Rooije R.H. Rumende, Eva L. Baideng, Fredine E.S. Rares, Laya M. Rares
ABSTRACT. The Percoll density gradient centrifugation (PDGC) method is frequently used in the sexing of spermatozoa. However, this method causes damage to the spermatozoa membranes, resulting in a decreased quality of spermatozoa. We analysed the impacts of phospholipid PC (phosphatidylcholine) and EGTA (ethylene glycol bis (β-aminoethyl ether) N,N,N’,N’-tetraacetic acid) Ca2+ free buffer on the quality of bovine spermatozoa after the PDGC process, using semen from Friesian Holstein (FH) bulls aged 5 – 8 years. The following variables were observed: spermatozoa motility, spermatozoa viability, spermatozoa membrane integrity, spermatozoa that have not experienced capacitation, spermatozoa that have experienced capacitation and spermatozoa that have undergone acrosomal reaction. The results showed that the administration of phospholipid PC + EGTA Ca2+ free buffer to spermatozoa, followed by the PDGC process, could maintain or further improve the values of all variables. In the PDGC process, phospholipid PC 10% + EGTA Ca2+ free buffer at 1 mM was most suitable.
Keywords: PDGC; PC phospholipids; EGTA; separation spermatozoa.
Cite
ALSE and ACS Style
Rumende, R.R.H.; Baideng, E.L.; Rares, F.E.S.; Rares, L.M. Analysis of spermatozoa quality using Percoll density gradient centrifugation through the administration of phospholipid + EGTA. Journal of Applied Life Sciences and Environment 2021, 54(3), 298-309.
https://doi.org/10.46909/journalalse-2021-026
AMA Style
Rumende RRH, Baideng EL, Rares FES, Rares LM. Analysis of spermatozoa quality using Percoll density gradient centrifugation through the administration of phospholipid + EGTA. Journal of Applied Life Sciences and Environment. 2021; 54(3): 298-309.
https://doi.org/10.46909/journalalse-2021-026
Chicago/Turabian Style
Rumende, Rooije R.H., Eva L. Baideng, Fredine E.S. Rares, and Laya M. Rares. 2021. “Analysis of spermatozoa quality using Percoll density gradient centrifugation through the administration of phospholipid + EGTA” Journal of Applied Life Sciences and Environment 54, no. 3: 298-309.
https://doi.org/10.46909/journalalse-2021-026
View full article (HTML)
Analysis of Spermatozoa Quality Using Percoll Density Gradient Centrifugation Through the Administration of Phospholipid + EGTA
Anastasia ȘTEFÎRȚĂ1,2, Ion BULHAC1, Lilia BRÎNZĂ1,3, Maria COCU1* and Vera ZUBAREVA1
Rooije R.H. Rumende1,*, Eva L. Baideng1, Fredine E.S. Rares2, Laya M. Rares3
1Department of Biology, Faculty of Mathematics and Natural Sciences, Sam Ratulangi University, Manado, Indonesia
2Section of Microbiology, Faculty of Medicine, Sam Ratulangi University, Manado, Indonesia
3Section of Ophthalmology, Faculty of Medicine, Sam Ratulangi University, Manado, Indonesia
*E-mail: rooije.rumende@gmail.com
Received: Oct. 27, 2021. Revised: Dec. 19, 2021. Accepted: Feb. 04, 2022. Published online: Feb. 15, 2022
ABSTRACT. The Percoll density gradient centrifugation (PDGC) method is frequently used in the sexing of spermatozoa. However, this method causes damage to the spermatozoa membranes, resulting in a decreased quality of spermatozoa. We analysed the impacts of phospholipid PC (phosphatidylcholine) and EGTA (ethylene glycol bis (β-aminoethyl ether) N,N,N’,N’-tetraacetic acid) Ca2+ free buffer on the quality of bovine spermatozoa after the PDGC process, using semen from Friesian Holstein (FH) bulls aged 5 – 8 years. The following variables were observed: spermatozoa motility, spermatozoa viability, spermatozoa membrane integrity, spermatozoa that have not experienced capacitation, spermatozoa that have experienced capacitation and spermatozoa that have undergone acrosomal reaction. The results showed that the administration of phospholipid PC + EGTA Ca2+ free buffer to spermatozoa, followed by the PDGC process, could maintain or further improve the values of all variables. In the PDGC process, phospholipid PC 10% + EGTA Ca2+ free buffer at 1 mM was most suitable.
Keywords: PDGC; PC phospholipids; EGTA; separation spermatozoa.
INTRODUCTION
Separation of the X and Y chromosomes of spermatozoa can be carried out using the Percoll density gradient centrifugation (PDGC) method. This approach is easy in its implementation and application and a valid and inexpensive method. However, results from several recent studies indicate that the PDGC method may cause damage to spermatozoa membranes, which can further decrease the quality of spermatozoa (Susilawati et al., 2017).
Damage to the spermatozoa membrane after the PDGC process is caused by three factors, namely loss of seminal plasma, the presence of chemical factors characterised by an increase in free radicals, as well as physical factors that occur due to friction/collision between spermatozoa. Of the three factors, the physical factor is the most important one. It is therefore assumed that once this is fixed, the other factors are also neutralised. To fix the physical factor, the integrity of the spermatozoa membrane, which is composed of lipids, proteins, carbohydrates and other substances, is crucial (Tucker and Jansen, 2002).
Although the PDGC method has been widely used and is proven to be efficient, there are factors that limit the success of separating X and Y spermatozoa, namely the occurrence of damage to the spermatozoa membrane due to mechanical forces during the centrifugation and washing process of spermatozoa, which decreases the quality of spermatozoa. Inappropriate speed and duration of centrifugation during spermatozoa separation will decrease the viability and motility of spermatozoa (Rasad et al., 2019). In addition, centrifugation can cause the generation of Reactive Oxygen Species (ROS), namely an increase in free radicals due to membrane damage, affecting the physiological processes of spermatozoa (Zanella et al., 2016). Rasad et al. (2019), who performed sexing using the PDGC method, found a decrease in motility by 39.87% for X spermatozoa and 34.73% for Y spermatozoa. This decrease occurred due to the influence of mechanical forces, the spermatozoa medium and the temperature during centrifugation. In addition, there was an increase in the percentage of abnormal spermatozoa due to collisions on the tube wall during the sexing process, resulting in damage to the morphology of spermatozoa.
To repair the damaged membrane structure, phospholipids have been administered based on the results of previous studies (Bowyer and Davies, 1979). Research has also been carried out on human spermatozoa by Djauhari (2009) through the addition of phospholipids in tris aminomethane diluent, which can maintain the quality of spermatozoa and increase the number of intact spermatozoa, inhibiting premature capacitation. In addition, Rumende et al. (2007) suggested that the addition of 10% PPC phospholipid (pure phosphatidyl choline 97.5%), in combination with the PDGC method, improved the quality of spermatozoa.
In bovine spermatozoa, damage to the structure of the spermatozoa membrane on the head, acrosomal hood or tail, caused by the PDGC process, affects the transport of calcium ions into and out of the spermatozoa membrane. An excessive increase in calcium ions in the cell causes the disruption of cell functions, resulting in a decreased quality of spermatozoa. Efforts to inhibit the entry of calcium ions into cells or to modulate the entry of calcium ions into cells, as well as attempts to conduct calcium chelation using various natural materials or more selective synthesis, have yielded promising results in the prevention and treatment of various diseases. For example, ethylene glycol bis (β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA) Ca2+-free buffer is a scavenger of extracellular and intracellular Ca2+ ions that selectively binds Ca2+ with the highest affinity level (150 nM at pH 7.2) (Ellis-Davies and Kaplan, 1994; Lemonnier et al., 2002). Another study has shown that 1 mM EGTA with free Ca2+ buffer in endothelial cell culture induced by various agonists, or administering 100 M H2O2, may cause Ca2+ depletion from endoplasmic reticulum depots and inhibit Ca2+ infusion from extracellular depots. The EGTA is a scavenger of Ca2+ ions that selectively binds Ca2+ with high affinity (Hu and Corda, 1988; Sadova and Klishin, 2000; Parekh, 2002).
The purpose of this study was to reveal the effect of phospholipid PC (phosphatidyl choline) and EGTA (ethylene glycol bis (β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid) Ca2+ free buffer on the quality of bovine spermatozoa after PDGC. The existence of X and Y chromosomes in the sexing results was not observed because this study was conducted only on the sexing process of spermatozoa and did not measure the separation of X and Y chromosomes. We looked at the impact of the sexing process on the quality of spermatozoa subjected to phospholipid + EGTA treatment. Based on previous studies, spermatozoa resulting from the sexing process generally show a decreased quality.
MATERIALS AND METHODS
This research was conducted at the Advanced Biology Laboratory, Department of Biology, Faculty of Math and Natural Sciences, Sam Ratulangi University, Manado, Indonesia. We used semen taken from 10 Friesian Holstein (FH) bulls aged 5 – 8 years and managed by the Sehati Farmers Group in Kakaskasen Dua Village, North Tomohon Sub-district, assisted by the Agriculture and Fisheries Service of Tomohon City. Semen was taken twice from each bull. The criteria for good-quality fresh semen were as follows: per-ejaculate volume ranging from 5 – 8 mL and concentrations between 700 and 2,000 million sperm per mL (Garner and Hafez, 2000; Berden and Fuquay, 1984). Also, semen with a motility percentage above 70% is more viable than that with lower motility (Susilawati et al., 2017). This has been supported by other studies: motility of at least 70% (Zenichiro et al., 2002), motility > 70% and pH level of 5.9 – 7.3 (Hafez, 2000); viability > 80% (Ax et al., 2000) and membrane integrity level of 72.90% (Check et al., 1992).
This research is a laboratory experimental study using phospholipid PC 10% + EGTA Ca2+ free buffer (0.5 mM, 1 mM, 2 mM), administered prior to the PDGC process. After the PDGC, the following variables were observed: spermatozoa motility, spermatozoa viability, spermatozoa membrane integrity, spermatozoa not capacitated, spermatozoa capacitated, spermatozoa subjected to acrosomal reaction. Statistical analysis was done using one-way classification analysis of variance (ANOVA) to determine which level had the best significance for each variable, followed by the LSD test to determine the significance between untreated spermatozoa and control spermatozoa after PDGC. Duncan’s multiple distance test was performed to determine the effect of each level on each variable. Data were analysed using the software package SPSS (Statistical Product and Service Solutions) version 22. Significance was set at 0.05 (p ≤ 0.05).
Percoll density gradient centrifugation method
Making a Percoll density gradient
The density gradients used were 1.036, 1.038, 1.043, 1.047, 1.052, 1.055, 1.057, 1.060, 1.065 and 1.070, obtained by diluting Percoll with Tris aminomethane phospholipid (Djati, 2005: 80 mL Tris aminomethane, 20 mL phospholipid PC) to the density levels of 20, 25, 30, 35, 40, 45, 50, 55, 60 and 65%, respectively. Subsequently, of each sample, an aliquot of 0.5 mL was taken and placed into a tube, arranged sequentially from the highest to the lowest density level.
Centrifugation
For this, 1 mL of the sample was placed into a tube containing a Percoll density gradient and centrifuged at 2,250 rpm (850 g) for 5 minutes. The obtained top layer was the seminal plasma, which was removed from the tube and second layer contained the Y spermatozoa. The third layer contained the X spermatozoa, which were taken out and placed into a tube containing 4% FBS in TCM 199, at a total volume of 3 mL, followed by centrifugation at 1,500 rpm (380 g) for 5 minutes. Subsequently, the supernatant was discarded, and a liquid containing the spermatozoa was obtained, of which 1 mL was taken. After treatment via PDGC for 30 minutes, the sample was stained for 15 minutes, followed by observing the quality of the spermatozoa.
Making coloring material
Eosin-Negrosin, as the staining agent in the viability test, was made by dissolving 1 g of eosin, 5 g of negrosin and 3 g of sodium citrate in 100 mL of distilled water.
Chlortetracycline (CTC) was made through the following steps: (1) 250 g of DABCO (Sigma, D-2522) was dissolved in 9 mL of glycerol and incubated in a waterbath at 37°C for 3 – 4 hours. After this, 1 mL of PBS was added, and the mixture was stored in a freezer; (2) 0.2422 g of Tris (Trisma base; Sigma T-1503) and 0.7592 g of NaCl were dissolved in 100 mL of deionised water and stored in a refrigerator; (3) 6.057 g of Tris was dissolved in 59 mL of deionised water and stored in a refrigerator; (4) 12.5 g of paraformaldehyde was dissolved in 50 mL of deionised water, followed by adding 1 M of NaOH until the colour became lighter; (5) the solution obtained from the fourth stage was mixed with 1 M tris buffer solution obtained from the third stage at a ratio of 1:1 (the pH was adjusted to 7.4 with 0.2 M HCl) and stored in a refrigerator; (6) 0.0044 g of CTC powder dye (Sigma, C-7880) was mixed with 0.22 g of L-cysteine (hydrochloric monohydrate); the mixture was mixed with 5 mL of CTC buffer from the second stage (pH was adjusted to 7.8 with 0.2 M HCl). Finally, three solutions were obtained, namely DABCO solution (stored in a freezer), CTC fixative solution (stored in a refrigerator) and CTC dye solution (stored in a refrigerator).
The hypoosmotic swelling test was performed by making a 150-m-osmos hypoosmotic solution that contained 7.35 g of sodium citrate 2H2O and 13.52 g of fructose, dissolved in 1,000 mL of distilled water. The phospholipid PPC (pure phosphatidyl choline 97.5%) was obtained from Viva.
Spermatozoa motility observation
Briefly, 10 µL of spermatozoa was dropped onto a concave glass object, covered with a glass cover measuring 22 × 22 mm and observed under a light microscope with 400x magnification using computer-assisted semen analysis (CASA). At least 200 motile spermatozoa were observed and divided into four groups: very good movement, good movement, non-progressive movement and not moving. The data obtained were used as a comparison in the observation of spermatozoa motility using CASA (Hafez, 2000).
Spermatozoa viability observations
The semen was dripped with 1 drop of eosin-negrosin solution, placed on a glass object, homogenised, smeared and dried. Observationswere made under a light microscope with a magnification level of 400x. As shown in Fig. 1, only living spermatozoa absorbed the colour (Hafez, 2000).
Capacitation observation
The method used to observe capacitation was based on Djati (2005), who modified the Fraser method as follows: (1) 100 µL of semen was placed in a 1.5-mL Eppendorf tube and mixed with 45 µL of CTC dye solution. Subsequently, the tube was covered with aluminium foil and vortexed for 1 minute; (2) 8 µL of CTC fixative was added into the Eppendorf tube, and the mixture was vortexed for 1 minute; (3) 10 µL of DABCO was added into the Eppendorf tube and mixed carefully; (4) the Eppendorf tube was then covered with a cover glass and thick tissue paper, which were pressed carefully. The edges of the cover glass were covered with nail polish to protect the solution from drying. Observations were made with an epifluorescence microscope (Nikon) with 400x magnification. The characteristics are shown in Fig. 2. Spermatozoa that had not experienced capacitation showed a light colour on the entire head, whereas spermatozoa that had experienced capacitation showed a light colour only on the upper half of the head. Spermatozoa subjected to acrosomal reaction showed either a light colour or a circular ring appearance at the central part of the head.
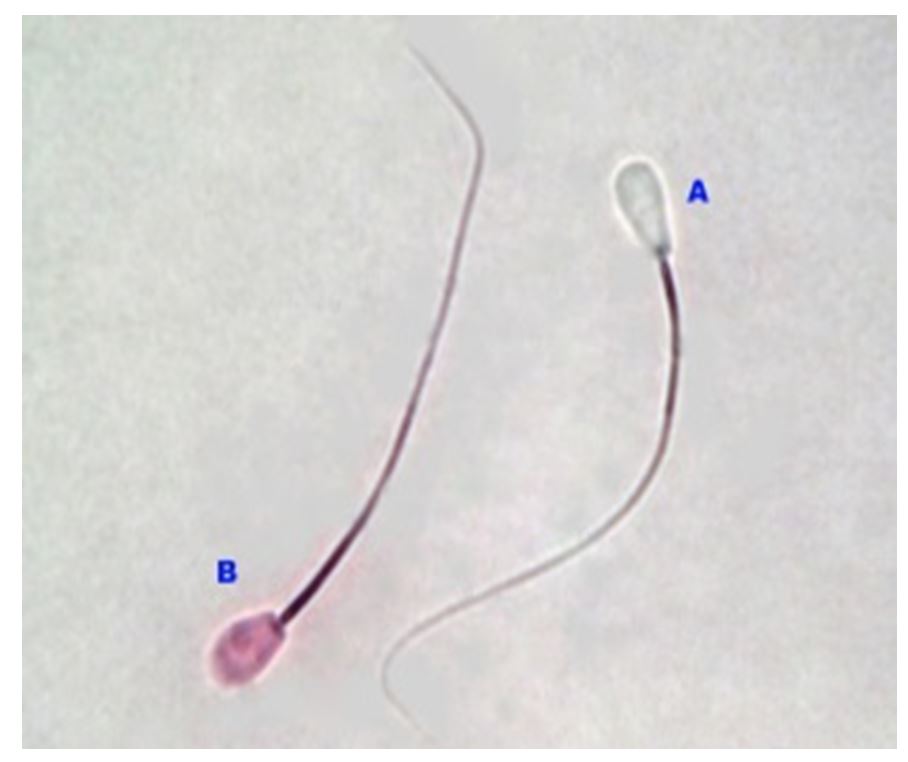
Notes: (A) Living spermatozoa (do not absorbe the dye); (B) Dead spermatozoa (absorbed the dye)
Figure 1 – Observation of spermatozoa viability with Eosin-Negrosin using a light microscope (OLYMPUS) with 1000X magnification
Observation of membrane integrity with hypoosmotic swelling test
For this, 1 mL of a 150-mL osmotic hypoosmotic solution was added to 0.1 mL of spermatozoa and the mixture was incubated at 370°C for 30 minutes. As shown in Fig. 3, when observed at 400x magnification, there was a characteristic membrane damage, namely swelling of the spermatozoa or a circular tail end (Correa et al., 1997).
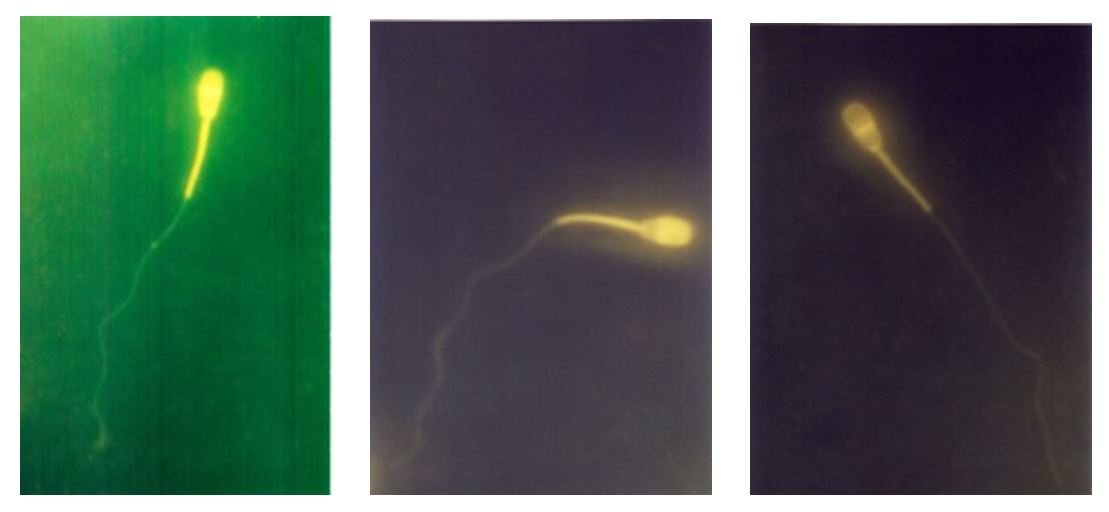
Notes: (A) Uncapacitated spermatozoa; (B) Capacitated spermatozoa; (C) Spermatozoa that have undergone acrosomal reaction
Figure 2 – Observation of the physiological conditions of spermatozoa after CTC staining using epifluorescence microscope (NIKON) with 400x magnification
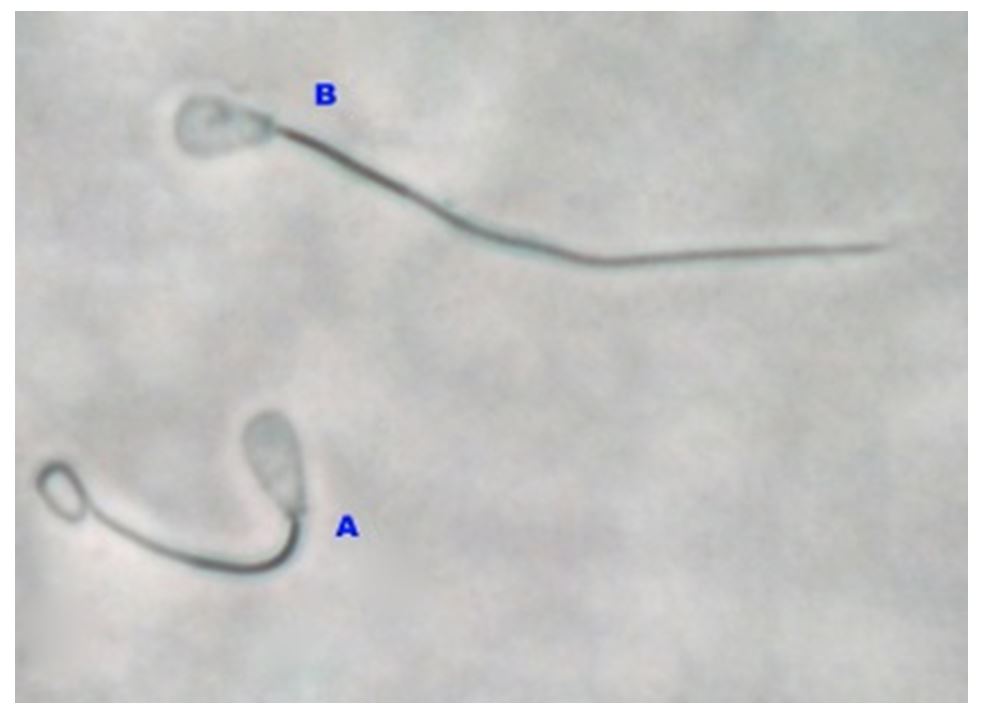
Notes: (A) The coiled spermatozoa are those with intact membrane integrity; (B) Spermatozoa without a coiled shape are those with damaged membrane integrity
Figure 3 – Observation of the spermatozoa membrane integrity using a light microscope (OLYMPUS)
RESULTS AND DISCUSSION
Fresh semen quality
The fresh semen samples that were taken from 10 Friesian Holstein (FH) bulls did not contain abnormal sperm, as shown in Table 1.
Based on the examination of the quality of fresh semen (Table 1), the semen was normal. The per-ejaculate volume ranged from 5 – 8 mL, with concentrations between 700 and 2,000 million sperm per mL (Garner and Hafez, 2000; Berden and Fuquay, 1984). According to the viability indicators, the semen may be used for further treatment.
Effect of Percoll density gradient centrifugation on cow spermatozoa quality
The results of the statistical analysis (Fig. 4) showed that the percentage of spermatozoa quality for all tested variables, using the PDGC method, decreased significantly (LSD test p≤ 0.001), when compared with the control (without PDGC).
In other words, the PDGC method significantly decreased the quality of spermatozoa in all variables studied.
Table 1
Results of quality inspection of fresh semen taken from 10 Friesian Holstein (FH) bulls before treatment with phospholipid 10%+EGTA Ca2+ free buffer (0.5 mM, 1 mM, 2 mM)
|
Parameter |
Mean ± SD |
|
Volume (ml) |
9.71 ± 0.95 |
|
pH |
6.30 ± 0.08 |
|
Concentration (106) |
1518.70 ± 303.25 |
|
Motility (%) |
75.00 ± 0.00 |
|
Viability (%) |
93.15 ± 2.78 |
|
Membrane integrity (%) |
74.99 ± 4.13 |
|
Uncapacitated spermatozoa (%) |
54.09 ± 4.61 |
|
Capacitated spermatozoa (%) |
31.39 ± 2.92 |
|
Acrosomal reaction spermatozoa (%) |
14.51 ± 4.71 |
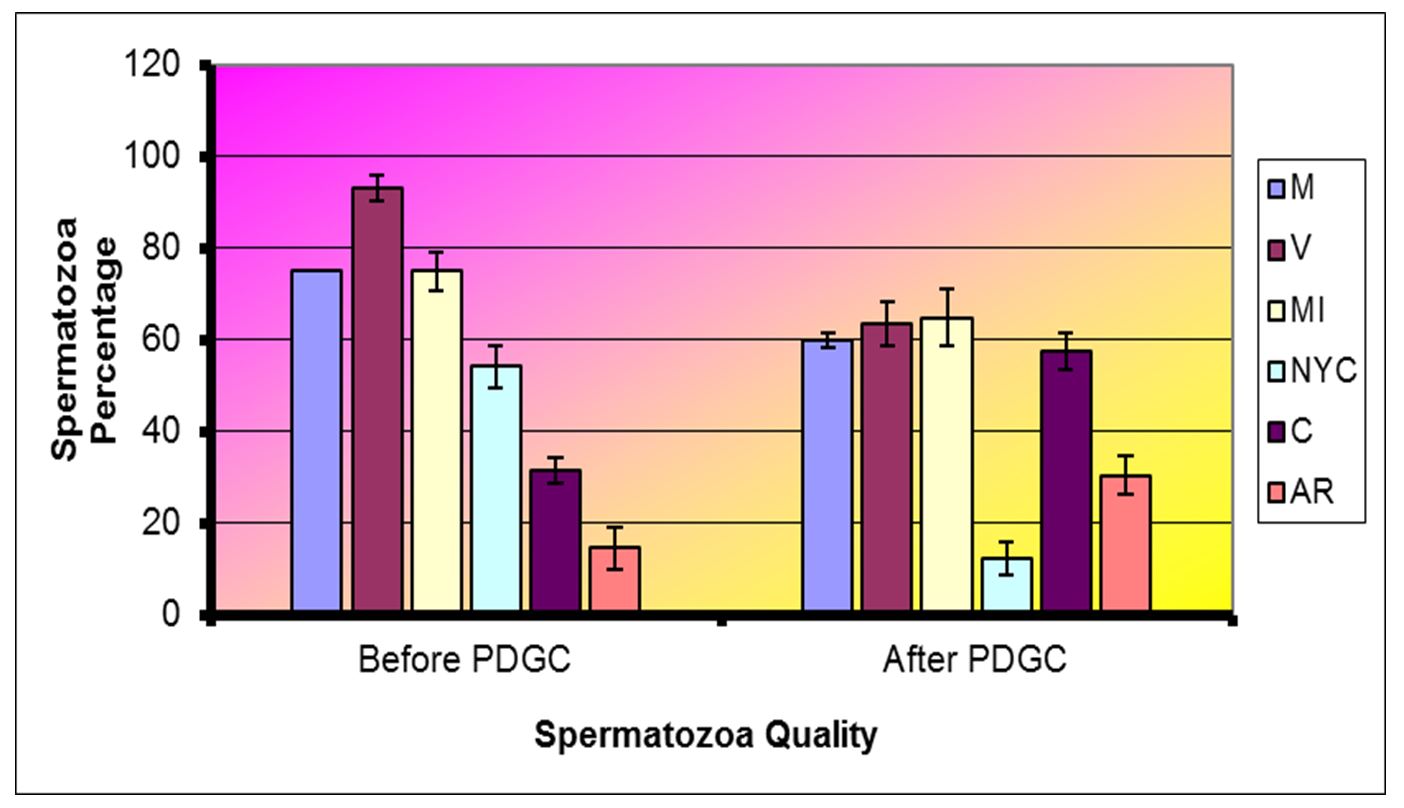
Notes: Motility (M), Viability (V), Membrane Integrity (MI), Not yet capacitated (NYC), Capacitated (C), Acrosomal reaction (AR)
Figure 4 – Percentage ± SD of all variables before PDGC treatment and after PDGC treatment (LSD test results, p≤ 0.01)
Effect of PC phospholipids 10% + EGTA Ca2+ free buffer (0.5 mM, 1 mM, 2 mM, respectively) on spermatozoa after PDGC
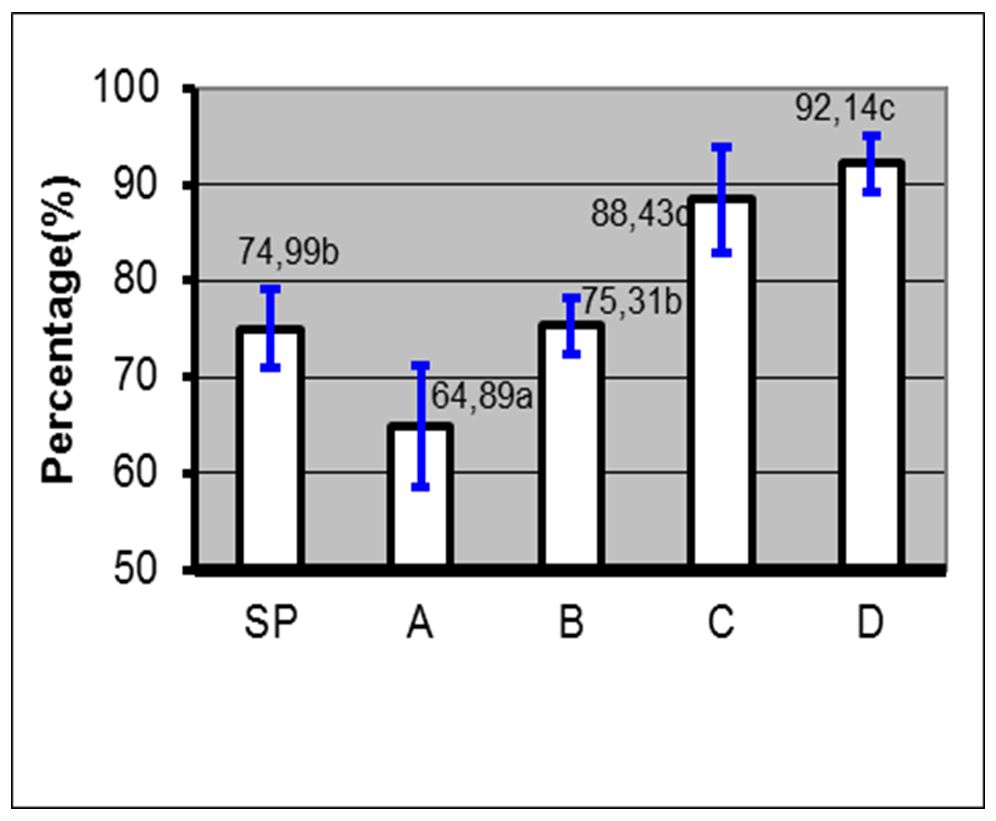
Based on the results, the administration of 10% PC phospholipid concentration + EGTA Ca2+ free buffer (0.5, 1 and 2 mM) in spermatozoa through the PDGC method, the spermatozoa quality in each variable spermatozoa motility (Fig. 5), spermatozoa viability (Fig. 6), spermatozoa membrane integrity (Fig. 7), spermatozoa that have not experienced capacitation (Fig. 8), spermatozoa that have experienced capacitation (Fig. 9) and spermatozoa that have undergone acrosomal reaction (Fig. 10) increased significantly (ANOVA result p≤ 0.001), when compared with the control variables. In addition, the administration of PC phospholipid 10% concentration + EGTA Ca2+ free buffer at 1 mM resulted in a more significant effect when compared to the other levels (0.5 or 2 mM).
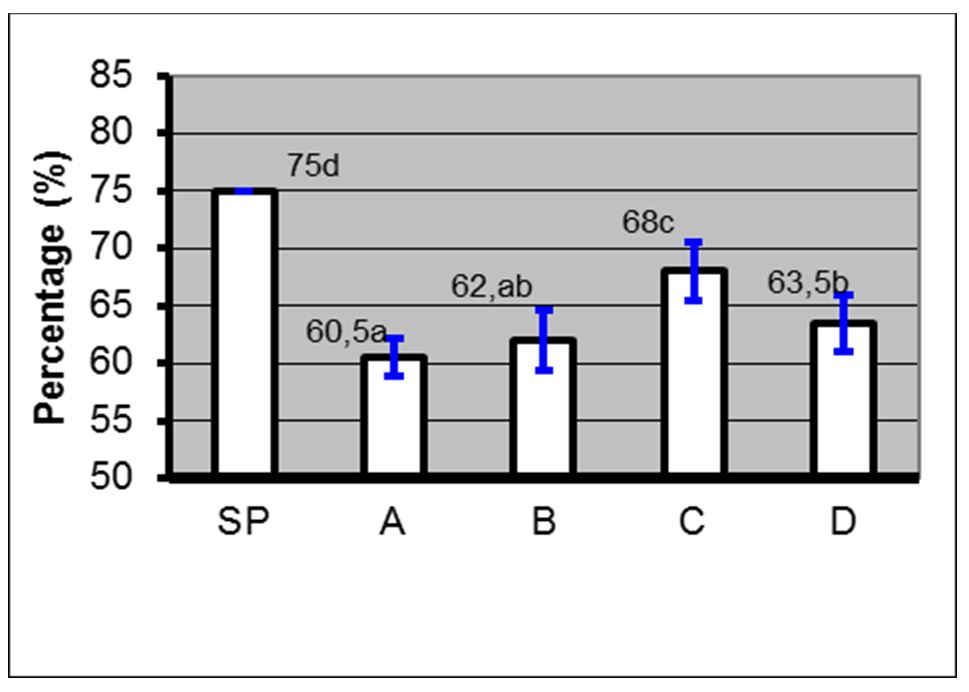
Notes: SP=Before treatment; A=Phospholipids 0% + EGTA Ca2+ free buffer (0 mM); B=Phospholipid 10%+ EGTA Ca2+ free buffer (0.5 mM); C=Phospholipid 10% + EGTA Ca2+ free buffer (1 mM); D=Phospholipids 10% + EGTA Ca2+ free buffer (2 mM); (a, b, c); Duncan’s multiple distance test results 5%
Figure 5 – Average percentage ± SD of motility before and after treatment with phospholipids + EGTA Ca2+ free buffer
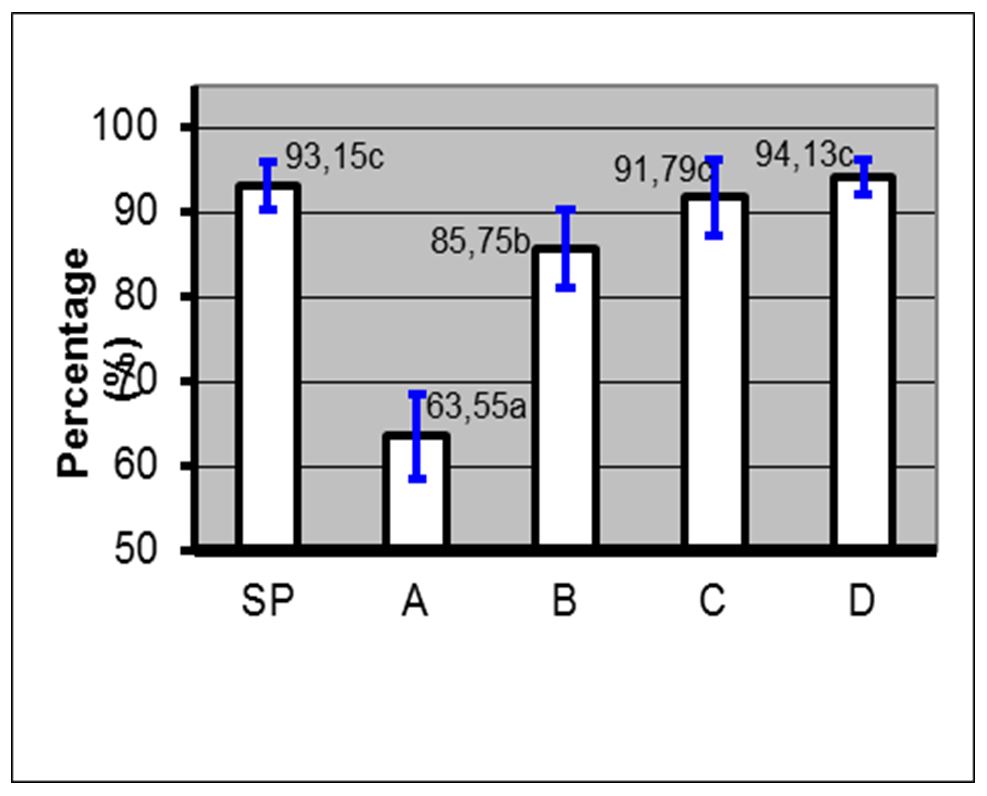
Notes: SP=Before treatment; A=Phospholipids 0% + EGTA Ca2+ free buffer (0 mM); B=Phospholipid 10%+ EGTA Ca2+ free buffer (0.5 mM); C=Phospholipid 10% + EGTA Ca2+ free buffer (1 mM); D=Phospholipids 10% + EGTA Ca2+ free buffer (2 mM); (a, b, c)=Duncan’s multiple distance test results 5%
Figure 6 – Average percentage ± SD viability before and after treatment with phospholipids + EGTA Ca2+ free buffer
Notes: SP=Before treatment; A=Phospholipids 0% + EGTA Ca2+ free buffer (0 mM); B=Phospholipid 10%+ EGTA Ca2+ free buffer (0.5 mM); C=Phospholipid 10% + EGTA Ca2+ free buffer (1 mM); D=Phospholipids 10% + EGTA Ca2+ free buffer (2 mM); (a, b, c)=Duncan’s multiple distance test results 5%
Figure 7 – Average percentage ± SD of membrane integrity before and after treatment with phospholipids + EGTA Ca2+ free buffer

Notes: SP=Before treatment; A=Phospholipids 0% + EGTA Ca2+ free buffer (0 mM); B=Phospholipid 10%+ EGTA Ca2+ free buffer (0.5 mM); C=Phospholipid 10% + EGTA Ca2+ free buffer (1 mM); D=Phospholipids 10% + EGTA Ca2+ free buffer (2 mM); (a, b, c)=Duncan’s multiple distance test results 5%
Figure 8 – Average percentage ± SD of uncapacitated spermatozoa before and after treatment with phospholipids + EGTA Ca2+ free buffer
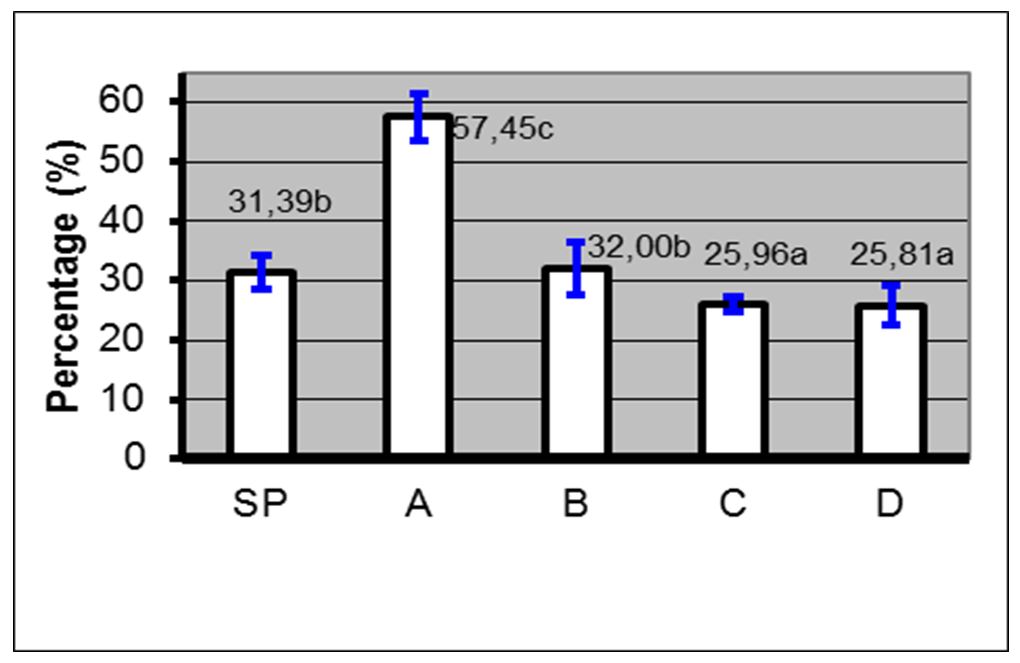
Notes: SP=Before treatment; A=Phospholipids 0% + EGTA Ca2+ free buffer (0 mM); B=Phospholipid 10%+ EGTA Ca2+ free buffer (0.5 mM); C=Phospholipid 10% + EGTA Ca2+ free buffer (1 mM); D=Phospholipids 10% + EGTA Ca2+ free buffer (2 mM); (a, b, c)=Duncan’s multiple distance test results 5%
Figure 9 – Average percentage ± SD of capacitated spermatozoa before and after treatment with phospholipids + EGTA Ca2+ free buffer
Based on the obtained results, the improvement of spermatozoa quality in all study variables after the administration of 10% PC phospholipid concentration + EGTA Ca2+ free buffer at either 0.5, 1 or 2 mM was caused by a significant positive effect of PC phospholipids on spermatozoa quality; the balance of the calcium ion concentration between inside and outside the cell was well maintained through the administration of EGTA Ca2+ free buffer. The EGTA Ca2+ free buffer functions as a scavenger of extracellular and intracellular Ca2+ ions and selectively binds Ca2+, with the highest affinity level of 150 nM at pH 7.2.
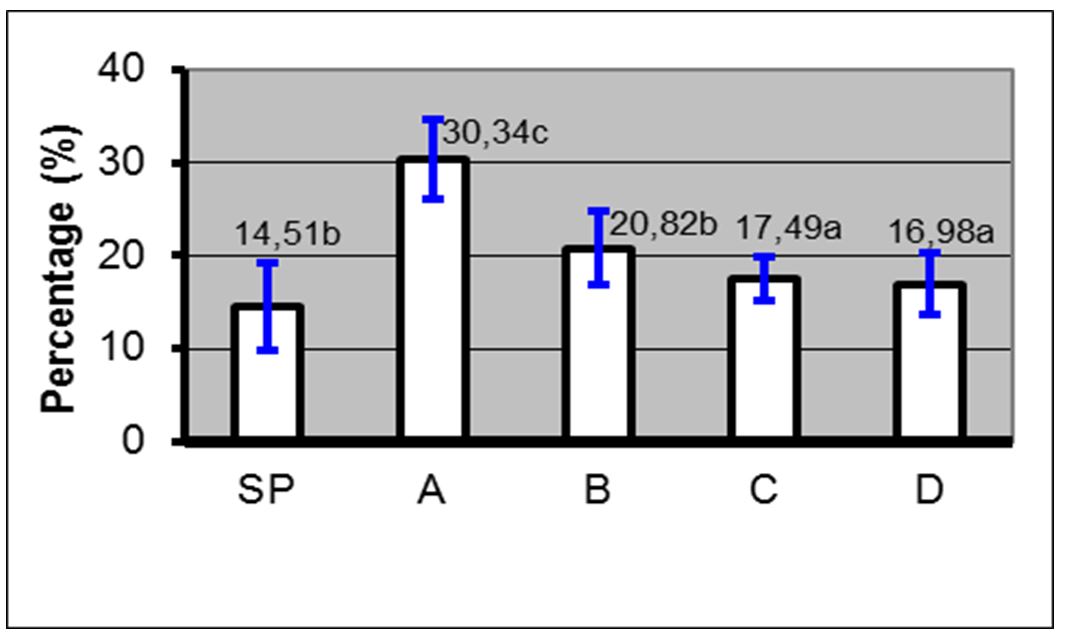
Notes: SP=Before treatment; A=Phospholipids 0% + EGTA Ca2+ free buffer (0 mM); B=Phospholipid 10%+ EGTA Ca2+ free buffer (0.5 mM); C=Phospholipid 10% + EGTA Ca2+ free buffer (1 mM); D=Phospholipids 10% + EGTA Ca2+ free buffer (2 mM); (a, b, c)=Duncan’s multiple distance test results 5%
Figure 10 – Average percentage ± SD of spermatozoa that have undergone acrosome reaction before and after treatment with phospholipids + EGTA Ca2+ free buffer
The results are in agreement with the findings of Ellis-Davies and Kaplan (1994) and Lemonnier et al. (2002). In another study, 1 mM EGTA with free Ca2+ buffer in an endothelial cell culture induced by various agonists or administration of 100 M H2O2 may cause Ca2+ depletion from endoplasmic reticulum depots and inhibits Ca2+ infusion from the extracellular matrix. In addition, EGTA was able to sharply reduce cytosolic Ca2+ with a long glucose exposure of 7 days. These results are in accordance with the findings of Hu et al. (1988), Sadova and Klishin (2000) and Parekh (2002). This contributed greatly to the increase in cytosolic Ca2+, and on day 7 of exposure, the increase in cytosolic Ca2+ was caused by influx from the extracellular matrix. Ellis-Davies and Kaplan (1994) obtained similar results.
Our findings support the hypothesis that the results obtained by other researchers may also be applied to spermatozoa cells. This is based on the understanding that calcium ions (Ca2+) are present in the cell matrix, via molecule adhesion, inside cell membranes and inside cell organelles, either bound to protein receptors in the cytosol or free in the cytosol. The free calcium ion concentration inside the cell is only 10-7 M, whereas outside the cell, it is 10-3 M. This huge difference between the free calcium ion concentration in the cytoplasm and in the extracellular fluid causes an abundant flow of calcium ions into the cytoplasm when calcium ion channels both inside the cell and in the organelle membrane are opened. This assumption is supported by Carrafoli et al. (1990) and Dedman and Kaetzel (1998).
The concurrent role of PC phospholipids and EGTA Ca2+ free buffer in this study showed that the membrane remained stable and could function properly because PC phospholipids maintain and protect the spermatozoa membranes, including building structures and metabolic processes, whereas EGTA Ca2+ free buffer functions as a scavenger of extracellular and intracellular Ca2+ ions. Synchronisation of the role of PC phospholipids and EGTA Ca2+ free buffer minimises the possibility of extracellular matrix deformation and changes in the ion regulation system in spermatozoa membranes that are caused by the PDGC process. As a results, the membranes may function properly and ultimately, the quality of the spermatozoa is maintained or even improved. The mechanism underlying the increase in calcium ions in the cytoplasm is two-fold: 1) calcium ions enter when the canal is opened due to changes in membrane voltage (voltage-operated calcium channel, VOCC) and 2) calcium ions enter when the canal is opened due to binding of the ligand with the receptor (receptor-operated calcium channel, ROCC).
The opening of these two types of channels will cause the entry of calcium ions from outside the cell into the cell because of the large concentration gradient. The second pathway is by opening the ion channels in the cell organelle membrane. This channel is an ROCC; when a ligand binds to a receptor on the membrane, it will cause the release of calcium ions from the organelle into the cell cytoplasm. Ion channels exist in two forms, namely in the closed and in the open state. In accordance with the findings of Breitbart and Naor (1999), calcium release-activated calcium channel (CRAC) is the main route of calcium entry in spermatozoa that do not have voltage-dependent calcium channels with a capacitive calcium entry (CCE) mechanism. This channel is related with the calcium deposition phase into the internal depot. The emptying of internal depots appears to increase the calcium permeability in the plasma membrane, which causes the calcium plateau in spermatozoa cells to be highly dependent on the concentration of extracellular calcium. Cytosolic calcium release and CRAC activation are closely related to the important role of acrosome calcium in regulating various cell functions, which is in line with Breitbart and Spungin (1997).
Furthermore, the incoming calcium will cause the intracellular calcium levels to increase or will fill the calcium storage. In addition, ligand binding to certain receptors will produce second-messenger IP3, which will bind to ROCC on the acrosome and cause the release of calcium into the cytoplasm. Increased levels of calcium in the cytosol will inhibit the ability of IP3 to activate the channel. The increased calcium level in the cytoplasm will enter the mitochondria, where calcium is used for various oxidative phosphorylation enzymes for ATP synthesis.
Calcium will also bind to various protein receptors in cells, including protein kinase C. In the acrosomal membrane, there is also a transport system that requires energy to transport calcium into the acrosome. Calcium from the acrosome will bind to PLC – phosphatidylinositol 4,5-bisphosphate (PIP2) – diacyglycerol (DAG), opening the plasma membrane so that calcium enters the cytoplasm. Calcium derived from the acrosome will affect CCE activity on the plasma membrane, which can enter through this pathway. Agonists that bind to certain receptors on the cell membrane will activate the PLC enzyme to cleave the PIP2 precursor molecule into DAG and inositol (1,4,5) triphosphate (IP3). The DAG, which also acts as a second messenger, will stimulate the activity of protein kinase-C (PKC), whereas IP3 causes the release of calcium in the acrosome. The mechanism by which IP3 releases intracellular calcium involves its interaction with specific receptors on the acrosomal membrane and the opening of acrosomal calcium channels. These results are supported by the findings of Toole et al. (1996), Breitbart and Spungin (1997), Breitbart and Naor (1999), Dragileva et al. (1999) and Baker et al. (2000).
Based on the results, it can be concluded that the optimal phospholipid concentration of PC 10% + EGTA Ca2+ free buffer is 1 mM, which can be justified based on the following results: (1) the concentrations of phospholipid PC 10% + EGTA Ca2+ free buffer 1 and 2 mM have the same notation for variables: spermatozoa viability, spermatozoa membrane integrity, spermatozoa that have not experienced capacitation, spermatozoa that have experienced capacitation, and spermatozoa that have undergone acrosomal reaction; (2) phospholipid concentration of PC 10% + EGTA Ca2+ free buffer at a concentration of 1 mM for spermatozoa motility variable, produced the best notation and was significantly different from the concentration of phospholipid PC 10% + EGTA Ca2+ free buffer 2 mM.
CONCLUSIONS
Phospholipid PC 10% + Ca2+ free buffer at 0.5, 1 and 2 mM, combined with PDGC, significantly improved the quality of spermatozoa. The optimal concentration regarding all variables was PC 10% + Ca2+ free buffer at 1 mM.
It is necessary to develop an effective and accurate method to measure the movement of calcium ions in spermatozoa when treated with 10% PC phospholipid concentration + EGTA Ca2+ free buffer + FURA2-AM.
A proportion of 10% PC phospholipid concentration + EGTA Ca2+ free buffer (1 mM) or 10% PC phospholipid concentration + EGTA Ca2+ free buffer (2 mM) can be used to obtain productive spermatozoa.
The best concentration of phospholipids found in this study may be recommended for breeding quality livestock with reproductive biology technology.
REFERENCES
Ax, R.L., Dally, M., Didion, B.A., Lenz, R.W., Love, C.C., Varner, D.D., Havez B. & Bellin, M.E. (2000). Semen evaluation. In: Reproduction in Farm Animals, 7th ed., E.S.E. Hafez and B. Hafez (Eds.), Lea and Febiger, Philadelphia, pp. 365-375.
Baker, H.W.G., Liu, D.Y. & Martic, M.G. (2000). The human acrosome reaction. Asian J. Androl., 2(3): 172-178.
Bowyer, D.E. & Davies, P.F. (1979). Effect of concentration of perfusing free fatty acid on arterial lipid synthesis in perfused normal and atherosclerotic rabbit aortas. Atherosclerosis, 31(4):409-419, DOI: 10.1016/0021-91 50(78)90136-3
Berden, H.J. & Fuquay, J.W. (1984). Applied animal reproduction. 2nd ed., Res Pub. Co., Inc. Prentice-Hall, Reston, Virginia.
Breitbart, H. & Spungin, B. (1997). The biochemistry of the acrosome reaction. Mol. Hum. Reprod., 3(3): 159-202, DOI: https://doi.org/10.1093/molehr/3.3.195
Breitbart, H. & Naor, Z (1999). Protein kinase in mammalian sperm capacitation reaction. Rev. Reprod., Sep.4(3): 151-159, DOI: 10.1530/ror. 0.0040151
Carrafoli, E., Chiesi, M. & Gazzotti, P. (1990). The membrane carriers related, and membrane potential in human endothelial cells. Circulation, 13: 1719-1725.
Check, J.H., Katsoff, D., Rozak, J. & Lurie, D. (1992). Effect of swim-up, Percoll and Sephadex sperm separation methods on the hypo-osmotic swelling test. Human Reprod., 7.(1): 109-111, DOI: 10.1093/oxfordjournals.humrep. a137540
Correa, J.R., Heersche, G. & & Zavos, P.M. (1997). Sperm membrane functional integrity and response of frozen-thawed bovine spermatozoa during hypoosmotic swelling test incubation at varying temperatures. Theriogenology, 47(3): 715-721, DOI: 10.1016/s0093-691x(97)00029-0
Dedman, J.R. & Kaetzel, M.A. (2012). Calcium as an intracellular second messenger. mediation by calcium-binding proteins – Chapter 7, Cell Physiology Source Book (4th ed.), 99-109, DOI: 10.1016/B978-0-12-387738-3.00007-X
Djati, M.S. (2005). Peran Buffer NaHCO3 pada BO Medium Dalam Menginduksi Kapasitasi, Reaksi Akrosom, Viabilitas Sperma, dan Penetrasi Sperma Terhadap Oosit pada Fertilisasi Invitro Sapi (Role of NaHCO3 buffer in BO medium in inducing capacitance, acrosome reaction, sperm viability, and sperm penetration of oocytes in vitro fertilization of cattle). Jurnal Ilmu-Ilmu Hayati (Journal of the Biological Sciences), Vol. 6(1) (in Indonesian)
Djauhari, T. (2009). Pengaruh Penambahan Fosfolipid Terhadap Kualitas Spermatozoa Manusia (Effects of addition of phospholipids on the quality of human spermatozoa). Saintika Medika: Jurnal Ilmu Kesehatan dan Kedokteran Keluarga (Saintika Medika: Journal of Health and Family Medicine), Vol. 5(2) (in Indonesian)
Dragileva, E., Rubinstein, S. & Breitbart, H. (1999). Intracellular Ca2+ – Mg2+ -ATPase regulates calcium influx and acrosomal exocytosis in bull and ram spermatozoa. Biol. Reprod., Vol. 61: 1226-1234.
Ellis-Davies, G.C. & Kaplan; J.H. (1994). Nitrophenyl-EGTA, a photolabile chelator that selectively binds Ca2+ with high affinity and releases it rapidly upon photolysis. Proc. Natl. Acad. Sci., 91(1): 187-191, DOI: 10.1073/pnas. 91.1.187
Garner, D.L. & Hafez., E.S.E (2000). Spermatozoa and seminal plasma. In: Reproduction in Farm Animals (7th ed.), Hafez, B., Hafez, E.S.E. (Eds.), Lea and Febiger, Philadelphia, pp. 96-109.
Hafez, E.S.E. (2000). X- and Y- chromosome-bearing spermatozoa. In: Reproduction in Farm Animals (7th ed.), Lea & Febiger, Philadelphia, pp. 440-446.
Hu, Q., Corda, S., Zweier, J.L., Capogrossi, M.C. & Ziegelstein, R.C. (1988). Hydrogen peroxide induces intracellular calcium oscillation in human aortic endothelial cells. Circulation, 97(3): 268-275, DOI: 10.1161/01.cir.97.3.268
Lemonnier, L., Vitko, Y., Shuba, Y.M., Abeele, F.V., Prevarskaya, N. & Skryma, R. (2002). Direct modulation of volume-regulation anion channels by Ca2+ chelating agents. FEBS Letter, 521: 152-156.
Parekh, A.B. (2002). The welcome prize lecture. Store-operated Ca2+ entry: dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane. J Physiology., 547: 333-348, DOI: 10.1113/jphysiol.2002.034140
Rasad, S.D., Setiawan, R., Solihati, N., Widyastuti, R. dan Nugraha, I. (2019). Derajat Pemulihan dan Persentase Spermatozoa X dan Y Kambing Peranakan Etawah Setelah Separasi dengan Gradient Percoll (Degree of recoverand percentage of spermatozoa X and Y Etawah Crossbreeds after separation with Percoll gradients). Jurnal Veteriner (Veterinary Journal). Vol. 20(1): 14-19 (in Indonesian).
Rumende, R.R.H., Kalim, H., Widodo, A. dan Djati, M.S. (2007). Peningkatan Kualitas Spermatozoa Sapi pada Proses Pemisahan Kromosom X dan Y dengan Sentrifugasi Gradien Densitas Percoll Melalui Pemberian Fosfolipid (Improving the quality of bovine spermatozoa in the separation process of X and Y chromosomes by centrifugation of Percoll density gradients by giving phospholipids). Jurnal Kedokteran Brawijaya (Brawijaya Medical Journal). Vol. 23(2): 71-81 (in Indonesian).
Sadova, M., Klishin, A., Huser, J. & Blatter, L.A. (2000). Capacitative calcium entry is graded with degree of intracellular calcium store depletion in bovine vascular endothelial cells. J. Physiol., 523(Pt 3): 549-559, DOI: 10.1111/ j.1469-7793.2000.t01-3-00549.x
Susilawati, T., Kusumawati, E.D., Isnaini, N., Yekti, A.P.A, Sudarwati, H. & Ridhowi, A. (2017). Effect of sexing process using Percoll density gradient centrifugation and frozen on motility and damage to spermatozoa membrane of Filial Ongole. Atlantis Press, Brawijaya University, Malang, Indonesia.
Toole, Ch., Roldan, M.B.O., Hampton, E.R.S. & Fraser, P. (1996). A role for diacylgycerol in human sperm acrosomal exocytosis. Mol. Hum. Reprod., 2(5): 317-326.
Tucker, K.E. & Jansen, C.A.M. (2002). Sperm separation techniques: comparison and evaluation of gradient product. In: Proceedings 2nd International Workshop for Embryologists: Troubleshooting Activities in the ART Lab., R. Basuray and D Mortimer (Eds.).
Zanella, E., Zanella, R., Poetini, M.R., Marques, M.G., Soares, J.C.M. & Bondan, C. (2016) Oxidative status of boar semen during storage. Am. J. Biochem. Biotechnol., 12(2): 95-101, DOI: 10.3844/ajbbsp.2016.95.101
Zenichiro, K., Herliante dan Sarastina (2002). Intruksi Praktis Teknologi Prosesing Semen Beku pada Sapi. (Technology of frozen semen processing for cattle). Balai Besar Inseminasi Buatan Singosari, Malang, Indonesia.
Baideng Eva L., Rares Fredine E.S., Rares Laya M., Rumende Rooije R.H.



