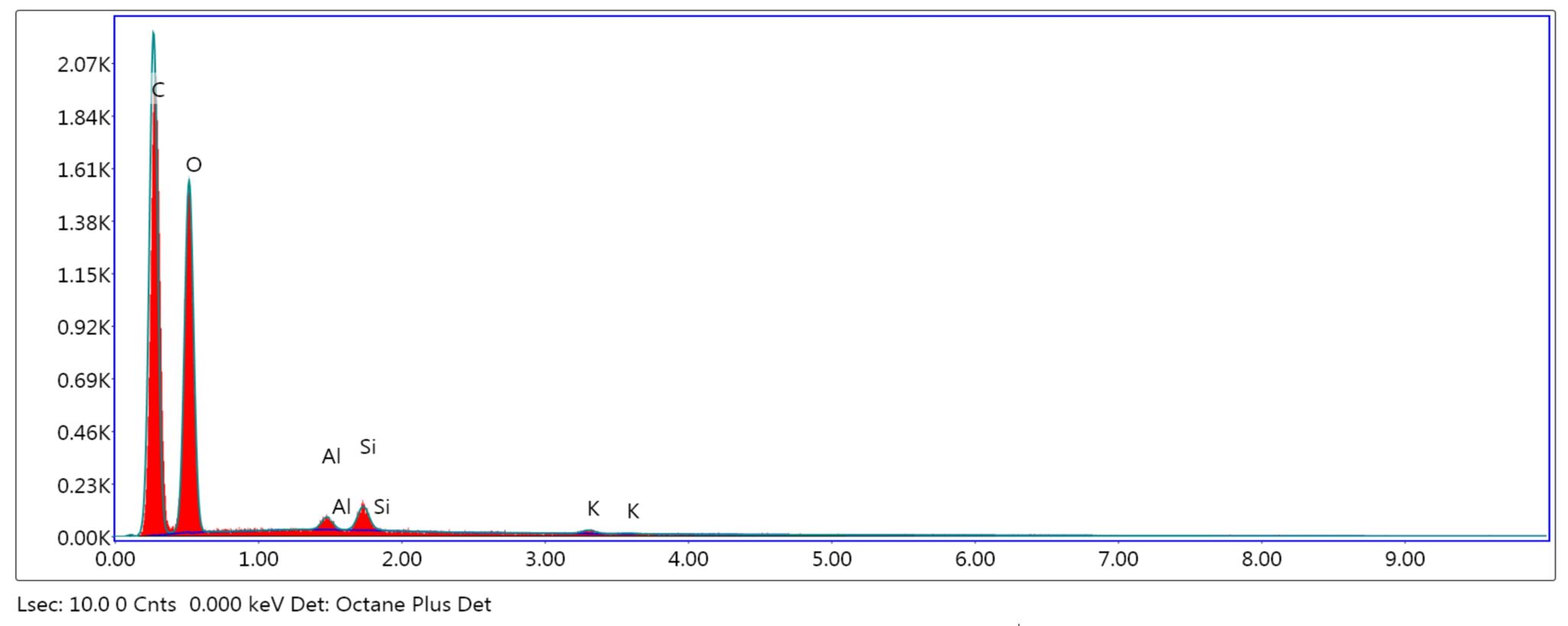
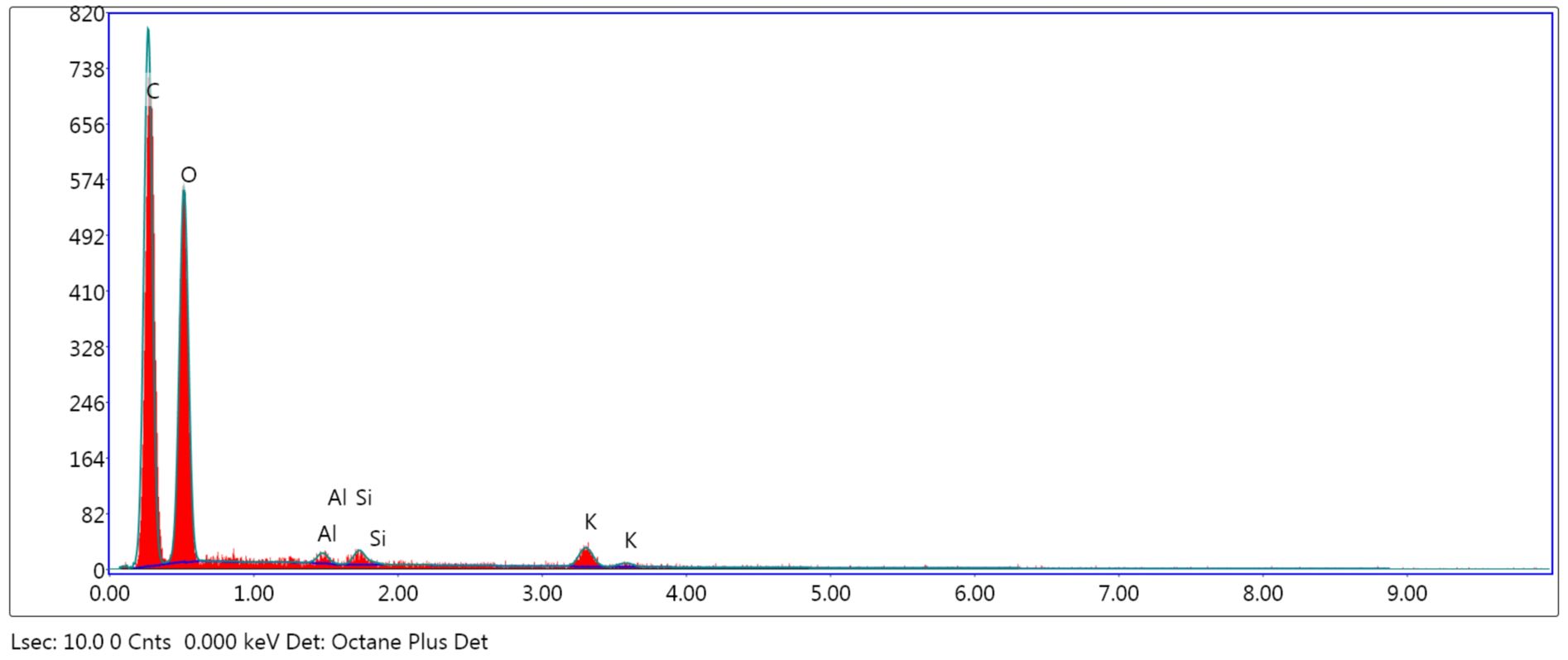
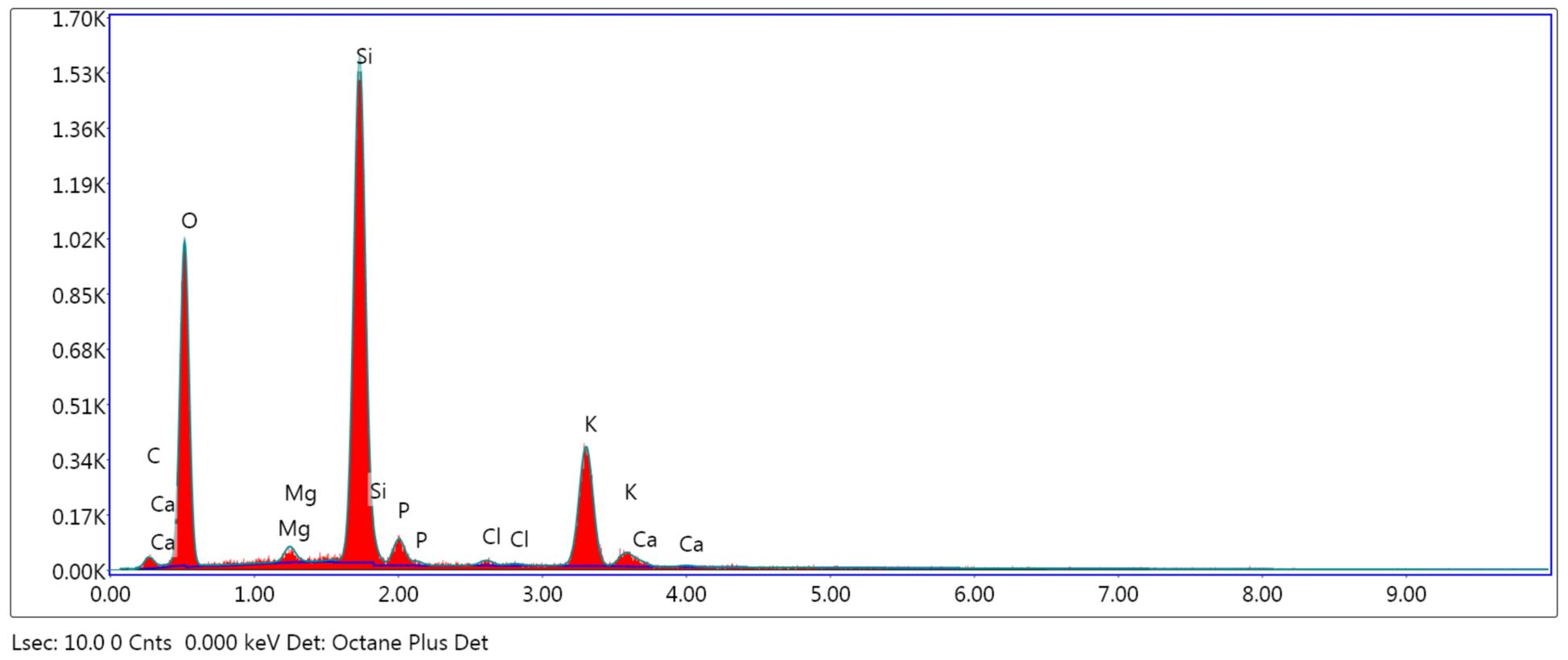
The adsorbents were subjected to surface and structure analysis by scanning electron microscopy (SEM) as seen in Figure 1 (a, b and c) and elemental analysis through EDAX TEAM, as seen in Figure 2, Figure 3 and Figure 4 and in Table 1.
The nitrite solutions were prepared from NaNO2 of analytical purity, purchased from Chemicalls Lab Supplier Romania and ultrapure water obtained with water purifier Biobase SCSJ-I, RO, DI, 10 L/h purchased from Amex Laboratory Romania and had concentrations of 10, 20, 30, 40 and 50 mg·L-1 NO2–. The contact times between the adsorbent and the solution were 30, 60, 90, 120 and 150 minutes for all analyzed concentrations. In the batch experiments, three repetitions were performed for each sample; 0.1 g of adsorbent was added to a glass containing 20 mL of nitrite solution, the mixture being subjected to magnetic stirring at 150 rotation·minute-1 for the considered time period, at a temperature of 20ºC.
The reagent used for nitrite recovery was HCl in a concentration of 3.7 g·L-1, provided also by Chemicalls Lab Supplier Romania. 20 mL of HCl solution were stirred with 0.1 g of used adsorbent for 15 minutes and the nitrite concentration was calculated using the same procedure steps and method as the samples.
After separating the filtrate from the adsorbent, the concentrations of the analyzed solutions were determined using the Griess method described in literature (Moorcroft et al., 2001; Wang, 2010). 0.03% solution of α-naphtylamine·HCl and 0.6% solution of sulphanilic acid from analytical grade reagents provided also by Chemicalls Lab Supplier Romania were prepared and mixed in equal volumes, to obtain the diazotization reagent for nitrite ions in the samples, and a VIS spectrophotometer V1000 SN, model YA07151909217 and glass vats 1 cm wide were used.
For each sample, three repetitions were made, considering the average value of the absorbance provided by the spectrophotometer’s software. 2 mL of filtrate were mixed with 2 mL of Griess reagent, maintained 30 minutes for the colour to develop properly and after that the absorbance was determined at 520 nm wavelength.
Comparing the surface’s aspect of the adsorbents taken into consideration and the elements’ percentages, an increase of the surface’s coarseness is observed in the treated powders, due to alkalization and calcination. Therefore, an increase of nitrite retention was expected to occur. In ACHP, the content in K was higher, as potassium hydroxide induced the modification of the vegetal particles’ surfaces and was partially retained. The CHBC powder had a different elemental distribution due to oxidation at high temperatures and C loss, mainly.
RESULTS
Thermodinamic study of nitrite adsorption Freundlich isotherm
The equation attached to the Freundlich model for the data obtained after an adsorption is generally suitable for processes that take place on heterogeneous surfaces where the particles are retained in several layers (Nimibofa et al., 2017).
This equation can be expressed mathematically as follows (Equation 1):
![]()
Table 1
eZAF Smart Quant results for NCHP, ACHP and CHBC
|
Adsorbent |
Element |
Weight % |
Atomic % |
Error % |
|
NCHP |
C K |
48.60 |
55.96 |
6.50 |
|
O K |
50.35 |
43.53 |
10.15 |
|
|
Al K |
0.35 |
0.18 |
14.27 |
|
|
Si K |
0.58 |
0.28 |
9.18 |
|
|
K K |
0.13 |
0.05 |
34.78 |
|
|
ACHP |
C K |
48.10 |
55.56 |
6.82 |
|
O K |
50.62 |
43.90 |
10.65 |
|
|
Al K |
0.28 |
0.14 |
27.11 |
|
|
Si K |
0.32 |
0.16 |
21.61 |
|
|
K K |
0.69 |
0.24 |
17.53 |
|
|
CHBC |
C K |
5.12 |
8.04 |
19.47 |
|
O K |
59.51 |
70.12 |
9.72 |
|
|
Mg K |
0.97 |
0.75 |
16.77 |
|
|
Si K |
22.62 |
15.18 |
4.93 |
|
|
P K |
1.79 |
1.09 |
11.17 |
|
|
Cl K |
0.32 |
0.17 |
38.68 |
|
|
K K |
9.18 |
4.43 |
4.33 |
|
|
Ca K |
0.48 |
0.23 |
33.98 |
In Equation 1, KF and n represent the Freundlich constants, qeq is the equilibrium adsorption capacity (mg·g-1) on the adsorbent’s surface and Ceq is the equilibrium concentration of the analyte in solution (mg·L-1). KF is related to the adsorption capacity of the material surface, and n is an indicator that shows how favourable and efficient the adsorption is. The ratio 1/n is a measure of the heterogeneity of the surface. The smaller the values of this ratio are, tending towards 0, the more heterogeneous the surface (Fytianos et al., 2000).
The linear form of the Freundlich isotherm (Equation 2) was applied for the data interpretation:
![]()
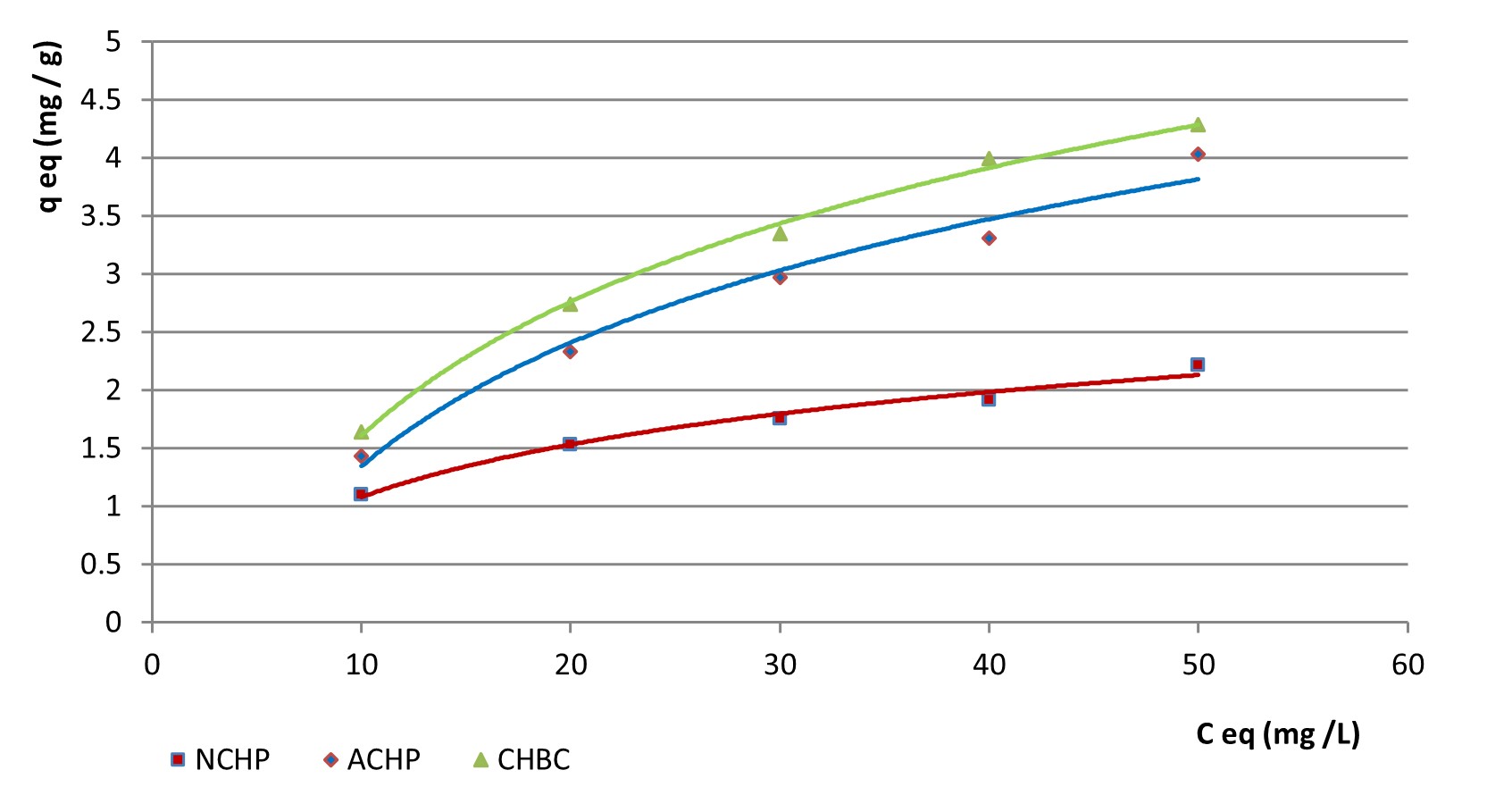
According to this isotherm model, the graph plotted with the retained quantity (mg nitrite·g-1) versus initial concentration expressed in mg nitrite·L-1 in Figure 5 shows that the adsorption process on the surfaces of the three cornhusk-based powders follows the Freundlich isotherm form with regression coefficients between 0.9763 for ACHP and 0.9967 for CHBC.
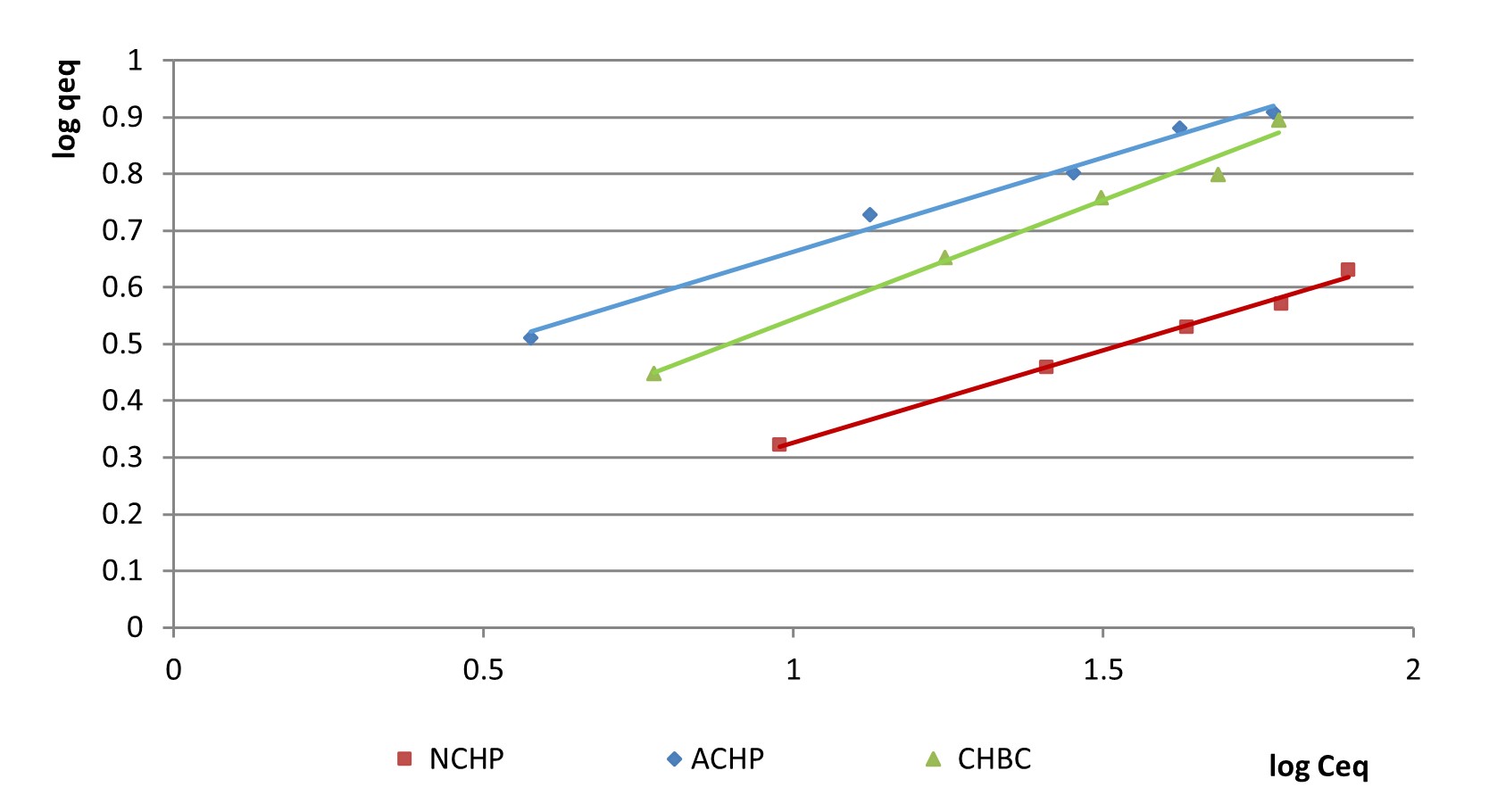
The plotting of log qeq versus log Ceq (as shown in Figure 6) allows the observation that this model suits appropriately the adsorption process of NO2– on the surfaces of NCHP, ACHP and CHBC. The considered contact time for the linear variations in Figure 6 is 90 minutes, as the amounts retained after that time were small compared to the ones already removed from the initial solution, for all concentrations.
Langmuir isotherm
Another isotherm that serves for the quantification of the adsorption potential of different materials is the Langmuir isotherm, described by the following mathematical Equation 3:
![]()
The linear form of the Langmuir isotherm (Equation 4) was also applied to the obtained data from NO2– adsorption on the three materials taken into consideration:
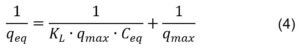
In Equation 3, qeq is the analyte equilibrium concentration (mg·g-1), qmax is the maximum amount of retained analyte (mg·g-1) and Ceq is the concentration of the analyte’s solution (mg·L-1) when equilibrium is established. The capacity and the energy of adsorption are related to the Langmuir constants KL and qmax.
A separation factor denoted RL is usually calculated in relation to the Langmuir isotherm characteristics (Equation 5):
![]()
In Equation 5, the Langmuir constant KL is expressed in mg·g−1 and the initial concentration C0 of the analyte is expressed in mg·L−1. When 𝑅𝐿 is bigger than 1, the retention process is declared unfavourable and if the values of RL range between 0 and 1, the adsorption is favourable (Cara et al., 2015).
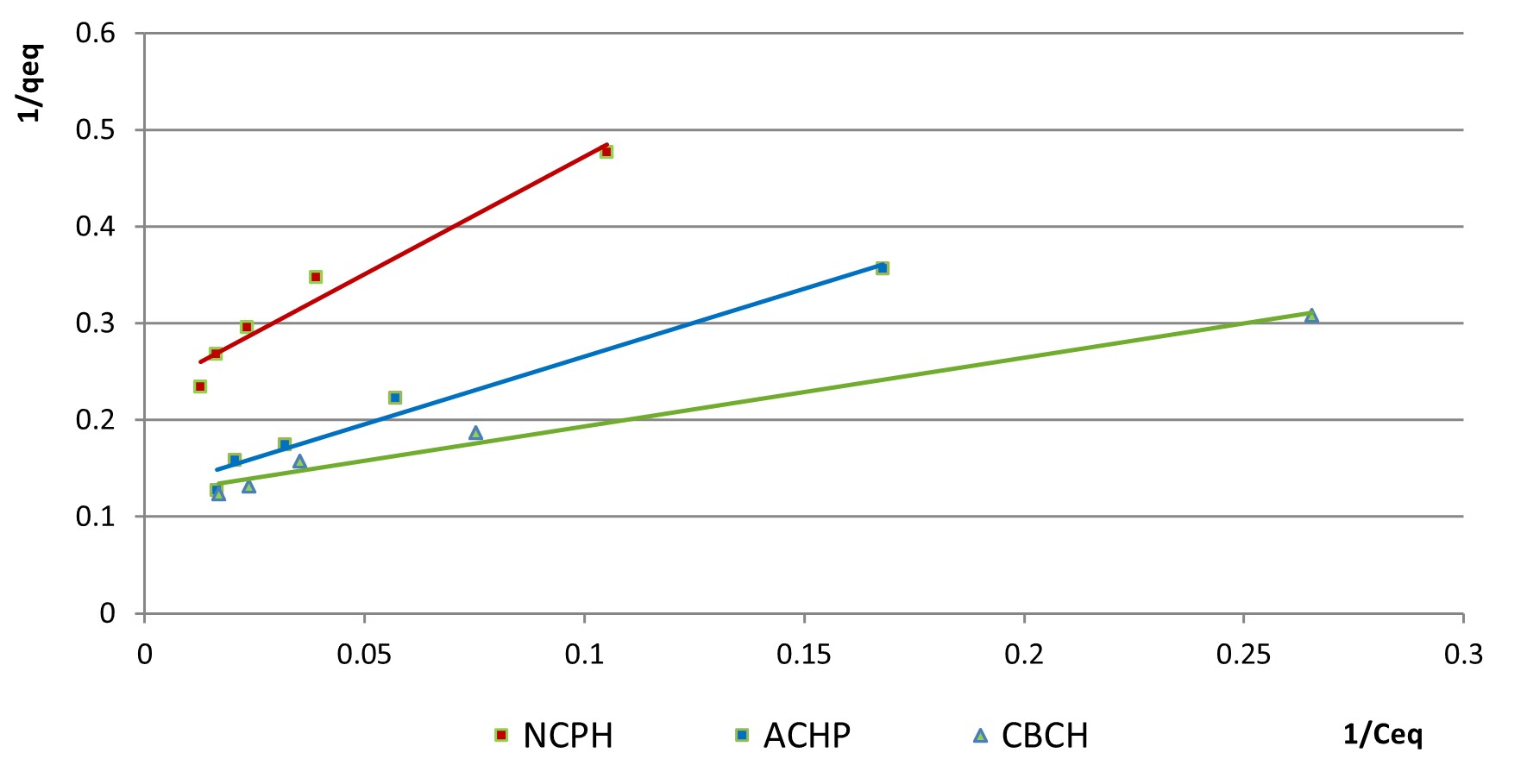
1/qeq was plotted versus 1/Ceq for the contact time of 90 minutes in Figure 7, to allow a comparison between the values of the parameters determined for both isotherm models for all three materials.
The extracted values for KF and 1/n (for Freundlich model) and the values of KL, qmax and RL (for Langmuir model) are presented in Table 2.
Five mechanisms are proposed for the adsorption of ions from aqueous solutions on different types of surfaces:
– ion exchange between the analyzed solution and other ions retained on the surface of the adsorbent material;
– electrostatic attractions between the ions in the solution and the superficial functional groups on the surface;
– the formation of complexes between retained ions and certain functional groups in the structure of the adsorbent;
– the deposition of ions in the form of insoluble combinations and
– the reduction of the oxidation state of the ions in order to retain them more easily on the surface. The adsorption mechanisms and the percentage of retained ions from the initial solution vary mainly with the properties of the surface and the working parameters selected to achieve the adsorption (Bulgariu et al., 2016).
NO2– retention on the surface of cornhusk-based materials occurs following two stages in the sorption mechanism. At the beginning, ions are retained quite rapidly on the surface’s active sites, the initial concentration of the analyte in solution decreasing with a higher rate; the second stage which involves intra particle diffusion is next, the retained ions diffusing into the pores of the adsorbent.
Kinetic study of nitrite adsorption
From a kinetic point of view, the adsorption process represents a sequence of diffusion stages, starting with the migration of the analyte from the initial solution to the liquid layer around the adsorbent particles, followed by its diffusion to the adsorbent’s surface and finally, the migration of the analyte into the pores of the adsorbent, where the active sites can physically or chemically retain the dissolved substance.
Table 2
Isotherm parameters determined in the adsorption of NO2–ions on NCHP, ACHP and CHBC
|
Adsorbent |
Time |
Freundlich isotherm |
Langmuir isotherm |
|||||
|
1/n |
kF |
R2 |
qmax |
kL |
RL |
R2 |
||
|
NCHP |
30 |
0.6049 |
0.5482 |
0.9689 |
4.6620 |
0.0269 |
0.4267 |
0.9387 |
|
60 |
0.3599 |
0.9203 |
0.9789 |
4.1876 |
0.0833 |
0.1936 |
0.9106 |
|
|
90 |
0.3262 |
0.9997 |
0.9944 |
4.3725 |
0.0937 |
0.1758 |
0.9614 |
|
|
120 |
0.307 |
1.04843 |
0.9875 |
4.4346 |
0.1053 |
0.1597 |
0.962 |
|
|
150 |
0.3073 |
1.05263 |
0.9892 |
4.4783 |
0.1053 |
0.1596 |
0.9646 |
|
|
ACHP |
30 |
0.487 |
0.8275 |
0.9707 |
6.8634 |
0.0369 |
0.3514 |
0.9984 |
|
60 |
0.4328 |
1.0684 |
0.9857 |
7.6220 |
0.0458 |
0.3041 |
0.9733 |
|
|
90 |
0.4195 |
1.1329 |
0.9859 |
7.9936 |
0.0889 |
0.1837 |
0.9748 |
|
|
120 |
0.4219 |
1.1482 |
0.9914 |
8.2919 |
0.0891 |
0.1833 |
0.9804 |
|
|
150 |
0.4217 |
1.1536 |
0.9907 |
8.3542 |
0.0899 |
0.1820 |
0.9806 |
|
|
CHBC |
30 |
0.3697 |
1.0824 |
0.9951 |
5.9773 |
0.0986 |
0.1686 |
0.9477 |
|
60 |
0.3263 |
1.3400 |
0.9922 |
6.9784 |
0.1985 |
0.0915 |
0.9655 |
|
|
90 |
0.3312 |
1.3938 |
0.9899 |
8.1766 |
0.1718 |
0.1043 |
0.9814 |
|
|
120 |
0.3354 |
1.4092 |
0.9906 |
8.5690 |
0.1675 |
0.1067 |
0.9842 |
|
|
150 |
0.3301 |
1.4212 |
0.9918 |
8.7413 |
0.1621 |
0.1098 |
0.9841 |
|
The kinetic models applied in the study of nitrite adsorption on the powders obtained from cornhusks (Lagergren and Ho&McKay) characterize in a suitable manner the mechanisms of the adsorption processes (Ho et al., 2002).
Pseudo first order Lagergren model
Specific for the liquid phase – solid surface adsorption processes, is characterized by the linear expression presented in Equation 6:
![]()
where K1 is the rate constant of Lagergren model (min-1), qeq is the analyte concentration at equilibrium (mg·g-1) and qt is the amount of retained ion at a specific time (mg·g-1).
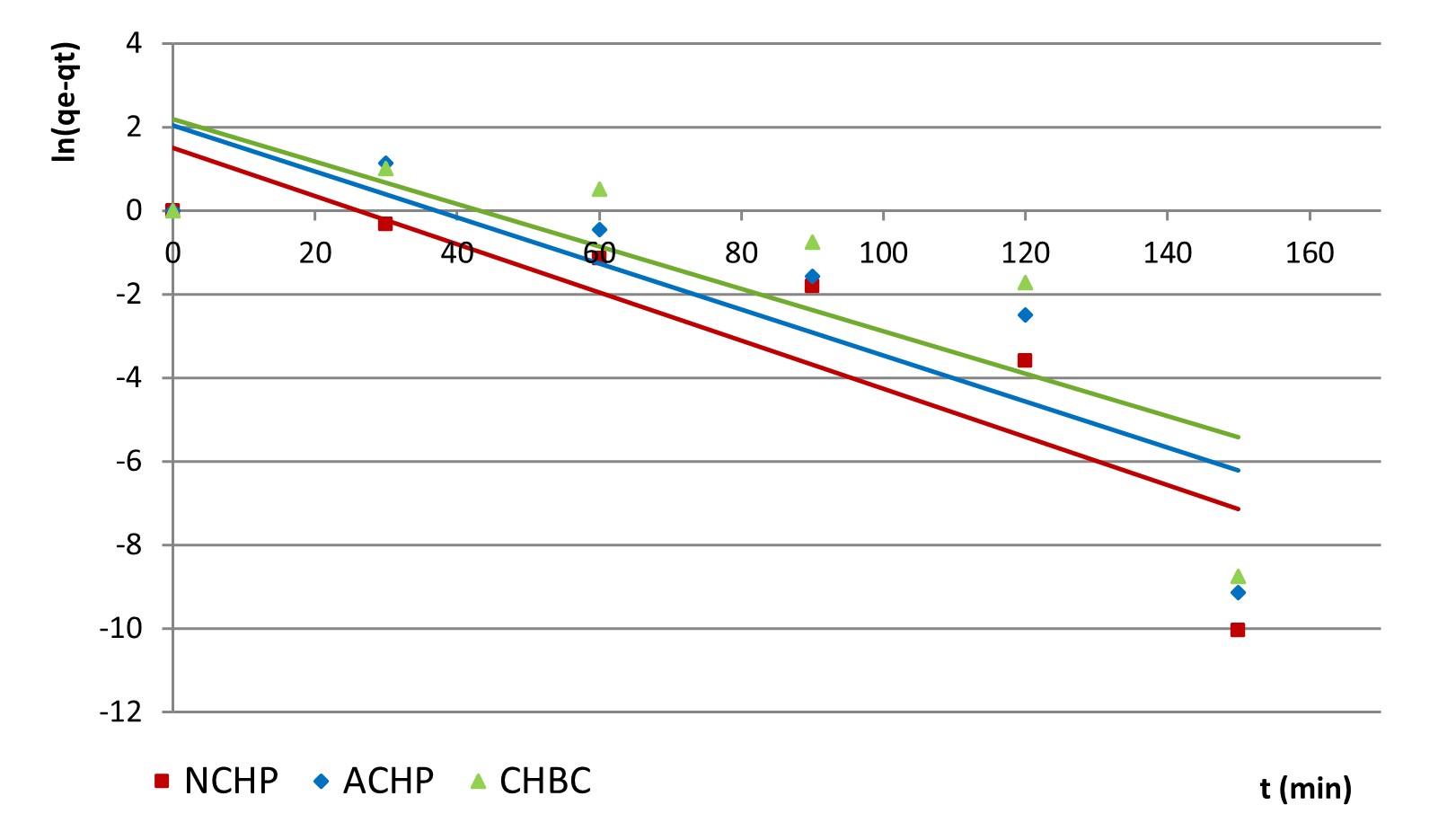
t (min) versus ln(qe − qt) plotting for Lagergren model allowed the calculation of estimated equilibrium quantity qeq, rate constant K1 and regression coefficient R2 values for the five initial concentration in nitrite ion: 10, 20, 30, 40 and 50 mg·L-1. In Figure 8, the above-mentioned dependency is presented for NCHP, ACHP and CHBC and the highest concentration of nitrite ions in solution – 50 mg·L-1.
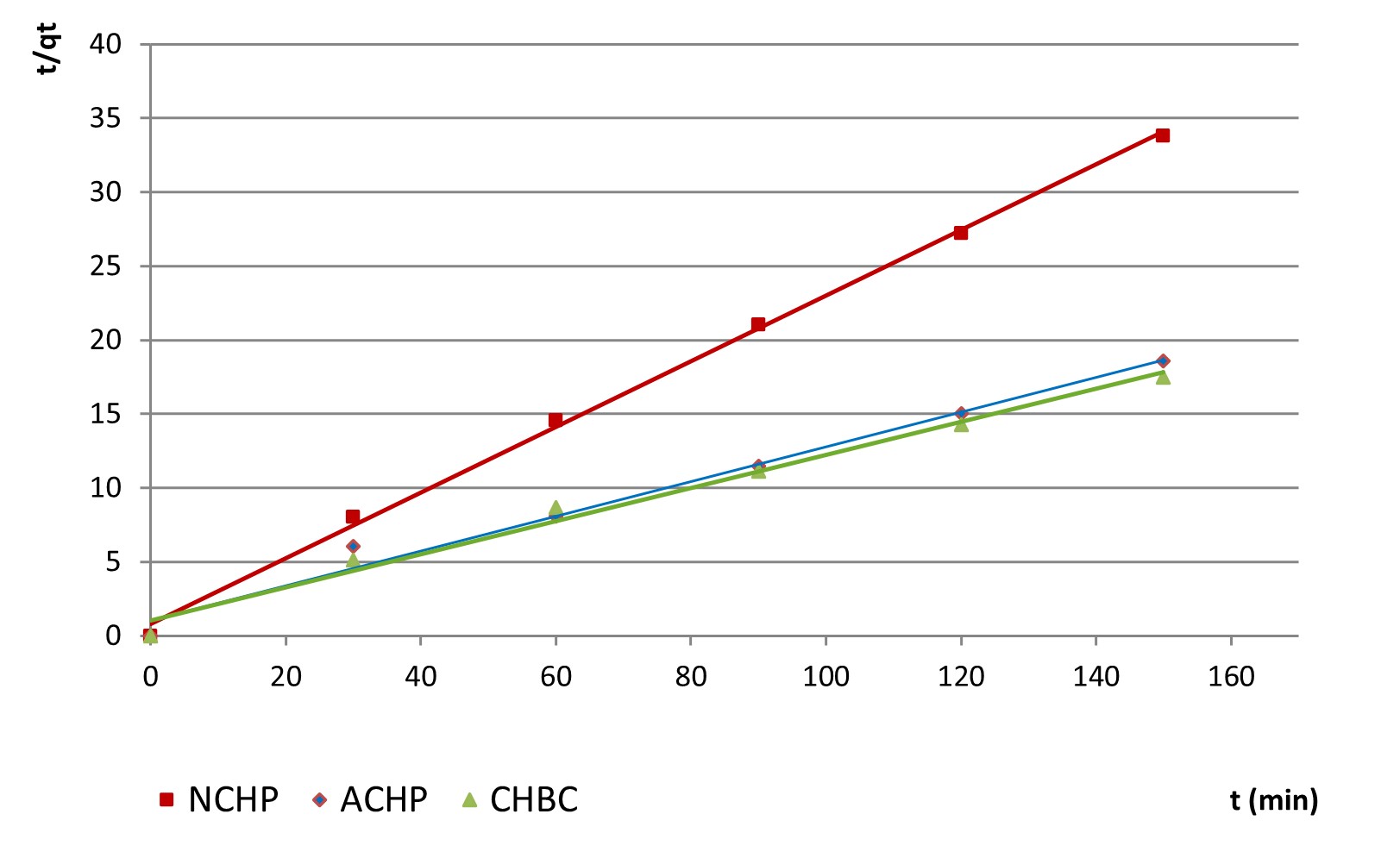
Pseudo second order Ho & McKay model, characterising the adsorption capacity of the solid surfaces, is expressed in a linear form by Equation 7:
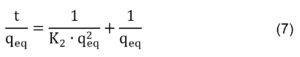
where K2 is the rate constant of Ho & McKay model (mg g-1 min-1) (Hanif et al., 2017).
For this model applied to the adsorption data, t (min) versus t/qt plotting allowed the calculation of the specific parameters: estimated equilibrium quantity qeq, rate constant K2 and regression coefficient R2.
Also for the highest considered concentration in nitrite ions, the variations for the three tested adsorbents are exemplified in Figure 9.
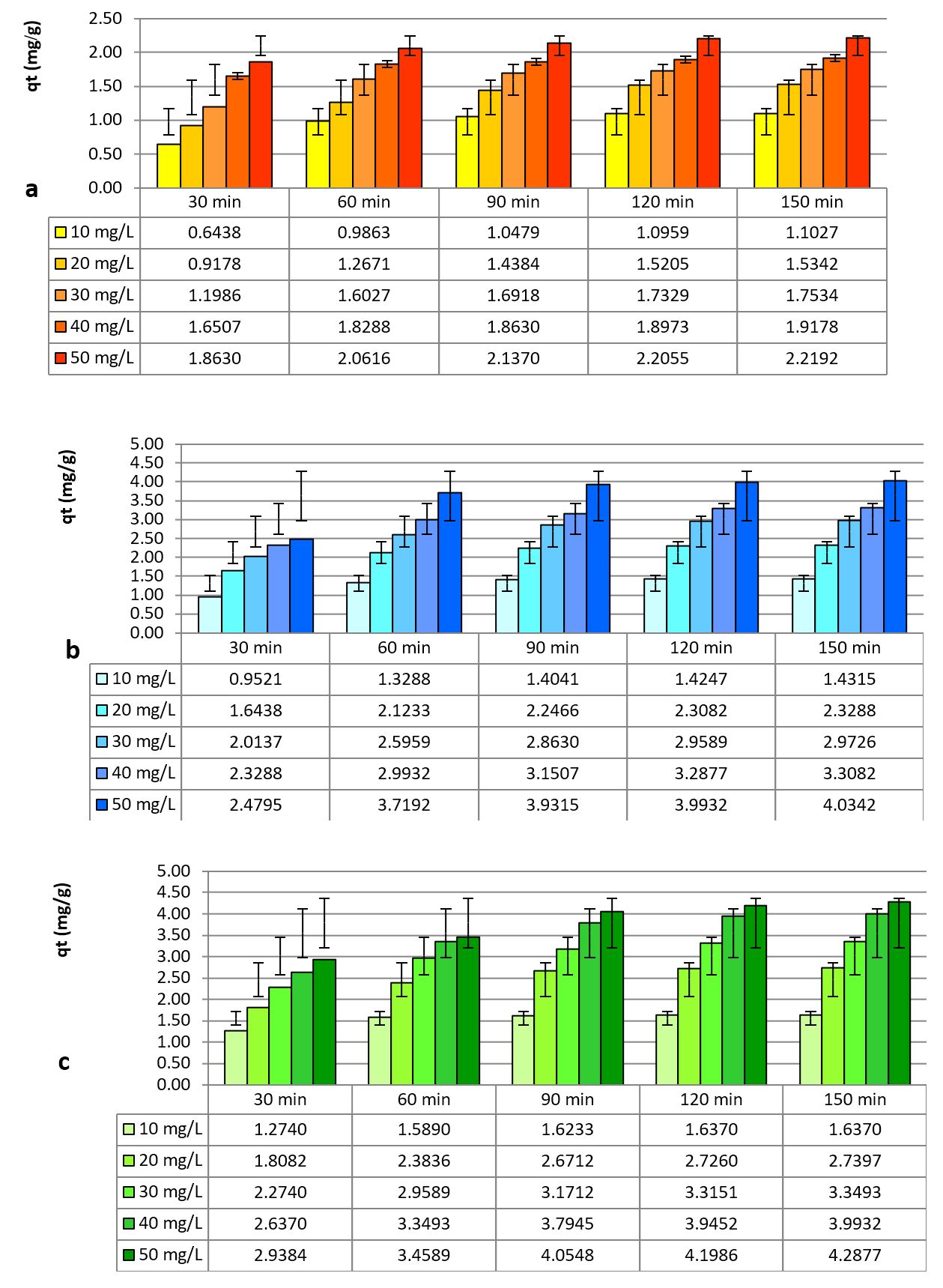
Considering the regression coefficients for both models, the pseudo-second order model shows a better description of the adsorption process, with smaller differences between the estimated retained quantity (qe) and the actually retained one (qt), as seen in Table 3. In what regards the efficiency of nitrite removal from the initial solutions, data inserted in Figures 10 (a, b and c) presents the mg of ions adsorbed per g adsorbent, for the considered materials.
The ion desorption process on the considered materials was carried out on the adsorbents obtained after filtration, considering the maximum period of 150 minutes, from each type of powder used, which were rinsed on the filter with distilled water, then treated with 20 mL HCl 3.7 g L-1 per 0.1 g adsorbent for 15 minutes under mild stirring.
The solution obtained after a new filtration led to the recovery of the following percentages of the initially retained amounts, calculated for 1 g of adsorbent: in the case of NCHP, for the retained ions from the five initial concentrations, 22.36, 28.57, 32.81, 38.93 and 42.90% were recovered; in the case of ACHP, the values were 12.92, 17.94, 20.51, 25.67 and 29.88% and in the case of CHBC, the percentages obtained were 10.88, 14.50, 18.40, 22.81 and 26.84%.
DISCUSSION
In general, two types of adsorption are described: physical and chemical. For the first type, the equilibrium state is established quickly, at low temperatures, the interactions between adsorbent and adsorbed being weak, van der Waals type, with low binding energies of 1-2 kcal mol-1). The second type, chemisorption, takes place through much stronger bonds, with higher bond energies, dependent on the chemical reaction taking place.
Table 3
Kinetic parameters extracted from the pseudo-first and pseudo-second models applied to the adsorption of NO2–ions on NCHP, ACHP and CHBC
|
Adsorbent |
Initial concentration (mg·L-1) |
qt (mg·g-1) |
Lagergren model |
Ho&McKay model |
||||
|
qeq (mg·g-1) |
K1 |
R2 |
qeq (mg·g-1) |
K2 |
R2 |
|||
|
NCHP |
10 |
2.2055 |
5.0789 |
-0.0004 |
0.8000 |
2.3535 |
0.0395 |
0.9800 |
|
20 |
3.0685 |
71.0796 |
-0.0137 |
0.6959 |
3.2669 |
0.0257 |
0.9803 |
|
|
30 |
3.5068 |
4.9062 |
0.0004 |
0.7434 |
3.6443 |
0.0384 |
0.9920 |
|
|
40 |
3.8356 |
5.3731 |
-0.0005 |
0.6703 |
3.8760 |
0.0933 |
0.9990 |
|
|
50 |
4.4384 |
4.5114 |
-0.0004 |
0.7433 |
4.5065 |
0.0612 |
0.9982 |
|
|
ACHP |
10 |
2.8630 |
3.7337 |
-0.0004 |
0.8550 |
2.9958 |
0.0456 |
0.9903 |
|
20 |
4.6575 |
5.5979 |
-0.0004 |
0.7397 |
4.8263 |
0.0312 |
0.9934 |
|
|
30 |
5.9452 |
7.1879 |
-0.0004 |
0.7542 |
6.2189 |
0.0197 |
0.9904 |
|
|
40 |
6.6164 |
7.6500 |
-0.0004 |
0.7098 |
6.8587 |
0.0210 |
0.9931 |
|
|
50 |
8.0685 |
7.6316 |
-0.0004 |
0.7044 |
8.5179 |
0.0130 |
0.9849 |
|
|
CHBC |
10 |
3.2740 |
4.3868 |
-0.0005 |
0.8812 |
3.3591 |
0.0772 |
0.9969 |
|
20 |
5.4795 |
7.6339 |
-0.0004 |
0.7552 |
5.7604 |
0.0201 |
0.9887 |
|
|
30 |
6.6986 |
5.8556 |
-0.0003 |
0.7203 |
6.9735 |
0.0179 |
0.9910 |
|
|
40 |
7.9863 |
6.3484 |
-0.0003 |
0.7035 |
8.3752 |
0.0126 |
0.9877 |
|
|
50 |
8.5753 |
8.8083 |
-0.0003 |
0.6160 |
8.9445 |
0.0119 |
0.9871 |
|
This type of adsorption can be activated (in this case a variation of the speed of the process with temperature is observed) or not activated (it takes place very quickly, with minimal energy requirements for the reactants) (Aksu, 2002). Adsorption in a single layer is usually found at low concentrations of the analyte, when the surface has enough active sites to retain it from the solution.
At higher concentrations, interactions between particles are frequent, which can explain the retention on the surface in multiple layers (Pan et al., 2021). The folding of the experimental data on the Freundlich model shows the tendency of the adsorption to take place in multiple layers, due to the heterogeneous surface of the adsorbent substrate (Karnib et al., 2014). Adsorption becomes favourable when 1/n has values between 0 and 1, and the surface is considered more heterogeneous the lower these values are. In the variation graphs 1/qeq versus 1/Ceq from the Freundlich model, the explanation of the fact that the line does not pass through the origin can be the transfer of the analyte to the surface with different speeds during the adsorption or the encounter of an initial resistance in accessing the active sites (Xia et al., 2019). In the case of the considered adsorbents, 1/n is between 0.6 and 0.3, which indicates the adequate development of the multilayer adsorption process of nitrites on the surface.
According to the parameters’ values presented in Table 2, the experimental data obtained in the process of NO2– ions adsorption on all three materials considered showed a better overlap with the Freundlich isotherm. This fact allows the observation that in the process of removing nitrite ions from the solution, chemisorption is favoured by the superficial functional groups in the surface structure of the adsorbents.
The percentages removed by the three materials, at pH 7.0 and a temperature of 20ºC, varied for NCHP between 55.14% at a concentration of 10 mg·L-1 and 22.19% at a concentration of 50 mg·L-1, for ACHP between 71.56% at the concentration of 10 mg·L-1 and 40.34% at the concentration of 50 mg·L-1 and for CHBC between 81.85% at the concentration of 10 mg·L-1 and 42.88% at the concentration of 50 mg·L-1.
Both Freundlich and Langmuir isotherms are usually applied in estimation of adsorption processes, and for vegetal materials as wheat straws, wood chips, cornhusks or corncobs, sugarcane (Diriba et al., 2014), Freundlich equation describes better the adsorption of nitrite and nitrate ions from aqueous solutions of different concentrations.
The biosorption of the nitrite ion is estimated to take place with the retention on the surface in the initial stage, through equilibrium processes, followed by a slow intra-particle migration, when the retention of the ions is favoured and the establishment of hydrogen bonds occur between the ions and the superficial functional groups in the adsorbents’ structure.
The relatively short time after which most of the analyte present in the solution is retained (90 minutes) attests to the evidence of strong attractions established with the surface of the materials.
Considering the previous observations presented in the literature (Ahmadpari et al., 2019; Asl et al., 2016; Ouakouak et al., 2013; Xu et al., 2013) regarding the influence of pH on the retention process of this ion, with minor variations in the range of 4-10, the neutral value was selected for the simplification of the procedure and a higher applicability with the decrease in the costs of reagents involved in the processing of adsorbents.
Both the retention on the surface and the migration of ions from the solution to the functional groups on the surface are involved in the adsorption of the considered analyte, and from the kinetic point of view, the obtained data allowed the calculation of the characteristic coefficients of the two applied models, Lagergren and Ho&McKay.
The linear dependencies applied for each individual kinetic model, for the adsorption of NO2– ions from aqueous solutions, allow the selection of the kinetic model that best fits the experimental data. For the Lagergren pseudo-first order model, the regression coefficients have small values, ranging between 0.6703 – 0.8 for NCHP, between 0.7044 – 0.8550 for ACHP and between 0.6160 – 0.8812 for CHBC; therefore, the Lagergren model does not appropriately describe the retention of ions on the surface, chemisorption being most probably involved as the predominant process instead of physical adsorption.
The Ho&McKay pseudo second-order model, which also showed a good applicability for describing the adsorption of nitrate and nitrite ions in the case of other adsorbents prepared from materials similar to cornhusks (Asl et al., 2016; Battas et al., 2019; Chabani et al., 2006; Hanafi et al., 2016; Ogata et al., 2018; Xu et al., 2013; Zhang et al., 2014) fitted better on experimental data, attesting to the fact that the retention of NO2– on the materials considered in this study occurs through activated adsorption on the active sites in the surface structure, much more intense initially and then with decreasing rates, once the active sites of the surface are occupied, confirming the nature of the interactions between dissolved ions and solid adsorbents.
The values of the regression coefficients calculated from the linear variation of the Ho&McKay model were between 0.980 – 0.9990 for NCHP, between 0.9849 – 0.9934 for ACHP and between 0.9871 – 0.9969 for CHBC.
Considering the wide availability of cornhusks, both in terms of the quantity produced globally and the very low costs of processing the raw material in order to obtain useful adsorbents, the present study offers an alternative to the use of this type of agricultural waste, in addition to composting and cellulose extraction.
As one of the quality parameters of natural waters, the concentration of NO2– ions must be controlled and kept within the limits accepted by the legislation, in order to avoid problems created as a result of environmental pollution. In this sense, low-cost adsorbents for the purification of industrial waters, those used for irrigation or even for population consumption can replace other more expensive products, being easily removed after use by incineration, if their recovery is not desired.
CONCLUSIONS
Three cornhusks-based powders were characterized and used for the removal of NO2– ions from aqueous solutions with concentrations varying between 10 and 50 mg·L-1, at neutral pH and room temperature, under gentle stirring at 150 rotations per minute. The amount of adsorbent used was 5 g·L-1 and the NO2– ions concentrations were determined from the solutions by classic spectrophotometric method.
The study of the isotherms described by the Langmuir and Freundlich equations for the adsorption processes allowed the evaluation of the ion retention mechanism on the considered adsorbents, and the experimental data matched the Freundlich isotherm better, with R2 correlations between 0.97 and 0.99 for all three adsorbents. Accordingly, the retention of ions was mainly achieved by chemisorption on the superficial functional groups of the surface, in detriment of physical adsorption. The estimated retained quantities (mg·g-1) determined from isotherms were 4.4783 (NCHP), 8.3542 (ACHP) and 8.7413 (CHBC) compared to the retained quantities from the normalized initial concentrations: 4.44 mg·g-1 (NCHP), 8.06 mg·g-1 (ACHP) and 8.58 mg·g-1 (CHBC).
The contact time of the solution with the adsorbent varied between 30 and 150 minutes, until the equilibrium was established, but the quantities retained after 90 minutes were much smaller; therefore, 90 minutes was the time interval for the adsorption, when most of the maximum adsorbed amount is retained (between 95 and 99%).
From a kinetic point of view, the adsorption process was analyzed by two models, pseudo first order Lagergren and pseudo second order Ho&McKay. The characteristic coefficients of the Ho&McKay model confirmed the development of the nitrite adsorption process on the surface predominantly by chemisorption, with R2 values between 0.98 and 0.999 for the adsorbents used; this model better describes the experimental results, which correlates with the observations of the studies cited in the literature regarding to the retention of nitrites and nitrates on natural or modified plant-based materials.
Also, the amounts estimated to be retained, calculated using this model, were closer to the amounts actually retained, being 2.25 mg·g-1 compared to 2.22 mg·g-1 (NCHP), 4.26 mg·g-1 compared to 4.03 mg·g-1 (ACHP) and 4.47 mg·g-1 compared to 4.29 mg·g-1 (CHBC). The nitrite uptake in the experiments reached a maximum of 2.2192 mg·g-1 on NCHP, 4.0342 mg·g-1 on ACHP and 4.2877 mg·g-1 on CHBC.
Considering that the parameters for the adsorption process are accessible, the quantities of adsorbents used are small and the preparation of the cornhusks based powders is easy, the current study shows that cornhusks, generally considered to be agricultural waste, can be valorised as adsorbent, not only as compost material or as source of cellulose; they can be considered as alternative to other adsorbents, more expensive or harder to obtain.
In the current context, when environmental pollution and the search for new methods and useful materials for soil, air and water remediation are general concerns, the studied powders NCHP, ACHP and CHBC can be used to remove nitrites from contaminated waters and will also be considered for adsorption for other toxic compounds in solutions.
Author Contributions: conceptualization (T.A.E., U.E.); methodology (T.A.E., U.E., L. C. T); analysis (T.A.E., U.E., M. I.); investigation (T.A.E., U.E., M. I.); resources (T.A.E., U.E., E. D. B., Ț. D. C.); data curation (T.A.E., M. I.); writing, review; supervision (T. A. E., E. D. B.). All authors declare that they have read and approved the publication of the manuscript in this present form.
Funding: There was no external funding for this study.
Conflicts of Interest: There are no conflicts of interest regarding this article.
REFERENCES
Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and disadvantages of bioplastics production from starch and lignocellulosic components. Polymers. 2021, 13, 2484. https://doi.org/10.3390/polym13152484.
Ahmadpari, H.; Noghany, M.E.; Ladez, B.R.; Mehrparvar, B.; Momeni, S. Kinetics modelling and Isotherms for adsorption of nitrate from aqueous solution by wheat straw. Tecnologia e Ambiente. 2019, 25, 203-214. https://doi.org/10.18616/ta.v25i0.5301.
Asl, M.K.; Hasani, A.H.; Naserkhaki, E. Evaluation of nitrate removal from water using activated carbon and clinoptilolite by adsorption method. Biosciences Biotechnology Research Asia. 2016, 13, 1045-1054. https://doi.org/10.13005/bbra/2131
Aksu, Z. Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of Nickel(II) ions onto Chlorella vulgaris. Process Biochemistry. 2002, 38, 1, 89-99. https://doi.org/10.1016/S0032-9592(02)00051-1.
Bai, L.; An, Y.; Wang, A.; Zhang, H.; Pam, X.; Ba, X. Kinetics of nitrites adsorption on AB2 type hyperbranched poly(aminoester) modified by tricholoctadecylsilan. Advanced Materials Research. 2012, 446-449, 537-541. https://doi.org/10.4028/www.scientific.net/AMR.446-449.537.
Battas, A.; El Gaidoumi, A.; Ksakas, A.; Kherbeche, A. Adsorption study for the removal of nitrate from water using local clay. The Scientific World Journal. 2019, 2019, 9529618. https://doi.org/10.1155/2019/9529618.
Bulgariu, L.; Bulgariu, D.; Macoveanu, M. Adsorptive performances of alkaline treated peat for heavy metal removal. Separation Science and Technology. 2011, 46, 1023-1033. https://doi.org/10.1080/01496395.2010.536192.
Cara, I.G.; Trincă, L.C.; Trofin, A.E.; Cazacu, A.; Țopa, D.C.; Peptu, C.A.; Jităreanu, G. Assessment of some straw-derived materials for reducing the leaching potential of Metribuzin residues in the soil. Applied Surface Science. 2015, 358, 586-594. https://doi.org/10.1016/j.apsusc.2015.08.141.
Chabani, M.; Amrane, A.; Bensmaili, A. Kinetic modelling of the adsorption of nitrates by ion exchange resin. Chemical Engineering Journal. 2006, 125, 111-117. https://doi.org/10.1016/j.cej.2006.08.014.
Dahliyanti, A.; Yunitama, D.A.; Rofiqoh, I.M.; Mustapha, M. Synthesis and characterization of silica xerogel from corn husk waste as cationic dyes adsorbent. F1000 Research. 2022, 11, 305. https://doi.org/10.12688/f1000research.75979.1.
De Carvalho Mendes, C.A.; Adnet, F.A.O.; Leite, M.C.A.M.; Furtado, C.; Furtado, A.M. Chemical, physical, mechanical, thermal and morphological characterization of corn husk residue. Cellulose Chemistry and Technology. 2015, 49,727-735.
Devi, S.; Poonia, P.K.; Kumar, V.; Tiwari, A.; Meena, R.K.; Kumar, U.; Gulnaz, A.; Al-Sadoon, M.K. Characterization of natural fiber extracted from corn (Zea mays L.) stalk waste for sustainable development. Sustainability. 2022, 14, 16605. https://doi.org/10.3390/su142416605.
Diriba, D.; Hussen, A.; Rao, V.M. Removal of nitrite from aqueous solution using sugarcane bagasse and wheat straw. Bulletin of Environmental Contamination and Toxicology. 2014, 93. https://doi.org/10.1007/s00128-014-1297-3.
Eperjessy D.B.; Trofin A.E.; Ungureanu, E.; Trincă L.C.; Sandu T. Observations on nitrite content of some fruits and vegetables during different storage conditions. Scientific Papers, Horticulture series. 2020, 63, 11-16.
EFSA (European Food Safety Authority). Nitrate in vegetables – Scientific Opinion of the Panel on Contaminants in the Food chain. EFSA Journal. 2008, 689, 1-7.
Fytianos, K.; Voudrias, E.; Kokkalis, E. Sorption-desorption behavior of 2,4-dichlorophenol by marine sediments. Chemosphere. 2000, 40, 3-6. https://doi.org/10.1016/s0045-6535(99)00214-3.
Gierak, A.; Łazarska, I. Adsorption of nitrate, nitrite and ammonium ions on carbon adsorbents. Adsorption Science & Technology. 2017, 35, 721-727. https://doi.org/10.1177/0263617417708085.
Hanafi, H.A.; Azeema, S.M.A. Removal of nitrate and nitrite anions from wastewater using activated carbon derived from rice straw. Journal of Environmental & Analytical Toxicology. 2016, 6, 346. https://doi.org/10.4172/2161-0525.1000346.
Hanif, M.A.; Tauqeer, H.M.; Aslam, N.; Hanif, A.; Yaseen, M.; Khera, R.A. Correct Interpretation of sorption mechanism by isothermal, kinetic and thermodynamic models. International Journal of Chemical and Biochemical Sciences. 2017, 12, 53-67.
Hashim, M.Y.; Amin, A.M.; Marwah, O.M.F.; Othman, M.H.; Yunus, M.R.M.; Chuan Huat, N. The effect of alkali treatment under various conditions on physical properties of kenaf fiber. Journal of Physics Conference Series. 2017, 914, 012030. https://doi.org/10.1088/1742-6596/914/1/012030.
Ho, Y.S.; Huang, C.T.; Huang, H.W. Equilibrium sorption isotherm for metal ions on tree fern. Process Biochemistry. 2002, 37, 1421-1430. https://doi.org/10.1016/S0032-9592(02)00036-5.
Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. The American Journal of Clinical Nutrition. 2009, 90. https://doi.org/10.3945/ajcn.2008.27131.
Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Extraction, chemical composition and characterization of potential lignocellulosic biomasses and polymers from corn plant parts. BioResources. 2019, 14, 6485-6500. https://doi.org/10.15376/biores.14.3.6485-6500.
Kalantary, R.R.; Dehghanifard, E.; Mohseni-Bandpi, A.; Rezael, L.; Esrafill, A.; Kakavandi, B.; Azari, A. Nitrate adsorption by synthetic activated carbon magnetic nanoparticles: kinetics, isotherms and thermodynamic studies. Desalination and Water Treatment. 2016, 57, 1-11. https://doi.org/10.1080/19443994.2015.1079251.
Karnib, M.; Kabbani, A.; Holail, H.M.; Olama, Z. Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia. 2014, 50, 113-120. https://doi.org/10.1016/j.egypro.2014.06.014.
Mohammed, A.A.B.A.; Hasan, Z.; Borhana Omran, A.A.; Kumar, V.V.; Elfaghi, A.M.; Ilyas, R.A.; Sapuan, S.M. Corn: its structure, polymer, fiber, composite, properties, and applications. Polymers. 2022, 14, 4396. https://doi.org/10.3390/polym14204396.
Moorcroft, M.J.; Davis, J.; Compton, R.G. Detection and determination of nitrate and nitrite: a review. Talanta. 2001, 54, 785-803. https://doi.org/10.1016/S0039-9140(01)00323-X.
Neşe, Ö.; Ennil, K.T. A kinetic study of nitrite adsorption onto sepiolite and powdered activated carbon. Desalination. 2008, 223, 174-179. https://doi.org/10.1016/j.desal.2007.01.209.
Nimibofa, A.; Newton Ebelegi, A.; Donbebe, W. Modelling and interpretation of adsorption isotherms. Journal of Chemistry. 2017, 3039817, 11. https://doi.org/10.1155/2017/3039817.
Nishant, K.; Kartick, K.S.; Basak, S.; Chattopadhyay, S.K.; Patil, P.G.; Deshmukh, R.R. Characterization of the corn husk fibre and improvement in its thermal stability by banana pseudostem sap. Cellulose. 2018, 25, 5241-5257. https://doi.org/10.1007/s10570-018-1931-z.
Ogata, F.; Noriaki Nagai, N.; Kariya, Y.; Nagahashi, E.; Kobayashi, Y.; Nakamura, T.; Kawasaki, N. Adsorption of nitrite and nitrate ions from an aqueous solution by Fe–Mg-type hydrotalcites at different molar ratios. Chemical and Pharmaceutical Bulletin. 2018, 66, 458-465. https://doi.org/10.1248/cpb.c17-01044.
Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petru, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers. 2021, 13, 231. https://doi.org/10.3390/polym13020231.
Ouakouak, A.K.; Youcef, L.; Achour, S. Removal of nitrates by adsorption on powdered activated carbon (in French). Courrier du Savoir. 2013, 17, 93-97.
Pan, J.; Zhou, L.; Chen, H.; Liu, X.; Hong, C.; Chen, D.; Pan, B. Mechanistically understanding adsorption of methyl orange, indigo carmine, and methylene blue onto ionic/nonionic polystyrene adsorbents. Journal of Hazardous Materials. 2021, 418, 126300. https://doi.org/10.1016/j.jhazmat.2021.126300.
Reinik, M.; Tamme, T.; Roasto, M. Naturally occurring nitrates and nitrites in foods. In Bioactive compounds in foods, Blackwell Publishing Ltd., Oxford, United Kingdom, 2009, 225-253.
Rusu (Nacu), G. Studies on the use of cellulosic waste in reducing environmental pollution (in Romanian), PhD Thesis, Technical University “Gheorghe Asachi” Iasi, Faculty of Chemical Engineering and Environmental Protection, 2015
Saleh, T.A. Trends in the sample preparation and analysis of nanomaterials as environmental contaminants. Trends in Environmental Analytical Chemistry. 2020, 28, e00101. https://doi.org/10.1016/j.teac.2020.e00101.
Saleh, T.A. Protocols for synthesis of nanomaterials, polymers, and green materials as adsorbents for water treatment technologies. Environmental Technology & Innovation. 2021, 24, 101821. https://doi.org/10.1016/j.eti.2021.101821.
Sari, N.H.; Wardana, I.N.G.; Irawan, Y.S.; Siswanto, E. Characterization of the chemical, physical, and mechanical properties of naoh-treated natural cellulosic fibers from corn husks. Journal of Natural Fibers. 2018, 15, 545-558. https://doi.org/10.1080/15440478.2017.1349707.
Sari, N.H.; Fajrin, J.; Suteja, S.; Fudholi, A. Characterisation of swellability and compressive and impact strength properties of corn husk fibre composites. Composites Communications. 2020a, 18, 49-54. https://doi.org/10.1016/j.coco.2020.01.009.
Sari, N.H.; Pruncu, C.I.; Sapuan, S.M.; Ilyas, R.A.; Catur, A.D.; Suteja, S.; Sutaryono, Y.A.; Pullen, G. The effect of water immersion and fibre content on properties of corn husk fibres reinforced thermoset polyester composite. Polymer Testing. 2020b, 91, 106751. https://doi.org/10.1016/j.polymertesting.2020.106751.
Sari, N.H.; Suteja, S.; Fudholi, A.; Zamzuriadi, A.; Sulistyowati, E.D.; Pandiatmi, P.; Sinarep, S.; Zainuri, A. Morphology and mechanical properties of coconut shell powder-filled untreated cornhusk fibre-unsaturated polyester composites. Polymer. 2021, 222, 123657. https://doi.org/10.1016/j.polymer.2021.123657.
Sari, N.H.; Suteja, S.; Fudholi, A.; Sutaryono, Y.A.; Maskur, M.; Srisuk, R.; Rangappa, S.M.; Siengchin, S. Evaluation of impact, thermo-physical properties, and morphology of cornhusk fiber-reinforced polyester composites. Polymer Composites. 2022, 43. https://doi.org/10.1002/pc.26573.
Tan, X.; Peng, Q.; Yang, K.; Yang, T.; Saskova, J.; Wiener, J.; Venkataraman, M.; Militky, J.; Xiong, W.; Xu, J. Preparation and characterization of corn husk nanocellulose coating on electrospun polyamide 6. Alexandria Engineering Journal. 2022, 61, 4529-4540. https://doi.org/10.1016/j.aej.2021.10.011.
Trofin, A.E.; Ungureanu, E.; Trincă, L.C.; Fortună, M.E.; Eperjessy, D.B. Potential valorisation of Protobind 1000 as adsorbent for Pb2+ and Zn2+. Journal of Applied Life Sciences and Environment. 2022, 55, 31-44. https://doi.org/10.46909/alse-551044.
Ungureanu, E.; Trofin, A.E.; Trincă, L.C; Ariton, A.M.; Ungureanu, O.C.; Fortuna, M.E.; Jităreanu C.D.; Popa, V.I. Studies on kinetics and adsorption equilibrium of lead and zinc ions from aqueous solutions on Sarkanda grass lignin. Cellulose Chemistry and Technology. 2021, 55, 939-948. https://doi.org/10.35812/CelluloseChemTechnol.2021.55.80.
Wang, Z. Griess Diazotization in Comprehensive Organic Name Reactions and Reagents. John Wiley & Sons. 2010. https://doi.org/10.1002/9780470638859.conrr280.
WHO. Water Sanitation and Health. https://www.who.int/teams/environment-climate-change-and-health/water-sanitation-and-health/chemical-hazards-in-drinking-water/nitrate-nitrite (accessed on 10 May 2023)
Wubneh, F.; Gideon, R.K.; Wu, D.; Km, B. Extraction and Characterization of Fibers from Corn Husk. Journal of Natural Fibers. 2022, 19, 12862-12869. https://doi.org/10.1080/15440478.2022.2077885.
Xia, Y.; Yang, T.; Zhu, N.; Li, D.; Chen, Z.; Lang, Q.; Liu, Z.; Jiao, W. Enhanced adsorption of Pb(II) onto modified hydrochar: Modelling and mechanism analysis. Bioresource Technology. 2019, 288, 121593. https://doi.org/10.1016/j.biortech.2019.121593.
Xu, X.; Gao, B.; Yue, Q.; Li, Q.; Wang, Y. Nitrate adsorption by multiple biomaterial based resins: Application of pilot-scale and lab-scale products. Chemical Engineering Journal. 2013, 234, 397-405. http://dx.doi.org/10.1016/j.cej.2013.08.117.
Yara. Global production (in Romanian). https://www.yara.ro/nutritia-plantelor/porumb/world-production/ (accessed on 10 May 2023).
Yilmaz, N.D.; Sulak, M.; Yilmaz, K.; Kalin, F. Physical and chemical properties of water-retted fibers extracted from different locations in corn husks. Journal of Natural Fibers. 2016, 13, 397-409. https://doi.org/10.1080/15440478.2015.1029201.
Zhang, Y.; Song, X-L.; Huang, S-T.; Geng, B-Y.; Chang, C-H.; Sung, I-Y. Adsorption of nitrate ions onto activated carbon prepared from rice husk by NaOH activation. Desalination and Water Treatment. 2014, 52, 4935-4941. https://doi.org/10.1080/19443994.2013.809984.
Academic Editor: Dr. Isabela Maria Simion
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Eperjessy Diana Beatrice, Motrescu Iuliana, Țopa Denis Constantin, Trincă Lucia Carmen, Trofin Alina Elena, Ungureanu Elena