Ionela Voloseniuc, Kálmán Imre, Liviu Miron
ABSTRACT. Dornelor Basin is characterised by numerous high quality water sources, which is proven by the fact that the main mineral waters on the Romanian market come from this area. The study aimed to provide data on the occurrence and human infective potential of Giardia and Cryptosporidium, as the most important water-borne parasites, from Bistrița river tributaries of Dornelor basin, North-Eastern Romania. Water samples were collected from 10 tributaries of the Bistrita river, from the level of sampling stations set upstream and downstream from the anthropic communities. The harvested water samples were further processed using non-molecular methods in order to isolate (oo)cysts. Subsequently, the isolated Cryptosporidium and Giardia (oo)ccyst were molecularly characterized through PCR and genomic sequencing, which led to the identification of Giardia in order to identify them at species level. The outcomes revealed the fact that the waters of the emissaries under study have a low parasite load and that, upstream from the human settlements, the water is highly pure when related to the protozoa under study. The increased load of Giardia spp. and Cryptosporidium spp. corresponded to important animal husbandry activity. The obtained results underline a potential public health risk.
Keywords: Giardia spp. Cryptosporidium spp., parasite load, surface water.
Cite
ALSE and ACS Style
Voloseniuc. I.; Imre, K.; Miron, L. Characterisation of the parasite load of river Bistrita tributaries, in Dornelor Basin, Romania. Journal of Applied Life Sciences and Environment 2021, 54(4), 450-457.
https://doi.org/10.46909/journalalse-2021-039
AMA Style
Voloseniuc I, Imre K, Miron L. Characterisation of the parasite load of river Bistrita tributaries, in Dornelor Basin, Romania. Journal of Applied Life Sciences and Environment. 2021; 54(4): 450-457.
https://doi.org/10.46909/journalalse-2021-039
Chicago/Turabian Style
Voloseniuc, Ionela, Kálmán Imre, and Liviu Miron. 2021. “Characterisation of the parasite load of river Bistrita tributaries, in Dornelor Basin, Romania” Journal of Applied Life Sciences and Environment 54, no. 4: 450-457.
https://doi.org/10.46909/journalalse-2021-039
View full article (HTML)
Characterisation of the Parasite Load of River Bistrita Tributaries, in Dornelor Basin, Romania
Ionela Voloseniuc1, Kálmán Imre2, Liviu Miron3,*
1VALVIS S.A. – V. Dornei, Romania
2Banat University of Agricultural Sciences and Veterinary Medicine in Timisoara, Romania
3Iasi University of Life Sciences, Romania
*E-mail: livmiron@yahoo.com
Received: June 10, 2022. Revised: July 22, 2022. Accepted: Aug. 3, 2022. Published online: Aug. 17, 2022
ABSTRACT. Dornelor Basin is characterised by numerous high quality water sources, which is proven by the fact that the main mineral waters on the Romanian market come from this area. The study aimed to provide data on the occurrence and human infective potential of Giardia and Cryptosporidium, as the most important water-borne parasites, from Bistrița river tributaries of Dornelor basin, North-Eastern Romania. Water samples were collected from 10 tributaries of the Bistrita river, from the level of sampling stations set upstream and downstream from the anthropic communities. The harvested water samples were further processed using non-molecular methods in order to isolate (oo)cysts. Subsequently, the isolated Cryptosporidium and Giardia (oo)ccyst were molecularly characterized through PCR and genomic sequencing, which led to the identification of Giardia in order to identify them at species level. The outcomes revealed the fact that the waters of the emissaries under study have a low parasite load and that, upstream from the human settlements, the water is highly pure when related to the protozoa under study. The increased load of Giardia spp. and Cryptosporidium spp. corresponded to important animal husbandry activity. The obtained results underline a potential public health risk.
Keywords: Giardia spp. Cryptosporidium spp., parasite load, surface water.
INTRODUCTION
The importance of water for the existence of ecosystems no longer needs to be argued. Pollution of this vital resource can cause unwanted effects on living organisms (Abel, 2014; Mahmud et al., 2017).
Economic development in recent years has put pressure on water quality in many countries around the world. Therefore, the specialists in the field are concerned with estimating and mitigating the environmental risks produced by the wastewater discharged into the components of the hydrographic network (Mahmoudi et al., 2015). The contamination of natural surface water bodies with pathogens, including Cryptosporidium spp. and Giardia spp., occurs especially through the introduction of human wastewater and livestock or wildlife origin feces (Baldursson and Karanis, 2011).
The extension and development of the mountain area of Dornelor Depression imposed the protection and preservation of certain habitats declared protected natural reservations, as well as of certain fauna and flora species of high ecological, scientific, landscaping and cultural importance, of national and international interest (Cojoc et al., 2016).
The area is characterized by high hydrologic potential, both as concerns surface waters (rivers Dorna, Bstrița and their tributaries) and underground waters. River Bistrița is 288 km long, having the longest mountain track among the Romanian rivers. River Dorna and River Neagra spring from Călimani Mountains, the former being 53 km long and the most important tributary of River Bistrița, and the latter being 34 km long.
The agricultural surface of the depression is 26.28% of the total surface. Ploughland occupies only 4.03% of the total surface and the rest is covered with pastures and meadows, thus supporting the animal husbandry sector, especially cattle breeding – 70.94%, followed by the breeding of sheep, horses, goats and other species. The valorisation of dairy products is a well-known national brand LaDorna, website www.ladorna.ro.
Presently, in Romania monitoring the presence of Cryptosporidium and Giardia (oo)cysts in different surface water types (rivers, wastewaters, brooks, irrigation channels, lakes and ponds) is limited to only two studies achieved in the western part of Romania (Imre et al., 2017a,b)The obtained results in this studies pointed out a potential public health risk, and the drawn conclusions suggested the necessity of a continuous monitoring of this water-borne parasites, in different regions of the country, with an unknown epidemiological situation, which can greatly contribute to their successful control in the environment. However, there are no scientific reports available about the occurrence of water-borne outbreaks in Dornelor basin.
Taking into account these considerations, the present study was conducted in order to provide data on the occurrence of Giardia spp. and Cryptosporidium spp. (oo)cysts from 10 workstations: Bancu, Secu, Arinu and Călimănel brooks, located in Dornelor hydrographic basin as main tributaries of Bistrita river. Also, their human infective potential was evaluated using molecular techniques.
MATERIALS AND METHODS
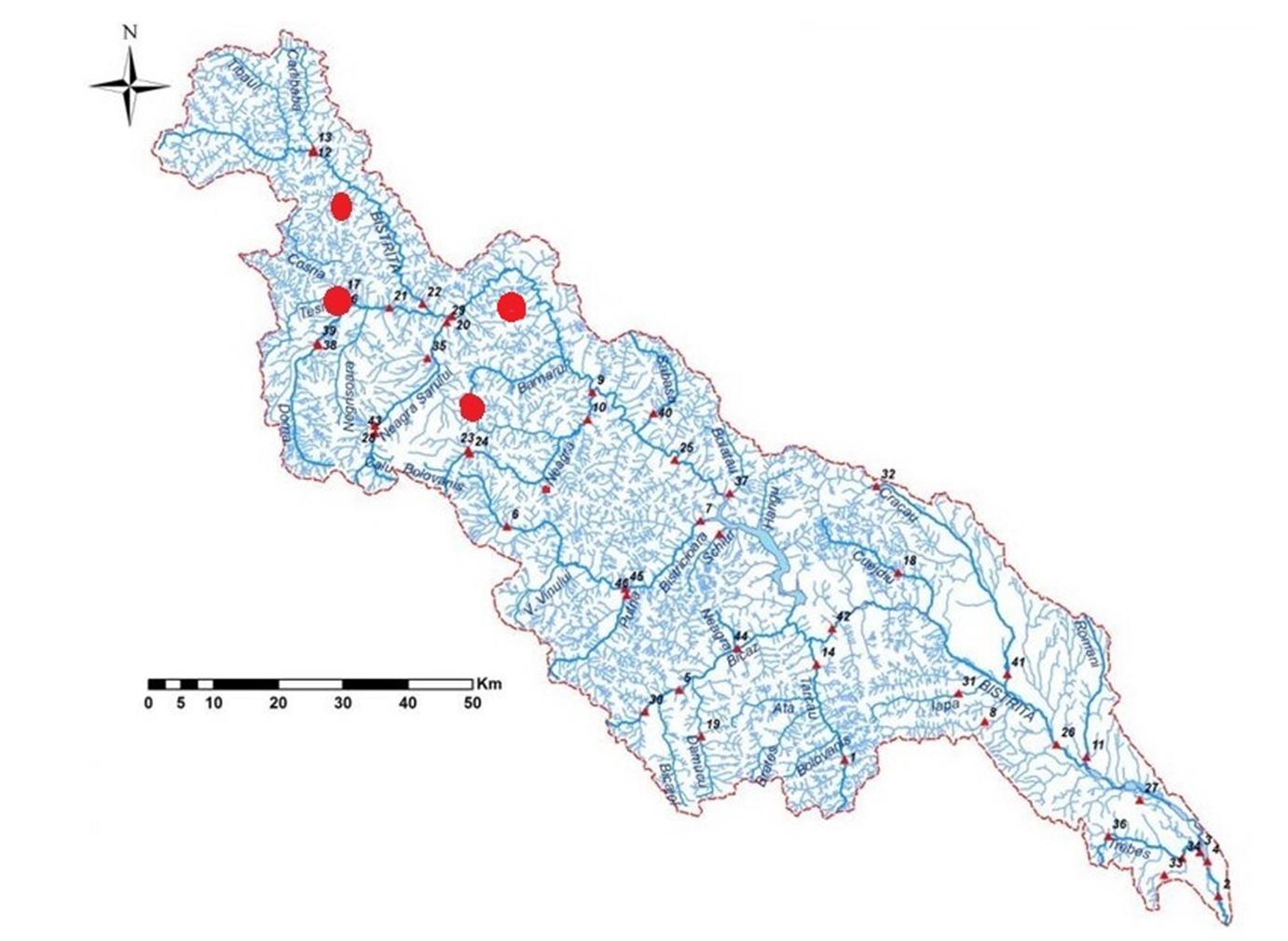
Samples were collected from four brooks – Bancu, Secu, Arinu and Călimănel, from ten stations set upstream and downstream from the anthropic areas with potential pollution due to the private animal farms (Fig. 1), as follows:
- Two sampling stations were set on Brook Bancu, of which one in the village close to the outfall into river Teșna and the other ~ 10 km upstream, in an area with low influence from the anthropic factor.
- Two sampling stations were set on Brook Secu, of which one in the village, close to the outfall into River Dorna and the other ~ 5 km upstream, in an area slightly affected by the anthropic factor.
- Two sampling stations were set on Brook Arinul, of which one in the village, at the outfall into River Bistrița, and the other ~ 7 km upstream, in an area where waters are very little affected by human pollution, due to the lack of the anthropic factor.
- Four sampling stations were set as follows: one in Coverca village and the other three ~ 3 / 5 / 7 km upstream, from three feeding brooks, in areas with different degrees of anthropic influence, on Brook Călimănel, which is a 12 km long, right-bank tributary of River Neagra.
The raw water samples were collected through microfiber filtration. Filters, having a pore size of 2 μm were used and the filtered water volume varied from 2.0 to 50.0 liter, depending on water turbidity. Next, the samples concentration was carried out in accordance with the description of Plutzer et al., (2010). The water concentrate resulted from the filtration and centrifuging of the samples, ~ 8 ml, was processed according to the method described and used by the U.S. Environmental Agency (USEPA method 1623). The oocysts of Cryptosporidium spp. and Giardia spp. were isolated by immunomagnetic separation (IMS), using the GC-DynabeadsTM Combo (Thermo Fisher Scientific Baltics UAB, Vilnius, Lithuania) kit, in accordance with the producer’s guidelines. The final concentration (100 µl) was divided in half. The first half was used to identify and count the Cryptosporidium spp. and Giardia spp. (oo)cysts based on immunofluorescence microscopy (IFA), (Motic-031, Motic Corporation LTD., Wetzlar, Germany), after colouring the mononuclear antibodies (Merifluor®, Cryptosporidium/ Giardia, Meridian Bioscience, Inc., Cincinnati, USA) with a fluorescent marker (FITC), in accordance with the producer’s guidelines.
The isolation and identification of the Cryptosporidium spp. (oo)cysts and the Giardia spp. cysts involved the following stages: collecting the water sample, filtering the water sample, carefully washing the laboratory filter, consecutively centrifugation of the concentrate, immunomagnetic separation of (oo)cysts, colouring by the immunofluorescence technique, examining using the fluorescence microscope, quantifying the presence or absence of (oo)cysts, carrying out a molecular characterisation of species as well as a genomic sequencing for the samples with a positive result (Mahmoudi et al., 2015). The used specific primer sets and cycling parameters in case of Crysptosporidium spp. were adopted as have been previously described by Xiao and Ryan (2008), targeting the small subunit (SSU) ribosomal RNA (rRNA) (18S) gene of the parasite and followed by restriction fragment length polymorphism (RFLP) analyses of the amplicons with the VspI (Promega, Madison, WI, USA) and SspI (Promega, Madison, WI, USA) digestion enzymes. In case of Giardia duodenalis the glutamate dehydrogenase (gdh) gene (~432 bp) was amplified and in case of positive results the amplicons were digested with the NlaIV restriction enzyme as has been previously indicated by Read et al., (2004). The results of PCR reactions were analyzed on 2.2% agarose gel, and aplicons indicated positive results were purified (Isolate II PCR and Gel Kit, Bioline®) and submitted for bidirectional sequencing (Macrogen Europe®, Amsterdam, the Netherlands).
RESULTS AND DISCUSSION
One hundred twenty water samples were collected from 10 sampling stations set at the beginning of the study, during year 2017, from May to October, as well as during the same period of year 2018. Between May and October 2017, 39 samples were found positive for Giardia spp., with 108 cysts visualized under the microscope, which means an average of 18 cysts / months. Among the 10 sampling stations, the highest load was detected in Călimănel downstream (Panaci), with a total of 25 cysts identified during the entire period in 2017, with an average of 4.17 cysts / month, followed by the Secu (downstream) station, with a total of 20 cysts visualized in 2017 and an average of 3.3 cysts / month.
By contrast, the lowest load was detected in Călimănel upstream (brook), where the total of visualized cysts was 2, with an average of 0.33 cysts / month, followed by the Bancu upstream station, with a total of 4 cysts identified and an average of 0.67 cysts / month. In both stations there were several months during which no cysts of Giardia spp. were identified. The stations Arinu downstream and Bancu downstream registered a steady parasite load of Giardia spp., considering that every month there were positive samples, but of low values.
Between May and October 2018, 23 samples were found positive for Giardia spp., with 31 cysts visualized under the microscope, which means an average of 5.1 cysts / month. Among the 10 sampling stations, the highest load was registered in Călimănel downstream (Panaci), with a total of 8 cysts identified during the entire period of 2018, with an average of 1.33 cysts / month, followed by the station Arinu (downstream), with a total of 4 cysts visualized in 2018 and an average of 0.66 cysts / month.
By contrast, the lowest load was also registered in Călimănel upstream (brook), where the total of visualized cysts was 1 cyst, with an average of 0.16 cysts / month, as well as in station Bancu upstream, with the same total of 1 cyst identified and an average of 0.16 cysts / month. In both stations there were several months during which no cysts of Giardia spp. were identified.
Specimens of the samples found positive by visualization under the microscope were sent to the Faculty of Veterinary Medicine in Timișoara for PCR genetic identification.
A total number of 27 (22.5%) samples from the 120 processed samples showed bands specific to the Giardia duodenalis species (previously known as Giardia intestinalis), measuring 432 bp (Fig. 2). The techniques of sequencing the 15 amplicons of Giardia duodenalis with the” forward” chain were successfully performed for 13 isolates. The genotypic groups identified were Giardia duodenalis assemblage E (9 out of 13 isolates – 69.2%), Giardia duodenalis assemblage A (3 out of 13 isolates – 23.1%), and Giardia duodenalis assemblage D (1 out of 13 isolates – 7.7%), respectively.
Between May and October 2017, 13 samples were found positive for Cryptosporidium spp., with 13 oocysts visualized under the microscope, which means an average of 2.166 oocysts / month.
Figure 2 – Image of the gel electrophoresis in the molecular diagnosis of the Giardia duodenalis species; M – molecular marker; P1-P3 – Work samples showing migration bands characteristic to 432 base pairs
The month with the highest load of Cryptosporidium spp. was August, with 4 oocysts visualized, and in October all samples were negative. The positive samples revealed only 1 oocyst each.
Between May and October 2018, 6 samples were found positive for Cryptosporidium spp., with 6 oocysts visualized under the microscope, which means an average of 1 oocyst / month.
The month with the highest load of Cryptosporidium spp. was June, with 2 oocysts visualized, and in August all samples were negative. The positive samples revealed only 1 oocyst each. The samples collected in the stations Arinu downstream, Arinu upstream, Bancu downstream, Călimănel downstream (in Buciniș), Călimănel upstream (brook) and Călimănel upstream were all negative during year 2018.
Overall, the recorded contamination level of Giardia cysts (range 0.01 – 0.27) and Cryptosporidium (range 0.01 – 0.07) oocysts (minimum maximum) in the present survey are mostly lower than those have been reported for water sources generating water-borne outbreaks in Norway ((0.5 cysts per l)) (Nygård et al., 2004) and USA (0.067 –0.132 oocysts per l) (Mac Kenzie et al., 1994).
Specimens of the samples found positive by visualization under the microscope were sent to the Faculty of Veterinary Medicine in Timișoara for PCR genetic identification.
Based on the SSU-rRNA gene test, in 6 (5%) out of a total of 120 processed samples, the ”nested PCR” method revealed that the secondary amplicons measured ~ 850 bp (Fig. 3). The size of these bands suggested that the genetically characterized organisms belonged to the Cryptosporidium genus. The restriction fragment length polymorphism (RFLP) analysis of the “nested PCR” final product by means of the SspI endonuclease revealed two separate bands in regions 444 and 247 bp (Fig. 3). This suggests a parasitism caused either by Cryptosporidium parvum or by Cryptosporidium hominis.
The differentiation between these two species was done by means of the VspI restriction enzyme, which highlighted bands in regions of 629 and 104 bp (Fig. 3). These sizes clearly indicated a parasitism caused by Cryptosporidium parvum.
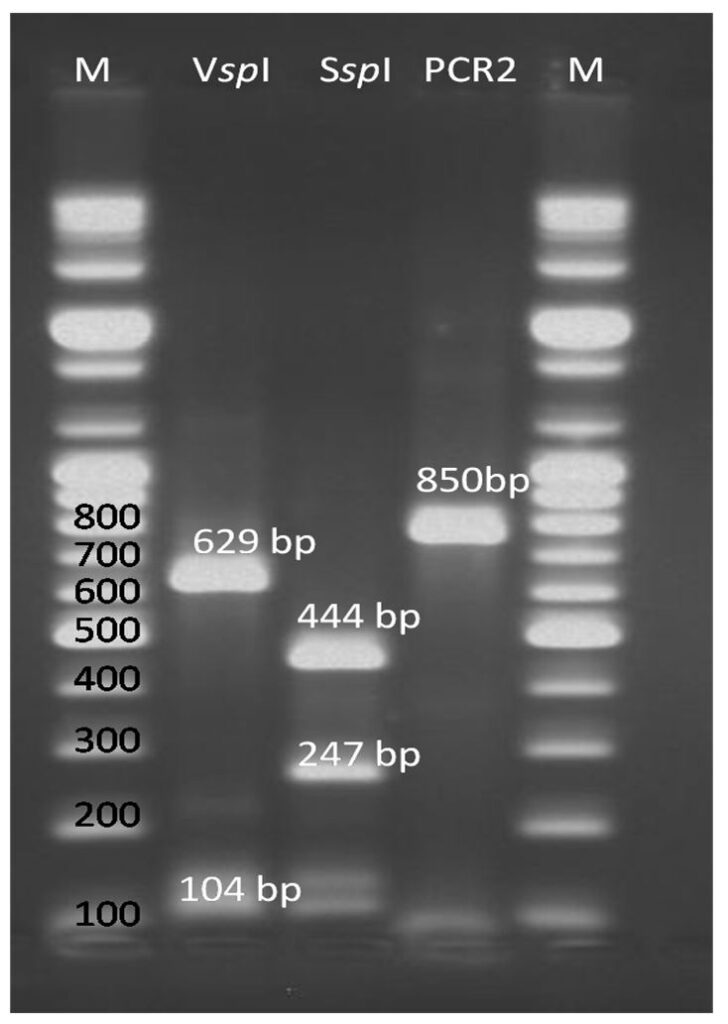
Figure 3 – Image of the gel electrophoresis in the molecular diagnosis of the Cryptosporidium parvum/hominis species, obtained following the amplification of the SSUrRNA gene; Columns 1 and 5 – molecular markers (every 100 bp); column 2 – digestion product resulted after the VspI enzyme action; column 3 – digestion product resulted after the SspI enzyme action; column 4 – amplicon of 850 base pairs specific to the molecular diagnosis of the Cryptosporidium genus
Similar to our results, the contamination of natural surface running water bodies with Giardia spp. and Cryptosporidium spp. with zoonotic potential have been previously demonstrated in Romania (Imre et al., 2017a,b), as well as in several other European countries such as the neighbouring Hungary (Plutzer et al., 2008) or Spain (Castro-Hermida et al., 2009), Portugal (Lobo et al., 2009) or Germany (Gallas-Lindemann et al., 2013).
The techniques of sequencing the 6 isolates of Cryptosporidium parvum were successfully performed and proved that four (66.6%) isolates belonged to the family subtype IIa, and two (33.3%) to subtype IId. Within these families, subtypes IIaA15G2R1 (n=3), IIaA17R1 (n=1), IIdA19G1 (n=1) and IIdA18G1 (n=1) were identified.
The recorded relatively low subtype diversity of the processed samples and their identity, is in agreement with the occurrence of these subtypes in livestock’s (cattle and sheep), as well us in surface waters reported in western region of the country (Imre et al., 2011; 2013; 2017a, b).
These results emphasize the impact of grazing livestock on natural surface bodies in the screened region. Further studies are recommended.
CONCLUSIONS
The results of the present study indicate the the screened surface water samples can harbor Giardia and Cryptosporidium (oo)cysts with zoonotic potential indicating a public health risk in the screened region.
The parasite load lower upstream than downstream proves the influence of human communities, and the exclusive identification of the Cryptosporidium parvum, a species with zoonotic potential, may be an indicator of the effect of the animal farms in the rural area, as well as of the wild animals on the mountain brooks. Also, the distribution of G. duodenalis assemblages suggest that human sewages as major pollution sources for the monitored water bodies.
REFERENCES
Abel, P.D. (2014). Water Pollution Biology (2nd ed.). CRC Press, https://doi.org/10.1201/9781482295368.
Baldursson, S., Karanis, P. (2011). Waterborne transmission of protozoan parasites: review of worldwide outbreaks—an update 2004–2010. Water Research, 45, 6603–6614, https://doi.org/10.1016/j.watres.2011.10.013.
Castro-Hermida, J.A., García-Presedo, I., Almeida, A., González-Warleta, M., Correia Da Costa, J.M., Mezo, M. (2009). Detection of Cryptosporidium spp. and Giardia duodenalis in surface water: a health risk for humans and animals. Water Research, 43, 4133-42, https://doi.org/10.1016/j.watres.2009.06.020.
Cojoc, G.M. (2016). Analysis of the hydrological regime of the Bistrita river in the context of hydrotechnical arrangements (in Romanian). Publishing House Terra Nostra, Iasi, ISBN: 978-606-623-061-2.
Dynabeads™ GC-Combo – Pub. no. MAN0014121 – Rev. B.0 (thermofisher.com).
Gallas-Lindemann, C., Sotiridou, I., Plutzer, J., Karanis, P. (2013). Prevalence and distribution of Cryptosporidium and Giardia in wastewater and the surface, drinking and ground waters in the Lower Rhine, Germany. Epidemiology and Infection, 141, 9–21, https://doi.org/10.1017/S0950268812002026.
Imre, K., Sala, C., Morar, A., Ilie, M.S., Plutzer, J., Imre, M., et al. (2017a). Giardia duodenalis and Cryptosporidium spp. as contaminant protozoa of the main rivers of western Romania: genetic characterization and public health potential of the isolates. Environmental Science and Pollution Research International, 24, 18672–9, https://doi.org/10.1007/s11356-017-9543-y.
Imre, K., Morar, A., Ilie, M.S., Plutzer, J., Imre, M., Emil, T., et al. (2017b). Survey of the occurrence and human infective potential of Giardia duodenalis and Cryptosporidium spp. in wastewater and different surface water sources of western Romania. Vector Borne Zoonotic Disease, 17, 685-91, https://doi.org/10.1089/vbz.2017.2155
Imre, K., Luca, C., Costache, M., Sala, C., Morar, A., Morariu, S., Ilie, M.S., Imre, M., Dărăbuş, G. (2013). Zoonotic Cryptosporidium parvum in Romanian newborn lambs (Ovis aries). Veterinary Parasitology, 191, 119–122, https://doi.org/10.1016/j.vetpar.2012.08.020.
Imre, K., Lobo, L.M., Matos, O., Popescu, C., Genchi, C., Dărăbuș, G. (2011). Molecular characterisation of Cryptosporidium isolates from preweaned calves in Romania: is there an actual risk of zoonotic infections? Veterinary Parasitology, 181, 321–324, https://doi.org/10.1016/j.vetpar.2011.04.042.
Lobo, M.L., Xiao, L., Antunes, F., Matos, O. (2009). Occurrence of Cryptosporidium and Giardia genotypes and subtypes in raw and treated water in Portugal. Letters in Applied Microbiology, 48, 732–737, https://doi.org/10.1111/j.1472-765X.2009.02605.x.
Mahmoudi, M.R., Nazemalhosseini-Mojarad, E., Karanis, P. (2015). Genotyping of Giardia lamblia and Entamoeba spp from river waters in Iran. Parasitology Research, 114, 4565-4570, https://doi.org/10.1007/s00436-015-4702-x.
Mahmud, R., Lim Ai, L.Y., Amir, A. (2017). Medical Parasitology. Springer International Publishing, https://doi.org/10.1007/978-3-319-68795-7.
Mac Kenzie, W.R., Hoxie, N.J., Proctor, M.E., Gradus, M.S., Blair, K.A., Peterson, D.E., Kazmierczak, J.J., Addiss, D.G., Fox, K.R., Rose, J.B., Davis, J.P. (1994). A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. The New England journal of medicine, 331, 161–167, https://doi.org/10.1056/NEJM199407213310304.
Nygård, K., Schimmer, B., Søbstad, Ø., Tveit, I. (2004). Waterborne outbreak of giardiasis in Bergen, Norway. Euro Surveill, 8, 2583, https://doi.org/10.2807/esw.08.46.02583-en.
Plutzer, J., Karanis, P., Domokos, K., Törökné, A., Márialigeti, K. (2008). Detection and characterization of Giardia and Cryptosporidium in Hungarian raw, surface and sewage water samples by IFT, PCR and sequence analysis of the SSurRNA and GDH genes. International Journal of Hygiene and Environmental Health, 211, 524–533, https://doi.org/10.1016/j.ijheh.2008.04.004.
Read, C.M., Monis, P.T., Thompson, R.C.A. (2004). Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases, 4, 125–130, https://doi.org/10.1016/j.meegid.2004.02.001.
Xiao, L., Ryan, U.M. (2008), Molecular epidemiology. In: Fayer R, Xiao L (eds) Cryptosporidium and Cryptosporidiosis, second edn. CRC Press, Boca Raton, 119–172.
www.ladorna.ro.