Iuliana-Maria Enache, Liliana Lucescu Ciurlă, Nicoleta Stănciuc, Antoanela Patraș, Camelia Vizireanu, Liviu Mihai Irimia
ABSTRACT. Cornus mas (CM) is one of the four edible fruits of the Cornus genus, a rich source of biologically active compounds (BACs) such as vitamins (like vitamin C), carotenoids, iridoids, and phenolics (phenolic acids, anthocyanins, and other flavonoids). This study aimed to analyse the improvement of the stability of CM anthocyanins by microencapsulation, in order to propose a new natural food dye. Microencapsulation using a mixture of whey protein isolate (WPI) and chitosan (CH) as wall materials has been applied to protect anthocyanins against external factors (e.g., light, temperature, storage, etc.). Two experimental variants of microencapsulated powders, WPI:CH = 1:1 (CH1) and WPI:CH = 1:2 (CH2), were realised by varying the wall materials ratio. The cornelian cherry fruit concentrated extract was evaluated for its phytochemical, colourimetric, and antioxidant capacities. Due to the excellent anthocyanin encapsulation effectiveness (74.29 – 88.71%), the wall materials utilised for both powders can be considered effective choices to safeguard the anthocyanins. All tests performed on the microencapsulated powders demonstrated that both suggested experimental forms can serve as a healthy substitute for artificial food additives. The incorporation of cornelian cherry fruit extract and microencapsulated powders into a food matrix (jelly candies) allowed examination of their effectiveness. The colour analysis rigorously characterised all the colour parameters related to red nuances (due to anthocyanins content, such as cyanidin-3-glucoside) and yellow nuances (associated with carotenoids content).
Keywords: chitosan; jelly candies; natural pigments; phenolic compounds; whey protein isolate.
Cite
ALSE and ACS Style
Enache, I.-M.; Lucescu Ciurlă, L.; Stănciuc, N.; Patraș, A.; Vizireanu, C.; Irimia, L.M. A new natural food dye: microencapsulated cornelian cherry bioactive compounds. Journal of Applied Life Sciences and Environment 2024, 57, 217-232.
https://doi.org/10.46909/alse-572133
AMA Style
Enache I-M, Lucescu Ciurlă L, Stănciuc N, Patraș A, Vizireanu C, Irimia LM. A new natural food dye: microencapsulated cornelian cherry bioactive compounds. Journal of Applied Life Sciences and Environment. 2024; 57 (2): 217-232.
https://doi.org/10.46909/alse-572133
Chicago/Turabian Style
Iuliana-Maria Enache, Liliana Lucescu Ciurlă, Nicoleta Stănciuc, Antoanela Patraș, Camelia Vizireanu, and Liviu Mihai Irimia. 2024. “A new natural food dye: microencapsulated cornelian cherry bioactive compounds” Journal of Applied Life Sciences and Environment 57, no. 2: 217-232.
https://doi.org/10.46909/alse-572133
View full article (HTML)
A New Natural Food Dye: Microencapsulated Cornelian Cherry Bioactive Compounds
Iuliana-Maria ENACHE1, Liliana LUCESCU CIURLĂ1, Nicoleta STĂNCIUC2, Antoanela PATRAȘ1*, Camelia VIZIREANU2 and Liviu Mihai IRIMIA3
1Department of Exact Sciences, Faculty of Horticulture, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 3, Mihail Sadoveanu Alley, 700490, Iasi, Romania; email: iuliana.enache@iuls.ro; liliana.ciurla@iuls.ro
2Department of Food Science, Faculty of Food Science and Engineering, “Dunărea de Jos” University of Galați, 111, Domnească Street, Galati, 800201, Romania; email: nicoleta.stanciuc@ugal.ro; camelia.vizireanu@ugal.ro
3Department of Horticultural Technologies, Faculty of Horticulture, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 3, Mihail Sadoveanu Alley, 700490, Iasi, Romania; email: liviu.irimia@iuls.ro
*Correspondence: antoanela.patras@iuls.ro
Received: Nov. 20, 2023. Revised: Feb. 22, 2024. Accepted: Mar. 08, 2024. Published online: Apr. 08, 2024
ABSTRACT. Cornus mas (CM) is one of the four edible fruits of the Cornus genus, a rich source of biologically active compounds (BACs) such as vitamins (like vitamin C), carotenoids, iridoids, and phenolics (phenolic acids, anthocyanins, and other flavonoids). This study aimed to analyse the improvement of the stability of CM anthocyanins by microencapsulation, in order to propose a new natural food dye. Microencapsulation using a mixture of whey protein isolate (WPI) and chitosan (CH) as wall materials has been applied to protect anthocyanins against external factors (e.g., light, temperature, storage, etc.). Two experimental variants of microencapsulated powders, WPI:CH = 1:1 (CH1) and WPI:CH = 1:2 (CH2), were realised by varying the wall materials ratio. The cornelian cherry fruit concentrated extract was evaluated for its phytochemical, colourimetric, and antioxidant capacities. Due to the excellent anthocyanin encapsulation effectiveness (74.29 – 88.71%), the wall materials utilised for both powders can be considered effective choices to safeguard the anthocyanins. All tests performed on the microencapsulated powders demonstrated that both suggested experimental forms can serve as a healthy substitute for artificial food additives. The incorporation of cornelian cherry fruit extract and microencapsulated powders into a food matrix (jelly candies) allowed examination of their effectiveness. The colour analysis rigorously characterised all the colour parameters related to red nuances (due to anthocyanins content, such as cyanidin-3-glucoside) and yellow nuances (associated with carotenoids content).
Keywords: chitosan; jelly candies; natural pigments; phenolic compounds; whey protein isolate.
INTRODUCTION
Due to their high concentration of bioactive compounds, the four edible species of the genus Cornus – Cornus mas (cornelian cherry), Cornus officinalis, Cornus controversa, and Cornus kousa – are primarily used in the food industry and in alternative medicine throughout the world (e.g., Romania, France, Spain, Germany, Turkey, etc.) (Dinda et al., 2016).
Over time, studies have highlighted the presence of some well-known biologically active compounds (BACs) in C. mas, including polyphenolic compounds, flavonoids (like kaempferol), catechins (like catechin), phenolic acids (like gallic acid), anthocyanins (like cyanidin-3-glucoside), carotenoids (like β-carotene, lutein), and vitamins (like vitamin C). Antioxidant activity is a trait that several bioactive substances share. The anti-inflammatory action, the anticancer and the anti-diabetic ability, the anti-anaemic capacity, and the protective effect for the gastrointestinal and excretory systems may be the most persuasive proof of the health benefits of BACs from cornelian cherry fruits (CCF) (Ahmadipour et al., 2017; Dinda et al., 2016; Enache et al., 2022a, b; Moldovan and David, 2014; Yilmaz et al., 2009).
According to a study by Visioli et al. (2011), eating foods with high polyphenol content (such as anthocyanins, flavonoids, etc.) is associated with several positive effects on human health, including a reduction in LDL cholesterol levels, as well as cardioprotective, anticancer, and antidiabetic effects. Due to the above-mentioned beneficial effects on health conditions, the anthocyanins (natural pigments of plants) from horticultural sources have been the subject of numerous recent research studies (Castañeda-Ovando et al., 2009; de Pascual-Teresa and Sanchez-Ballesta, 2007; Pojer et al., 2013).
The primary factors influencing consumer choice are the organoleptic parameters of the food (flavour, aroma, colour, etc.). In addition to the health promoting properties, anthocyanins are the pigments that give the colour of fresh, unprocessed, plant foods (fruits and vegetables), thus having the potential to be used as dyes in the food industry. These natural, colourful, water-soluble dyes have antioxidant properties but are also chemically unstable. Anthocyanins are hence sensitive to factors such as light, temperature, storage, the interaction of metal ions, pH, etc., (Castañeda-Ovando et al., 2009). In order to manifest their beneficial action on the human body, it is necessary to protect anthocyanins from the degrading action of external factors.
Due to their rapid deterioration, the protection of anthocyanins from environmental influences is one of the current concerns for specialists. By applying BACs microencapsulation using various methods (such as freeze-drying, spray-drying, etc.) this problem has been partially resolved (Turturică et al., 2016).
With a history spanning more than 70 years, encapsulation technology is now successfully used in a wide range of industries. The primary ones include the food industry, chemicals, printing products, cosmetics, pharmaceutics, and medicine (the work of Greaves from 1944, concerning the preservation of blood plasma was the first application of encapsulation technology) (Kumar et al., 2011). Many materials, bio-products, or waste products have been effectively encapsulated over the years, including fats and oils, aromatic compounds, oleoresins, vitamins, minerals, dyes, and enzymes. Freeze-drying is primarily used to stabilise food products like coffee, herbs, fruits, and vegetables, to pharmaceuticals (vaccines, antibiotics, vitamins), biopharmaceuticals (antibodies, enzymes, hormones), as well as medical applications (plasma preservation of rare blood groups, DNA, and RNA) (Desobry and Debeaufort, 2011; Kumar et al., 2011; Madene et al., 2006; Milea et al., 2019; Stoica et al., 2022; Vasile et al., 2020).
Microencapsulation of BACs from natural sources is also the interest of our research group. We previously reported the successful microencapsulation of bioactive compounds from various fruits (blackcurrant – Enache et al., 2020; red grape – Mihalcea et al., 2020; buckthorn – Roman et al., 2021), vegetables (black beans – Vasile et al., 2020), or wastes (red onion skin – Stoica et al., 2022; sweet cherries skins – Milea et al., 2019). Also, favourable results were obtained for the microencapsulation of cornelian cherry fruits, using a different wall material such as protein-rich (whey protein isolate) or polymer-rich materials (casein, inulin, pectin, etc.) (Enache et al., 2022a, b). For these reasons, we were encouraged to continue improving the bioavailability of the bioactive compounds in CCF, by microencapsulating them in a new original mixture of whey protein isolate (WPI) and chitosan (CH) as the wall material.
The ingredients used as wall materials are crucial to the encapsulation process. Whey protein isolate (WPI), which is obtained from whey and contains at least 90% proteins, is one of the two components used as wall material in this study. The by-product of milk processing with the highest quantitative weight is whey (approximately 90%) (Bonnaillie andTomasula, 2008; Tunick, 2008), consisting of lactic acid, fat, protein, lactose and minerals (Khezri et al., 2016). The high amount of protein (over 90%) in WPI makes it a desirable food additive. These high-quality proteins have the capacity to supplement foods with nutrients that are helpful to the human body (Tapas et al., 2008). According to studies, whey proteins have a variety of beneficial effects on the human body, including antioxidant, anti-inflammatory, anticancer, immunomodulatory, anti-hypertensive, anti-diabetic, osteoprotective, and dermoprotective properties. Whey protein isolate reduces muscle damage. (Brandelli et al., 2015; Cooke et al., 2010; Patel, 2015).
Chitosan represents the second wall material utilised for the powder’s microencapsulation in this study. By removing its acetyl group, the chitin, the most important derivative, is obtained. (Mourya and Inamdar, 2008). According to a recent study (Inanli et al., 2020), CH can be effective for food preservation and has anti-microbial, antioxidant, and preventive effects. Furthermore, according to Zhang (2022) many encapsulating techniques (emulsification, films, hydrogels, ionotropic gelation, layer-by-layer self-assembly, or spray-drying) were employed to shield enzymes from environmental variables by utilising chitosan.
Considering the aforementioned research products, the major objective of the present research was to safeguard the BACs extracted from CCF against external factors using an original mixture of wall materials, in order to deliver a new natural food dye with beneficial health properties. Therefore, two experimental varieties of microencapsulated powders of the cornelian cherry fruit concentrated extract (CE), based on WPI and CH as wall materials, were developed. Two powders were obtained, by varying the ratio of WPI and CH, namely 1:1 (CH1) and 1:2 (CH2).
The interest of the current work is to assess how the two experimental versions of microencapsulation, (CH1) and (CH2) improve the stability of anthocyanins from Cornus mas fruits, so that they can be employed as natural colour substitutes in the food sector. To accomplish the goals outlined in this work, for the concentrated cherry fruit extract tests were run to establish the phytochemical profile and colour, and to demonstrate the effectiveness of anthocyanin encapsulation.
Furthermore, the value of the cornelian cherry fruit extract and of the microencapsulated powders was evaluated by incorporating them into a food matrix (jelly candies). The naturally coloured jelly candies were analysed for phytochemical profile, colour parameters and sensory analysis.
MATERIALS AND METHODS
Materials
The reagents used in this study were: ethanol and distilled water (ratio 70:30, v/v used for bioactive compounds extraction); methanol; 2,2-Diphenyl-1-picrylhydrazyl (DDPH); 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) (antioxidant capacity); Folin-Ciocalteu reagent, sodium carbonate, and gallic acid (total polyphenolic content); cyanidin-3-glucoside, sodium acetate, and potassium chloride (total anthocyanins content); sodium nitrate, aluminium chloride, sodium hydroxide, and catechin (total flavonoids content); and acetic acid (encapsulation efficiency). The following analytical grade reagents were utilised for HPLC analysis: 3,4-dihydroxybenzoic acid, 4-hydroxybenzoic acid, vanillic acid, catechin, chlorogenic acid, syringic acid, coumaric acid, ferulic acid, salicylic acid, sinapic acid, and resveratrol, purchased from Sigma-Aldrich in Steinheim, Germany. One of the wall materials used in this study, CH, was also acquired from Sigma-Aldrich, while WPI 894 was from Fonterra (Clandeboye, New Zealand). For the preparation of jelly candies: a mixture of sweeteners, comprised of Stevia (produced by Sweet and Safe, containing erythritol 98% and steviol glycosides from stevia 2%), gelatine (Dr. Oetker) and citric acid (Dr. Oetker), was used. All the ingredients used for jelly candies were purchased from a local market of Iasi, Romania.
Methods
Extract preparation
The extraction of the cornelian cherry BACs was carried out using 70% ethanol. In this study, 30 g fresh fruits were mixed with 300 mL extraction solvent. The next step consisted of ultrasound-assisted extraction (30 min, 40 oC, 100 W, and 40 KHz – ultrasonic bath MRC Scientific Instrument). The obtained extract was filtered and centrifuged (5000 rpm for 30 min at 4 oC – Universal 320R, Tuttlingen, Germany), and then the solvent was removed under vacuum at 40°C (AVC 2-18, Christ, UK) to constant mass.
The resulting concentrated extract of cornelian cherry fruit (8.44 g) was kept in dark glass containers, hermetically sealed at 4°C, until further analysis.
Microencapsulated powders preparation
The CE was combined with two wall materials, WPI and chitosan, to create the experimental variants for the microencapsulated powders: CH1 (2.5 g CE, 1 g WPI, 1 g chitosan) and CH2 (2.5 g CE, 1 g WPI, 2 g chitosan), following a procedure similar to Enache et al. (2022b).
Jelly candies formulation
Cornelian cherry fruit concentrated extract or microencapsulated powder (CH1/CH2), sweetener mix, gelatine, citric acid, and demineralised water were the ingredients used to manufacture the jelly candies. Table 1 presents the obtained experimental variants.
Phytochemical analysis
The total phenolics (mg gallic acid equivalents (GAE)/ g dry matter (DM)), total anthocyanins (mg cyanidin-3-glucoside (C3G)/g DM), total flavonoids (mg catechin equivalents (CE)/g DM), and total antioxidant capacity (mMol Trolox equivalents (TE)/ g DM), as well as the encapsulation effectiveness and colourimetric parameters were analysed as previously described (Enache et al., 2022a).
Carotenoids content
The colour of the extract and the microencapsulated powders are associated with bioactive compounds such as carotenoids, polyphenols (especially anthocyanins) etc.
A quick total carotenoid assessment, based on UV-VIS spectra (ultraviolet-visible spectra) of the dissolved sample was performed using the method earlier described by Abou-Arab et al. (2010) and Roman et al. (2021), using an Analytik Jena spectrophotometer.
Briefly, lycopene content was measured at wavelength of λ=503 nm, β-carotene at λ=450 nm. The absorbance of the total carotenoid content at λ=470 nm was measured.
Table 1
Jelly candies formulation
|
Experimental variant |
Concentrated extract/ encapsulated powder (g) |
Sweetener mix (g) |
Gelatine (g) |
Citric acid (g) |
Water (mL) |
|
JE-1 |
1 (extract) |
6.00 |
7.50 |
0.01 |
100 |
|
JE-2 |
2 (extract) |
6.00 |
7.50 |
0.01 |
100 |
|
J1-CH1 |
1 (CH1) |
6.00 |
7.50 |
0.01 |
100 |
|
J2-CH1 |
2 (CH1) |
6.00 |
7.50 |
0.01 |
100 |
|
J1-CH2 |
1 (CH2) |
6.00 |
7.50 |
0.01 |
100 |
|
J2-CH2 |
2 (CH2) |
6.00 |
7.50 |
0.01 |
100 |
Colourimetric analysis
Colourimetric profile of the samples was performed using a colourimeter CR 410 Chroma Meter (Konica Minolta, Tokyo, Japan).The display of the scientific instrument indicated three parameters: brightness (L*) and chromatic parameters (a* and b*), while c* (chroma) and h (hue angle) were calculated by using the equations described by Enache et al. (2022b).
Chromatographic analysis of the phenolic compounds
Separation and quantification of the phenolic compounds from cornelian cherries were performed on a Waters Alliance HPLC system coupled with a PDA (photo diode array) detector, controlled by the Empower software. Separation was achieved on a Waters XBridge column C18 column (50 × 4.6 mm, 3.5 µm), maintained at 30 °C, with a gradient elution at the flow rate of 0.7 mL/min.
The mobile phase A consisted of a solution of 0.1 % TFA in water, while for mobile phase B a solution of 0.1 % TFA in acetonitrile was used. The chromatographic standards used in this study were: gallic acid, 3,4-dihydroxybenzoic acid, 4-hydroxybenzoic acid, vanillic acid, catechin, chlorogenic acid, syringic acid, coumaric acid, ferulic acid, sinapic acid, salicylic acid, and resveratrol.
The identification of phenolic compounds was realised by comparing retention time with available standards. Phenolic compound quantification was performed using the standard curves of external standards, obtained by plotting HPLC peak areas against the concentrations (µg/mL) (r2 > 0.99).
Sensory analysis
The formulation of jelly candies complies with the regulations of Order 121/382/2001 and their quality parameters meet the requirements of Order 523/808/351/2003 (Irimia, 2013).
Regarding the sensory analysis, the jelly candies prepared in this study were rated on a five-point scale based on unit numbering. The analysed characteristics were aspect, consistency, colour, smell, aroma, aftertaste, and overall appeal. A panel of 15 trained tasters (20 – 60 years old; 60% women; 40% men) took part in the sensory evaluation at 20°C, in accordance with all the rules involved.
Statistical analysis
Statistical analysis was conducted using Microsoft Excel software and the t-test. The experiments were run three times, and the results were presented as means ± standard deviations and expressed as mg/g dry matter (mg/g DM). To identify significant differences, experimental data were subjected to a t-test with a 95% confidence interval, p < 0.05, being considered statistically significant. Statistical analysis was performed using Minitab 18 software (Minitab, 2023).
RESULTS
Phytochemical parameters
Table 2 summarises the results obtained for the phytochemical analysis of the cornelian cherry fruit extract and of the microencapsulated powders. The effectiveness of powder encapsulation and the antioxidant capacity are also presented. From Table 2, it can be observed that the two corresponding powders have a similar global phytochemical profile and antioxidant activity.
However, CH2 powder showed a higher encapsulation efficiency, highlighting the role of chitosan in retaining the anthocyanins. In our previous study (Enache et al., 2022a), two WPI-based powders were developed, using as adjuvants for microencapsulation casein (CN) and inulin in an equal ratio.
The data obtained in our study were similar for the phytochemical content and antioxidant activity. For example, when using the same amount of wall materials, the total phenolic content reported in WPI:CN microencapsulated powder was 9.67 0.12 GAE/g DM, while for WPI:I powder, the total polyphenol content was 9.79 0.15 mg GAE/g DM.
The antioxidant activity obtained in this study is higher, probably due to the ability of WPI and chitosan to retain a higher concentration of anthocyanins. Enache et al. (2022a) reported values ranging from 77.97 to 79.03% when combining WPI and CN and WPI and inulin. The results highlights the superior microencapsulation efficiency of the WPI–CH combination in ratio of 1:2.
Carotenoid compounds of the extract and microencapsulated powders
The results obtained for this parameter show a significant level of carotenoids for the concentrated extract, for the microencapsulated powders, and all jelly candies formulation, as can be seen in Table 3.
The data obtained for total carotenoid content, as well as the data for the content of lycopene and β-carotene, can be correlated with the positive values obtained for the b* parameter, following colourimetric analysis. No significant differences were obtained in carotenoids content in the powders. The β-carotene content was around 3.7 mg/g DM. After the addition into jelly candies, no statistical differences were observed between the jelly candies with extract and the CH1 powders in different ratios.
However, the higher content of carotenoids in jelly candies obtained by adding powder CH2 similar to the powder content may be explained by the higher ability of CH2 to microencapsulate carotenoids as well.
HPLC phenolic compounds
The HPLC technique allowed the identification of 12 phenolic compounds in the cornelian cherries fruit extract sample namely: gallic acid, 3,4-dihydroxybenzoic acid, 4-hydroxybenzoic acid, vanillic acid, catechin, chlorogenic acid, syringic acid, coumaric acid, ferulic acid, sinapic acid, salicylic acid, and resveratrol (Figure 1).
The presence of most of these compounds in CM fruit was also identified in a previously reported study (Tenuta et al., 2022). The significant number of phenolic compounds identified emphasises the value of these fruits.
The content determined for each identified phenolic compound is shown in Table 4, and the results represent the average of two assays, and are presented as mean ± standard deviation.
Among the identified phenolic compounds, the highest content was observed for sinapic acid (160.62 µg/g), coumaric acid (122.51 µg/g), chlorogenic acid (68.58 µg/g), ferulic acid (64.05 µg/g), and syringic acid (56.46 µg/g).
Table 2
Phytochemical parameters of the concentrated extract of C. mas and microencapsulated powders
|
Sample Parameter |
CE |
CH1 |
CH2 |
|
Total phenolics (mg GAE/g DM) |
16.10 ± 0.67 |
9.59 ±0.57a |
9.72 ± 0.21a |
|
Total anthocyanins (mg C3G/g DM) |
12.18 ± 0.79 |
5.62 ± 0.23a |
5.65 ± 0.04a |
|
Total flavonoids (mg CE/g DM) |
5.51 ± 0.19 |
3.42 ± 0.25a |
3.76 ± 0.14b |
|
Total antioxidant capacity (mmol TE/g DM) |
188.06 ± 1.65 |
53.42 ± 0.21a |
53.65 ± 0.08a |
|
Encapsulation efficiency of anthocyanins (%) |
– |
74.29 ± 0.82a |
88.71 ± 0.11b |
The mean values which, for the same parameter, do not have the same superscript letter (a, b) are statistically different at p < 0.05 based on the paired t-test (CH1 and CH2).
Table 3
Carotenoid compounds of the concentrated extract, microencapsulated powders and jelly candies
|
Parameter Sample |
Lycopene (mg/g D.M.) |
β-carotene (mg/g D.M.) |
Total carotenoid content (mg/g D.M.) |
|
Concentrated extract (CE) |
4.47±0.13 |
5.89±0.13 |
11.94±0.36 |
|
Microencapsulated powders |
|||
|
CH1 |
3.69±0.09a |
5.17±0.15a |
9.78±0.06a |
|
CH2 |
3.72±0.08a |
5.15±0.09a |
9.94±0.15a |
|
Jelly candies |
|||
|
JE-1 |
2.31±0.08a |
4.01±0.09a |
7.60±0.08a |
|
JE-2 |
2.74±0.07a |
4.26±0.04a |
7.25±0.10a |
|
J1-CH1 |
1.88±0.07a |
3.30±0.17a |
5.90±0.08a |
|
J2-CH1 |
2.21±0.18b |
4.10±0.17b |
6.42±0.06b |
|
J1-CH2 |
3.04±0.03a |
4.80±0.06a |
7.95±0.01a |
|
J2-CH2 |
3.27±0.03a |
5.00±0.22b |
8.72±0.12b |
The mean values which, for the same parameter, do not have the same superscript letter (a, b), are statistically different at p < 0.05 based on the paired t-test (CH1 and CH2; JE-1 and JE-2; J1-CH1 and J2-CH1; J1-CH2 and J2-CH2). The corresponding values for the concentrated extract were not subjected to statistical analysis, to avoid the appearance of significant differences, as expected.
Table 4
HPLC quantification of phenolic compounds in cornelian cherry fruit extract
|
No. |
Phenolic compound |
Content (µg/g) |
|
1 |
Gallic acid |
14.77 ± 0.66 |
|
2 |
3,4-Dihydroxybenzoic acid |
0.17 ± 0.05 |
|
3 |
4-Hydroxybenzoic acid |
5.76 ±0.53 |
|
4 |
Vanillic acid |
1.13 ± 0.21 |
|
5 |
Catechin |
14.29 ± 0.68 |
|
6 |
Chlorogenic acid |
68.58 ± 0.73 |
|
7 |
Syringic acid |
56.46 ± 0.84 |
|
8 |
Coumaric acid |
122.51 ± 1.52 |
|
9 |
Ferulic acid |
64.05 ± 0.44 |
|
10 |
Sinapic acid |
160.62 ± 1.89 |
|
11 |
Salicylic acid |
29.02 ± 0.79 |
|
12 |
Resveratrol |
3.80 ± 0.16 |
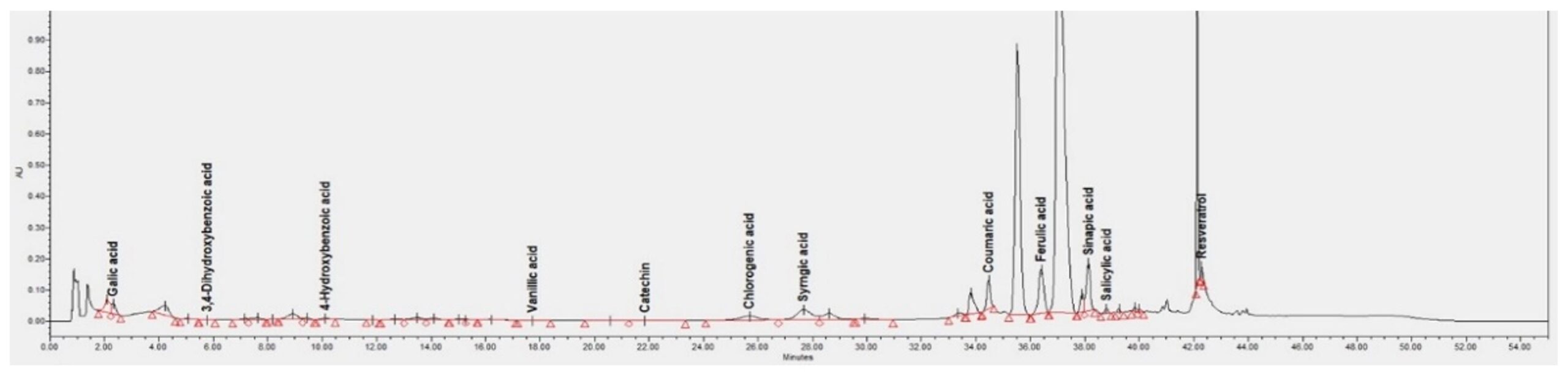
Figure 1 – HPLC-DAD (diode array detection) chromatogram at 280 nm of the cornelian cherry fruit extract
Colourimetric profile of the concentrated extract, microencapsulated powders and jelly candies
To the best of our knowledge, vibrant colour is associated with fresh foods. Spence (2015) has also shown that coloured food has a beneficial psychological effect and revealed that a food’s vivid colour, such as hues of red, can improve the consumer’s appetite. Unfortunately, to fulfill customer demand, food industry norms nowadays use an increasing amount of artificial food colouring chemicals. In this way, large quantities of synthetic food colouring are consumed and are linked to inflammatory conditions, stomach problems, and even cancer. One of the main objectives of the current work was to show that the suggested microencapsulated variations can be employed as a natural food colour for the food industry due to their vibrant colours. Table 5 shows the chromatic properties of the extract and microencapsulated powders expressed as brightness, green/red colour component, and blue/yellow colour component.
According to the colourimetric analysis, the obtained results showed that the concentrated extract had higher luminosity compared to the powders. However, when considering the powders, the use of white wall materials used in this study (WPI and CH) led to L* values of 30.89 ± 0.28 for CH1 and 32.63 ± 0.48 for CH2. Additionally, all the samples tested were red shades (positive value for a* parameters), possibly due to the anthocyanins content of cornelian cherry. Furthermore, positive values for b* parameters suggest the presence of carotenoids in the concentrated extract, in the microencapsulated powers, and in the prepared jelly candies. Overall, both microencapsulated powders represent a natural alternative for synthetic food additives due to their intense red-yellow shades.
Sensory analysis of the jelly candies
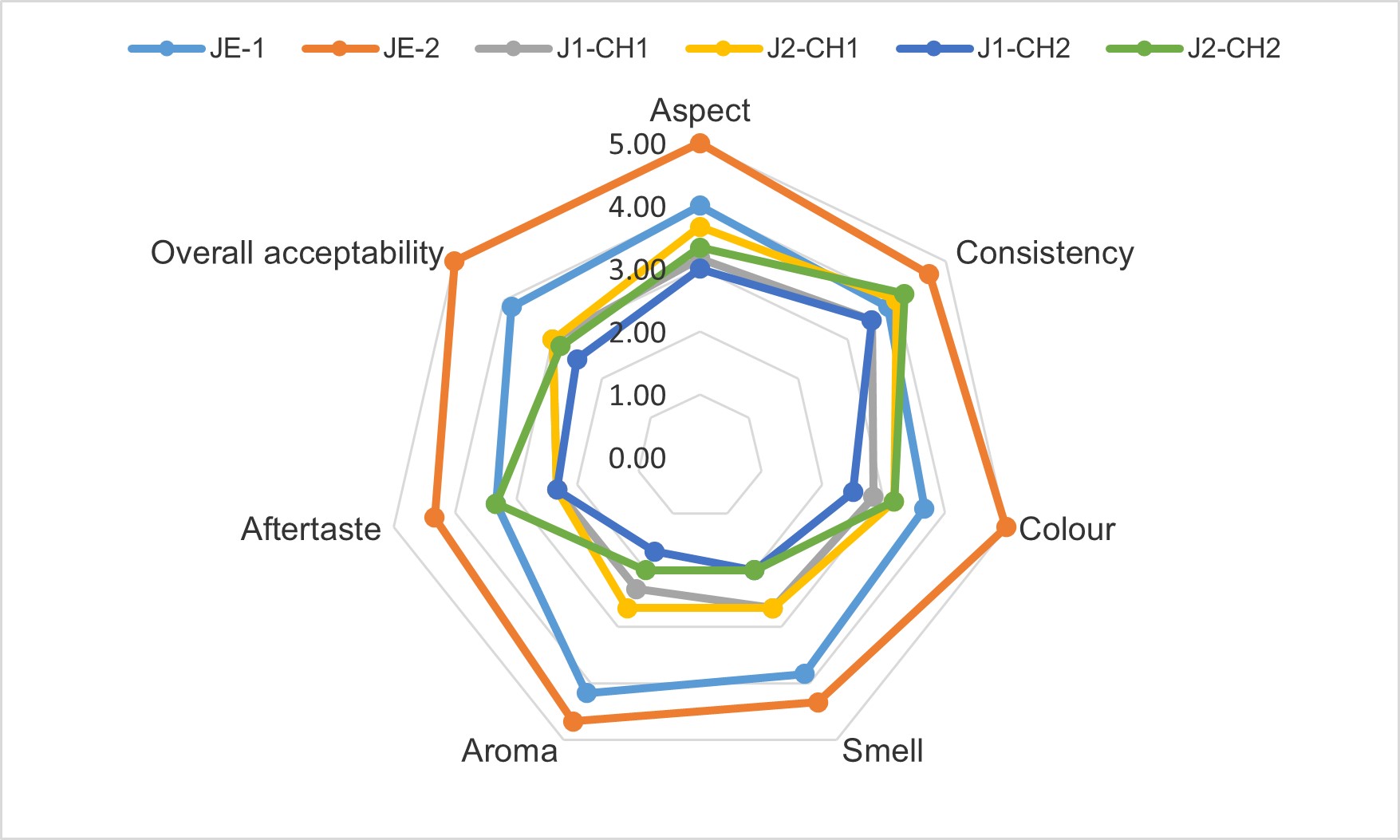
As can be seen in Figure 2, the consistency of all the experimental variants was specific for jelly candies, being in accordance with the scientific literature and food industry standards (Ghendov-Moșanu et al., 2016; Irimia, 2013). Furthermore, the colour attribute obtained by sensory analysis shows a strong red shade for the second variant with concentrated extract.
Additionally, our results prove that JE-2 obtained the best results for all the attributes tested and the results obtained for jelly candies performed with microencapsulated powders (CH1 or CH2) were similar for all the tested attributes.
Table 5
Colour parameters of the concentrated extract, microencapsulated powders and jelly candies
|
|
Parameter Sample |
Luminosity (L*) |
Green/red colour component (a*) |
Blue/yellow colour component (b*) |
Chroma colour intensity (c*) |
Tone (hue angle, h) |
|
Concentrated extract (CE) |
53.72 ± 0.83 |
20.22 ± 0.32 |
7.04 ± 0.03 |
21.41 ± 0.15 |
0.33 ± 0.01 |
|
|
Microencapsulated powders |
||||||
|
CH1 |
30.89 ± 0.28a |
10.34 ± 0.20a |
5.42 ± 0.23a |
11.68 ± 0.22a |
0.48 ± 0.02a |
|
|
CH2 |
32.63 ± 0.48b |
11.18 ± 0.14b |
5.59 ± 0.29a |
12.50 ± 0.22b |
0.46 ± 0.02a |
|
|
Jelly candies |
||||||
|
JE-1 |
16.42 ± 0.17a |
8.53 ± 0.44a |
2.50 ± 0.20a |
8.89 ± 0.41a |
0.29 ± 0.02a |
|
|
JE-2 |
33.07 ± 0.34b |
5.63 ± 0.42b |
3.38 ± 0.49b |
6.58 ± 0.18b |
0.54 ± 0.09b |
|
|
J1-CH1 |
18.24 ± 0.42a |
8.33 ± 0.06a |
9.92 ± 0.53a |
12.96 ± 0.37a |
0.87 ± 0.03a |
|
|
J2-CH1 |
14.87 ± 0.21b |
9.52 ± 0.05b |
3.22 ± 0.31b |
10.05 ± 0.05b |
0.33 ± 0.03b |
|
|
J1-CH2 |
11.27 ± 0.52a |
8.61 ± 0.12a |
2.89 ± 0.24a |
9.08 ± 0.14a |
0.32 ± 0.02a |
|
|
J2-CH2 |
9.13 ± 0.09b |
7.39 ± 0.06b |
2.58 ± 0.17b |
7.83 ± 0.10b |
0.34 ± 0.02b |
|
The mean values which, for the same parameter, do not have the same superscript letter (a, b) are statistically different at p < 0.05 based on t-test (CH1 and CH2; JE-1 and JE-2; J1-CH1 and J2-CH1; J1-CH2 and J2-CH2).
DISCUSSION
From the results shown in our study, it seems that the increase of chitosan concentration in the encapsulation materials led to powders with a higher ability to retain anthocyanins. Although the anthocyanins content in the two powders was similar, the CH2 showed a higher capacity to retain anthocyanins in the microparticles (approximatively 89%), which may be explained by the amplitude of polymer chain interactions due to electrostatic interactions. In both cases, the powders showed a tendency to appear yellow due to the carotenoid content. When compared with the concentrated extract, a reduction of phytochemicals was observed due to their instability during freeze-drying. For both experimental variants, good results were obtained regarding the total polyphenolic content (around 5.6 mg GAE/g DM), as well for the total flavonoid content (around 3.4 mg CE/g DM).
Compared to previous studies where whey protein isolate was included in the composition of the encapsulation material (Enache et al., 2022a), the current results for the total polyphenols are similar, whereas the values for total flavonoids are lower. Similar values were obtained for antioxidant activity, as a result of both polyphenols and carotenoids content.
Anthocyanin content is reflected in the red colour of the microencapsulated powders (positive value for a* component), while white wall materials can be related to brightness (L* parameter).
Nowadays, one of the major challenges in the food industry is finding natural food alternatives for children and teenagers.
Unfortunately, jelly candies are rich in synthetic dyes associated with many human diseases, such as children’s hyperactivity, cancer, diabetes, obesity etc. However, a rich diet of value-added natural dyes, based on biologically active compounds (e.g., carotenoids, betalaines, polyphenols including anthocyanins, etc.) from fruits or vegetables, provides antioxidants and cardioprotective, anti-inflammatory, anticancer effects, etc., (Boeing et al., 2012; Wrolstad and Culver, 2012).
Therefore, one of the main objectives of this study was to obtain a new natural food colourant rich in biologically active compounds and a high antioxidant potential.
Consequently, the powders were added as a natural dye in the jelly candy formulations, highlighting the higher content of carotenoids and anthocyanins, evidenced by the red and yellow shades.
Further, sensory analysis showed good results for all the experimental variants tested in this study, especially for JE-2. The good score obtained for sensorial attributes by the proposed jelly candies formulation make them a healthy alternative to be considered for commercial jelly candies prepared with synthetic ingredients.
The mechanisms via which the varying ratios of wall materials (1:1 or 1:2) affect the characteristics of the microencapsulated powders and jelly candies can be linked to the higher interfacial activity of the complex matrix compared to single compounds, as was also mentioned by Lopes et al. (2021). Thus, the bioactive peptides of whey protein isolate have a variety of physiological and functional effects on the human body, including mineral binding, reduced hunger, anti-ulcer, immunomodulatory, and antioxidant effects (Brandeli et al., 2015). On the other hand, because of its non-toxicity, high solubility, bioactivity, biocompatibility, and bioadhesivity, chitosan is widely employed as a food preservative (Inanli et al., 2020). The textural properties of jelly candies could be affected by the presence of the two wall materials used, in particular chitosan, but in order to provide an objective answer to this hypothesis, instrumental texture analysis of candies will be carried out in further studies. Furthermore, because of their high anthocyanin encapsulation effectiveness, whey protein isolate, and chitosan were employed in this study as wall materials for microencapsulating biologically active components from cornelian cherry fruit extract.
Moreover, it is important to mention that jelly candies have been used as experimental matrices for microencapsulated powders, but the study can be extended to other matrices. Furthermore, besides colour, the powders bring antioxidant activity with beneficial effects, but further studies are needed to test the cytocompatibility and multifunctional potential of the powders, such as controlled release upon digestion and better bioavailability of polyphenols.
CONCLUSIONS
The present study aimed to evaluate the effect of a mixture of two biopolymeric matrices (whey protein isolate and chitosan) as wall material on the pigments and other bioactive compounds of cornelian cherry, following the microencapsulation procedure. The present research revealed that the encapsulation efficiency for both experimental powders was higher than 70%. The polyphenolic content of the extract revealed by the HPLC analyses showed important quantities of bioactive phenols such as sinapic, coumaric, chlorogenic, and ferulic acids, as well as catechin, resveratrol, etc., which are known to have cardiovascular protective, anticancer, and anti-diabetic effects. This composition characterises cornelian cherry fruits as an important source of bioactive compounds. Furthermore, the results obtained for antioxidant activity, a remarkable property of the biologically active compounds mentioned above, were high for both powders. Our data provides insight into the use of cornelian cherry microencapsulated powders as a natural food colouring or nutraceutical due to the rich phytochemical profile, and to the results obtained from colourimetric analysis and encapsulation efficiency of the anthocyanins. Moreover, the successful incorporation of these powders into food matrices such as jelly candies highlighted the potential to replace the synthetic dyes. All the jelly candies, prepared with concentrated extract or microencapsulated powders and gelatine as gelling agent, were tested by colourimetric analysis and the obtained results indicate red and yellow shades (positive values for a* and b* parameters).
In conclusion, cornelian cherry fruit extract and the studied microencapsulated powders can be successfully used as new natural food dyes with nutraceutical properties, according to the results obtained in the present study.
Author Contributions: Conceptualization, IME, CV and AP; methodology, IME, LLC CV, NS, AP; analysis, IME and LLC; data curation, NS, and LMI; writing, IME, LLC and AP; review: NS, CV and AP. The authors declares that they read and approved the publication of the manuscript in this present form.
Funding: project AUF-DRECO-7863_SER-ECO_USVIIBI_DECHETJUS (financed by the Francophone University Agency of Central and Eastern Europe, AUF DRECO)
Acknowledgments: The authors are grateful for receiving technical support from the Horticultural Research Center (https://www.uaiasi.ro/horticultura/centru_cercet.php) and from the Laboratory for Bioactive Compounds Analysis of “Ion Ionescu de la Brad” Iași University of Life Sciences (Romania), and also, from the Integrated Center for Research, Expertise and Technological Transfer in Food Industry (https://www.bioaliment.ugal.ro/index_en.html) of “Dunărea de Jos” University of Galați (Romania).
Conflicts of Interest: There was no conflict of interest.
REFERENCES
Ahmadipour, S.H.; Vakili, M.; Ahmadipour, S. Phytotherapy for children’s nocturnal enuresis. Journal of Medical and Biomedical Sciences. 2017, 6, 23-29. https://doi.org/10.4314/jmbs.v6i3.4
Abou-Arab, A.E.; Abou-Arab, A.A.; Abu-Salem, M.F. Physico-chemical assessment of natural sweeteners steviosides produced from Stevia rebaudiana bertoni plant. African Journal of Food Sciences. 2010, 4, 269-281.
Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A. Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. European Journal of Nutrition. 2012, 51, 637-663. https://doi.org/10.1007/s00394-012-0380-y
Bonnaillie, L.M.; Tomasula, P.M. Whey protein fractionation. In Whey Processing, Functionality and Health Benefits, First edition, Blackwell Publishing and the Institute of Food Technologists, 2008, pp. 15-38. https://doi.org/10.1002/9780813803845.ch2
Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Research International. 2015, 73, 149-161. https://doi.org/10.1016/j.foodres.2015.01.016
Castañeda-Ovando, A.; Pacheco-Hernández, M. de L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chemistry. 2009, 113, 859-871. https://doi.org/10.1016/j.foodchem.2008.09.001
Cooke, M.B.; Rybalka, E.; Stathis, C.G.; Cribb, P.J.; Hayes, A. Whey protein isolate attenuates strength decline after eccentrically-induced muscle damage in healthy individuals. Journal of the International Society of Sports Nutrition. 2010, 7, 1-9. https://doi.org/10.1186/1550-2783-7-30
de Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: from plant to health. Phytochemistry Reviews. 2007, 7, 281-299. https://doi.org/10.1007/s11101-007-9074-0
Desobry, S.; Debeaufort, F. Encapsulation of flavors, nutraceuticals and antibacterials. in Application of encapsulation and controlled release, First edition, CRC Press, 2019, 1-31.
Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. Journal of Ethnopharmacology. 2016, 193, 670-690. https://doi.org/10.1016/j.jep.2016.09.042
Enache, I.M.; Vasile, A.M.; Enachi, E.; Barbu, V.; Stănciuc, N.; Vizireanu, C. Co-microencapsulation of anthocyanins from black currant extract and lactic acid bacteria in biopolymeric matrices. Molecules. 2020, 25, 1700, 1-12. https://doi.org/10.3390/molecules25071700
Enache, I.M.; Vasile, M.A.; Crăciunescu, O.; Prelipcean, A.M.; Oancea, A.; Enachi, E.; Barbu, V.V.; Stănciuc, N.; Vizireanu, C. Co-microencapsulation of anthocyanins from cornelian cherry (Cornus mas L.) fruits and lactic acid bacteria into antioxidant and anti-proliferative derivative powders. Nutrients. 2022a, 14, 3458. https://doi.org/10.3390/nu14173458
Enache, I.M.; Lucescu Ciurlă, L.; Stănciuc, N.; Irimia, L.M.; Patraș, A.; Vizireanu, C. Microencapsulation of anthocyanins from cornelian cherry fruits in whey protein isolate and pectin, Journal of Engineering Science. 2022b, 29, 138-149. https://doi.org/10.52326/jes.utm.2022.29(4).11
Ghendov-Moșanu, A.; Sturza, R.; Chirița, E.; Patraș, A. Valorization of winemaking by-products in the production of jelly candies. Italian Food Materials and Machinery. 2016, 12-16.
Khezri, S.; Seyedsaleh, M.M.; Seyedsaleh, I.; Dastras, M.; Dehghan, P. Whey: characteristics, applications and health aspects. 3rd International Conference on Science and Engineering. 2016, 7, 1383-1389.
Kumar, G.P.; Prashanth, N.; Kumari, B.C. Fundamentals and applications of lyophilization. Journal of Advanced Pharmaceutical Research. 2011, 2, 157-169.
Inanli, A.G.; Tümerkan, E.T.A.; Abed, N.E.; Regenstein, J.M.; Özogul, F. The impact of chitosan on seafood quality and human health: A review. Trends in Food Science & Technology. 2020, 97, 404-416. https://doi.org/10.1016/j.tifs.2020.01.029
Irimia, L.M. Quality control and expertise of vegetables, fruits and derived products (in Romanian). Ion Ionescu de la Brad Publishing house, Iași, Romania, 2013, 277.
Madene, A.; Muriel, J.; Joël, S.; Stéphane, D. Flavour encapsulation and controlled release – A review. International Journal of Food Science and Technology. 2006, 41, 1-21. https://doi.org/10.1111/j.1365-2621.2005.00980.x
Mihalcea, l.; Barbu, V.; Enachi, E.; Andronoiu, D.G.; Râpeanu, G.; Stoica, M.; Dumitrașcu, L.; Stănciuc, N. Microencapsulation of red grape juice by freeze drying and application in jelly formulation. Food Technology and Biotechnology. 2020, 58, https://doi.org/10.17113/ftb.58.01.20.6429
Milea, A.Ș.; Vasile, A.M.; Cîrciumaru, A.; Dumitrașcu, L.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Valorizations of sweet cherries skins phytochemicals by extraction, microencapsulation and development of value- added food products. Foods. 2019, 8, 188. https://doi.org/10.3390/foods8060188
Minitab. https://www.minitab.com/en-us/ (accessed on 13 March 2023).
Moldovan, B.; David, L. Influence of temperature and preserving agents on the stability of cornelian cherries anthocyanins. Molecules. 2014, 19, 8177-8188. https://doi.org/10.3390/molecules19068177
Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. Reactive and functional polymers. 2008, 68, 1013-1051. https://doi.org/10.1016/j.reactfunctpolym.2008.03.002
Patel, S. Functional food relevance of whey protein: A review of recent findings and scopes ahead. Journal of Functional Foods. 2015, 19, 308-319. https://doi.org/10.1016/j.jff.2015.09.040
Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: a review. Comprehensive Reviews in Food Science and Food Safety. 2013, 12, 483-508. https://doi.org/10.1111/1541-4337.12024
Roman, D.; Condurache (Lazăr), N.N.; Aprodu, I.; Enachi, E.; Barbu, V.; Bahrim, G.E.; Stănciuc, N.; Râpeanu, G. Insights of sea buckthorn extract’s encapsulation by coacervation technique. Inventions. 2021, 6, 59; https://doi.org/10.3390/inventions6030059
Spence, C. On the psychological impact of food colour. Flavour. 2015, 4, 1-16. https://doi.org/10.1186/s13411-015-0031-3
Stoica, F.; Condurache, N.N.; Horincar, G.; Constantin, O.E.; Turturică, M.; Stănciuc, N.; Aprodu, I.; Croitoru, C.; Râpeanu, G. Value-added crackers enriched with red onion skin anthocyanins entrapped in different combinations of wall materials. Antioxidants. 2022, 11, 1048. https://doi.org/10.3390/antiox11061048
Tapas, A.R.; Sakarkar, D.M.; Kakde, R.B. Flavonoids as nutraceuticals: a review. Tropical Journal of Pharmaceutical Research. 2008, 7, 1089-99. https://doi.org/10.4314/tjpr.v7i3.14693.
Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Cuyamendous, C.; Bonesi, M.; Sicari, V.; Trabalzini, L.; Mitaine-Offer, A.-C.; Xiao, J.; Tun-dis, R. An Overview of Traditional Uses, Phytochemical Compositions and Biological Activities of Edible Fruits of European and Asian Cornus Species. Foods. 2022, 11, 1240. https://doi.org/10.3390/foods11091240
Tunick, M.H. Whey Protein Production and Utilization: A Brief History. In Whey Processing, Functionality and Health Benefits, First edition, Blackwell Publishing and the Institute of Food Technologists, 2008, pp. 1-13. https://doi.org/10.1002/9780813803845.ch1
Turturică, M.; Stănciuc, N.; Bahrim, G.; Râpeanu, G. Effect of thermal treatment on phenolic compounds from plum (Prunus domestica) extracts – A kinetic study. Journal of Food Engineering. 2016, 171, 200-207. https://doi.org/10.1016/j.jfoodeng.2015.10.024
Vasile, M.A.; Milea, Ș.A.; Enachi, E.; Barbu, V.; Circiumaru, A.; Bahrim, G.E.; Râpeanu, G.; Stănciuc, N. Functional Enhancement of bioactives from black beans and lactic acid bacteria into an innovative food ingredient by comicroencapsulation. Food and Bioprocess Technology. 2020, 13, 978-987. https://doi.org/10.1007/s11947-020-02451-8
Visioli, F.; Lastra, C.A.D.L.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; Llorach, R.; Vitaglione, P.; Zoratt, M.; Edeas, M. Polyphenols and human health: a prospectus. Critical Reviews in Food Science and Nutrition. 2011, 51, 524-546. https://doi.org/10.1080/10408391003698677
Wrolstad, R.E.; Culver, C.A. Alternatives to those artificial FD&C food colorants. Annual Review of Food Science and Technology. 2012, 3, 59-77. https://doi.org/10.1146/annurev-food-022811-101118
Yilmaz, K.; Ercisli, S.; Zengin, Y.; Sengul, M.; Kafkas, E. Preliminary characterisation of cornelian cherry (Cornus mas L.) genotypes for their physico-chemical properties. Food Chemistry. 2009, 114, 408-412. https://doi.org/10.1016/j.foodchem.2008.09.055
Zhang, H.; Feng, M.; Fang, Y.; Wu, Y.; Liu, Y.; Zhao, Y.; Xu, J. Recent advancements in encapsulation of chitosan-based enzymes and their applications in food industry. Critical Reviews in Food Science and Nutrition. 2022, 63, 11044-11062. https://doi.org/10.1080/10408398.2022.2086851
Academic Editor: Dr. Mihaela ROȘCA
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Enache Iuliana-Maria, Irimia Liviu Mihai, Lucescu Ciurlă Liliana, Patraș Antoanela, Stănciuc Nicoleta, Vizireanu Camelia