Liliana Rotaru, Vasile Răzvan Filimon, Roxana Mihaela Filimon, Mihai Mustea, Roberto Renato Bernardis, Lucia Cintia Colibaba
ABSTRACT. The monitoring of new grapevine varieties with superior agrobiological and technological characteristics, in relation to the evolution of the climatic factors, represents an important and continuous objective of the worldwide viticultural research and breeding programs. Observations and determinations of the current study were performed on 11 new table grapes varieties created in Romania, growing in the Ampelographic collection of the University of Life Sciences Iasi, north-eastern area of Romania. The grapevine varieties were evaluated under the morpho-structural aspect, regarding the leaf area, the average weight of the grapes, the number and weight of the berries, rachis weight, the number and weight of the seeds, the weight of the skin and pulp, calculating the main technological indices. From a biochemical point of view, the content of photosynthetic pigments in leaves, the concentration of soluble dry solids and titratable acidity of the grapes were determined in the climatic condition of the Copou – Iasi vineyard. Therefore, the monitoring of genetic resources provides useful data for grape producers and researchers regarding the integration of new table grape varieties into the viticultural ecosystems, evaluating their yield and quality in correlation with the evolution and influence of the climatic factors.
Keywords: agrobiological and technological value; autochthonous grapevine variety; chemical composition; genetic resources; physico-structural characterization.
Cite
ALSE and ACS Style
Rotaru, L.; Filimon, V.R.; Filimon, R.M.; Mustea, M.; Bernardis, R.R.; Colibaba, L.C. Preliminary studies on some morpho-structural and biochemical characterization of some genotypes of Vitis vinifera L. cultivated in northeast Romania. Journal of Applied Life Sciences and Environment 2024, 57 (1), 69-90.
https://doi.org/10.46909/alse-571124
AMA Style
Rotaru L, Filimon VR, Filimon RM, Mustea M, Bernardis RR, Colibaba LC. Preliminary studies on some morpho-structural and biochemical characterization of some genotypes of Vitis vinifera L. cultivated in northeast Romania. Journal of Applied Life Sciences and Environment. 2024; 57 (1): 69-90.
https://doi.org/10.46909/alse-571124
Chicago/Turabian Style
Rotaru, Liliana, Vasile Răzvan Filimon, Roxana Mihaela Filimon, Mihai Mustea, Roberto Renato Bernardis, and Lucia Cintia Colibaba. 2024. “Preliminary studies on some morpho-structural and biochemical characterization of some genotypes of Vitis vinifera L. cultivated in northeast Romania” Journal of Applied Life Sciences and Environment 57, no. 1: 69-90.
https://doi.org/10.46909/alse-571124
View full article (HTML)
Preliminary Studies on Some Morpho-Structural and Biochemical Characterisation of Some Genotypes of Vitis vinifera L. Cultivated in Northeast Romania
Liliana ROTARU1, Vasile Răzvan FILIMON2, Roxana Mihaela FILIMON2, Mihai MUSTEA1, Roberto Renato BERNARDIS1 and Lucia Cintia COLIBABA1*
1Department of Horticultural Technologies, Faculty of Horticulture, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 3, Mihail Sadoveanu Alley, 700490, Iasi, Romania; email: liliana.rotaru@iuls.ro; musteaimihai@yahoo.com; roberto041069@yahoo.com
2Research Development Station for Viticulture and Winemaking, 48, Mihail Sadoveanu Alley, 700489, Iasi, Romania; email: razvan_f80@yahoo.com; roxanacotovanu@yahoo.com
*Correspondence: cintia.colibaba@iuls.ro
Received: Jan. 23, 2024. Revised: Feb. 19, 2024. Accepted: Feb. 20, 2024. Published online: Mar. 08, 2024
ABSTRACT. The monitoring of new grapevine varieties with superior agrobiological and technological characteristics, in relation to the evolution of the climatic factors, represents an important and continuous objective of the worldwide viticultural research and breeding programs. Observations and determinations of the current study were performed on 11 new table grapes varieties created in Romania, growing in the Ampelographic collection of the University of Life Sciences Iasi, north-eastern area of Romania. The grapevine varieties were evaluated under the morpho-structural aspect, regarding the leaf area, the average weight of the grapes, the number and weight of the berries, rachis weight, the number and weight of the seeds, the weight of the skin and pulp, calculating the main technological indices. From a biochemical point of view, the content of photosynthetic pigments in leaves, the concentration of soluble dry solids and titratable acidity of the grapes were determined in the climatic condition of the Copou – Iasi vineyard. Therefore, the monitoring of genetic resources provides useful data for grape producers and researchers regarding the integration of new table grape varieties into the viticultural ecosystems, evaluating their yield and quality in correlation with the evolution and influence of the climatic factors.
Keywords: agrobiological and technological value; autochthonous grapevine variety; chemical composition; genetic resources; physico-structural characterization.
INTRODUCTION
The viticultural genetic resources are constantly in the attention of European countries, the main concerns regarding the increase in the risks of genetic erosion, the impact of climate change, the anthropic factor, the fragmentation of viticultural ecosystems, the substitution of native varieties, as well as the uncontrolled transfer of planting material (Brault, 2021). The cultivation of a small number of “commercial” varieties over large areas has resulted in a rapid loss of grapevine germplasm worldwide. Also, the dominance of a small number of varieties has gradually led to an alarming reduction in the genetic variability of grapevine (Bortoló, 2012; D’onofrio, 2020). The Romanian range of varieties for table grapes has been enriched in recent years with valuable new genotypes, in the production of which old, established varieties were predominantly used, but whose individual characteristics are less and less appreciated (Bosoi et al., 2022).
The current problem of the viticulture, and viticultural breeding in particular, is related to the phenomenon whereby native varieties or new creations end up being very little exploited, many of them remaining of local significance or preserved in ampelographic germplasm collections. In parallel, as a consequence of genetic erosion, there is currently a constant loss of some varieties that were traditionally cultivated in certain wine-growing regions (Ollat et al., 2019). The only way to prevent this phenomenon is to locate, study and preserve the sources of wine germplasm, so that they can be precisely characterized and correctly identified. Thus, the characterization and evaluation of existing viticultural germplasm has become a priority task in the successful completion of breeding programs. The conservation of viticultural genetic resources is aimed at maintaining the authenticity and health of varieties, avoiding the natural or accidental disappearance of valuable genotypes. The possibility of preserving genetic resources is limited to ampelographic collections (eg. in situ), modern biotechnologies (in situ) and cryopreservation (apexes in liquid nitrogen at -196°C). Of these, the most common strategy remains the preservation of the viticultural material in the ampelographic collections that include old, valuable local varieties, but also new created genotypes, selections and clonal overselections as well as foreign varieties introduced through exchange of biological material (Filimon et al., 2021).
Considering all these aspects, permanent monitoring of the genetic resources, under the aspect of their integration into various viticultural ecosystems, concerning the agrobiological and technological characteristics, in permanent correlation with the evolution and influence of climatic factors, will allow the multiplication of valuable new or ancestral grapevine varieties (Bortoló, 2012; Filimon et al., 2019). Therefore, the current study provides important data for grape producers, researchers and consumers concerning the physico-chemical and physiological features of new table grapes varieties, created in Romania and recommended for planting within the temperate climate vineyards.
MATERIALS AND METHODS
Grapevine varieties and growing conditions
The samples were collected from the Ampelographic Collection of the University of Life Sciences in Iași, in the year 2023. The biological material was represented by leaves and grapes from 11 autochthonous grapevine varieties (for table grapes and with mixed function), new creations belonging to the species Vitis vinifera L. genus Vitis (Table 1). The varieties are between 15 and 25 years old, grafted on Kober 5 BB rootstock (V. berlandieri × V. riparia). Planting distances were: 2.2 m between rows and 1.2 m between plants per row. Trellising was semi-high (trunk of 0.7-0.8 m), bilateral cordons with pruning in canes and spurs (2 buds spurs and 4-5 buds canes), which ensures an average load of 40–45 buds/plant. The soil maintenance system within the plantation was “black field” (ploughed) and the maintenance works applied to the vines were those specific to the industrial viticultural system.
Table 1
The grapevine varieties analysed in the study
|
Grapevine variety |
Origin |
Institution of homologation |
Homologation year |
|
‘Gelu’ |
free fertilization of the ‘Coarnă neagră’ variety whose hybrid seeds were irradiated with X-rays |
SCDVV Iași |
1998 |
|
‘Milcov’ |
‘Coarnă neagră’ × ‘Muscat de Hamburg’ |
SCDVV Odobești |
1988 |
|
‘Someșan’ |
self-fertilization of the hybrid elite ‘Muscat de Hamburg’ × ‘Regina viilor’ |
SCH Cluj-Napoca |
1987 |
|
‘Cetățuia’ |
Crâmpoșie x Frumoasă de Ghioroc |
SCH Cluj-Napoca |
1979 |
|
‘Napoca’ |
‘Alphonse Lavallée’ × (‘Regina viilor’ × ‘Muscat Hamburg’) |
SCH Cluj-Napoca |
1984 |
|
‘Splendid’ |
‘Black rose’ × ‘Regina viilor’ |
SCH Cluj-Napoca |
1984 |
|
‘Transilvania’ |
‘Black rose’ × ‘Cardinal’ |
SCH Cluj-Napoca |
1984 |
|
‘Purpuriu’ |
‘Ceauş’ × ‘Villard blanc’ |
ICDVV Valea Călugărească |
1985 |
|
‘Radames’ |
(‘Regina viilor’ × ‘Villard blanc’) × ‘Traminer roz’ |
SCDVV Blaj |
1994 |
|
‘Coarnă neagră selecționată’ |
individual selection obtained from seeds from the Coarnă neagră variety, with normal hermaphrodite flowers and fertile pollen |
IANB București |
1970 |
|
‘Coarnă Neagră’ |
the area of Asia Minor, the Anatolian Plateau |
– |
– |
SCDVV Iași – Research Development Station for Viticulture and Winemaking Iasi; SCDVV Odobești – Research Development Station for Viticulture and Winemaking Odobești; SCH Cluj-Napoca – Horticultural Research Station Cluj-Napoca; ICDVV Valea Călugărească – Research and Development Institute for Viticulture and Winemaking Valea Călugărească; SCDVV Blaj – Research and Development Station for Viticulture and Winemaking Blaj; IANB București – Agronomical Institute Bucharest.
To carry out the experimental determinations, a number of 30 plants of each grape variety, normally developed, with medium vigour, unaffected by diseases or pests, were selected and marked. Samples of leaves (in the period of bud burst – fall of leaves) and grapes (in the period of veraison – full maturity) were harvested manually, in the morning (9:00 am), on layers of ice (in polystyrene boxes), from both sides of the trunk. Except for the bud burst phenophase, when the leaves were collected from the subapical node, the samples were collected from the middle third of the shoots, where the ampelographic and age variability is the most reduced (Rotaru, 2009). The collected samples were clean, normally developed and unaffected by diseases; the time from collection to laboratory determinations was 15 minutes. The time of sample collection was established according to the international classification of phenological phases proposed by Baggiolini (1952) and Eichhorn and Lorenz (1977): bud burst (stage 07/E), shoots growth (stage 15), flowering (stage 21/I), berry growth (stage 31/K), veraison (stage 35/M), grape maturation (stage 38/N), leaf fall (stage P).
General climate conditions
The ampelographic collection of the U.S.V. Iaşi is located in the CIa wine-growing area, being part of the V. Adamachi Iaşi horticultural farm (NE of Romania), with geographical coordinates 27°53′ eastern longitude and 47°09′ northern latitude, benefiting from a favourable microclimate for grapevine growing (Coţovanu and Rotaru, 2011). The plot on which the ampelographic collection was established has an altitude of 150-160 m, with S-SW exposure, the inclination of the slope is 6–7%, and the orientation of the rows is in the N-S direction. The soil is cambic chernozem, formed on marl with intercalation of sands, with the depth of the water table above 2.5–3 meters.
For the investigation of climatic data, was used a weather station located near the experimental plot (Research – Development Station for Viticulture and Winemaking Iași), coupled with the AgroExpert® software.
The temperature is typical for the temperate-continental climate, with some excessive nuances. The multiannual (1980-2010) average air temperature is 9.6°C, the highest average monthly temperatures are recorded in July, and the lowest average monthly temperatures in January. The absolute minimum temperatures drop in winter to −26… −32°C and endanger the grapevine culture about 2 years out of 10 (Mustea, 2004). The absolute minimum in Iași was −30°C, recorded in 1929 and 1937 (Amăriucăi and Machidon, 2022).
Morpho-structural determinations
Leaf area was periodically determined by direct scanning of 10 leaves/variety, with the portable instrument ADC BioScientific AM 300 Area meter, Hertfordshire, UK (non-destructive method).
The average weight of a grape, as a character that constitutes an element of yield and quality in the grapevine analysis (Sestraş, 2004), was determined by weighing 25 grapes/variety, harvested from different grapevine stocks, the results being expressed in grams (g) (Dumitriu, 2008).
The average number of berries in bunches, as a structural determination which consists in establishing the average number of berries per 10 bunches harvested from each variety, the affected berries being counted separately.
The average weight of a berry was obtained by weighing the normally developed and healthy berries from 10 bunches harvested from each variety (g).
The weight of 100 berries was obtained by weighing 100 berries (g) from 10 grapes/variety, 10 berries each from the top, middle and base of the grape and recording the data; the values of this determination representing a variety characteristic.
The average weight of the rachis was determined by weighing the rachis from 10 grapes (g).
The number of seeds in the berry represents a usual determination, being a criterion of appreciation in the tasting sheets of table grapes.
The weight of the skin, pulp and seeds were performed to obtain the composition index. The result represents the average values recorded from 50 berries/variety and is expressed in grams (g). All weighing were performed using an ATX 224 (Shimadzu, Japan) analytical balance.
The structure index of the grape represents the ratio between the weight of the berries and the weight of the rachis. In grapes, the structure index has values between 12 and 50, the low values being characteristic of wine varieties, high values being typical for table grapes (Constantinescu, 1970).
The berry index represents the number of berries per 100 g of grapes and is lower for table varieties, where it can go down to 30, and higher for wine varieties, where it can exceed 100 (Constantinescu, 1970).
The composition index of the berry is given by the ratio between the mass of the pulp/the mass of the skin and seeds and has values between 10-15 for table varieties and between 5-8 for wine grape varieties (Constantinescu, 1970).
Biochemical determinations
Determination of the content of photosynthetic pigments in leaves:
For the extraction of chlorophyll (a and b) and carotenoids (xanthophylls and carotenoids), 0.5 g of freshly harvested leaves on ice were crushed, infused with 10 mL of acetone 99.98% and stored overnight in the cold (4°C) and dark. Small amounts of MgCO3 (0.5 mg) were added during the extraction to neutralize the acids responsible for the formation of pheophytin a from chlorophyll a (Lee and Schwartz, 2005). The obtained fractions were subsequently centrifuged (Nahita 2816 cooling centrifuge) for 15 minutes, 3000 rpm (10°C). Analytical determinations were carried out using a UV-vis spectrophotometer Shimadzu 1700 Pharmaspec, at wavelengths: 662 and 645 nm, for chlorophyll a and b and 470 nm for carotenoids (Davies, 2004). The pigment content was calculated in mg/g fresh weight, using the equations proposed by Lichtenthaler and Buschmann (2001) and the Carnegie Institute for Science through the Spectranomics Protocol (2011) (Eqs. 1-3):
|
Chl a (μg mL-1) = 11.24 × (A662 – A710) – 2.04 × (A645–A710) |
(1) |
|
Chl b (μg mL-1) = 20.13 × (A645–A710) – 4.19 × (A662–A710) |
(2) |
|
Carotenoids (μg mL-1) = (1000 × (A470–A710) – 1.90 × Chl a – 63.14 × Chl b)/214 |
(3) |
Determination of the concentration of soluble dry solids of grapes by refractometry (ºBrix) and titratable acidity by titrimetry (g/L as tartaric acid) were conducted according to the OIV’s Compendium of International Methods of Wine and Must Analysis (OIV, 2021). The correspondence between the ºBx measurement and the sugar concentration of the must (g/L) was carried out according to the tables presented by OIV (2021).
Statistical analysis
Analysis of variance (ANOVA test) was initiated to investigate significant differences between biometrical and biochemical data in XLSTAT®: Statistical software, within Microsoft® Excel software. The method used to discriminate among the means was Duncan’s multiple range test at 95% confidence level. P values lower than 0.05 (p<0.05) were considered to be significant. Statistical measure of the dispersion of data points around the mean was performed using the coefficient of variation (CV), which represents the ratio of the standard deviation (±) to the mean (%). If CV < 10% then the analysed data are very homogeneous in terms of dispersion, if 10%<CV<20% the data are homogeneous, and if CV > 20% then the data are not homogeneous, so they do not behave uniformly in relation to the characteristic studied.
All data were reported as means of a minimum of three replicates, with the standard deviation (±) specified.
RESULTS AND DISCUSSION
Analysis of the climatic data
In 2023, during the summer months, the values of the average temperatures recorded in the air were slightly higher than the normal values, the summer being considered “hot” from a thermal point of view. The average air temperature during the vegetation period recorded values close to the normal only in the months of June and July (20.3oC, in June and 23.0oC, in July). August of 2023 was the warmest so far with a monthly average of 24.6oC, exceeding the multi-year average of 21.0oC by more than 3oC. The maximum air temperature in 2023 was recorded in August (38.2oC).
Global, active and useful thermal balance values were lower than the multi-annual averages in April and May and much higher in July, August and September, with a maximum in August, when the global and active heat balance value was 762.3oC, and the value of the useful heat balance was higher by more than 100oC, compared to the multiannual value (328.6oC) (data not shown).
During the vegetation period of 2023, the amount of precipitation (rainfall) was unevenly distributed, 349.3 mm. The lowest amounts of precipitation were recorded in the months of September (8.6 mm) and August (14.6 mm). The most abundant precipitation was recorded in the months of April, 157.7 mm, and July, 107.8 mm (Table 2). During the vegetation period, 11 days with rain, in amounts greater than 10 mm, were recorded. The average air temperature during the ripening period had a positive influence on the phenophases of grape veraison and maturation, the number of days with temperatures above 30°C in the 3 months being 38 days. In August, were recorded 21 days with temperatures higher than 30°C, increasing the accumulation of sugars and phenolic compounds in grapes. Moreover, insolation, assessed by the number of hours of sunshine, recorded a maximum value in August (294.0 h), also contributing to the accumulation of sugars and phenolic compounds in grapes. The characterisation of the climatic potential of a vineyard or wine-growing center can be achieved with the help of synthetic indicators, which integrate the action of two or three climatic factors. The value of the cool nights index was obtained by the average of the minimum temperatures in September, the value obtained was 14.3, corresponding to the climate class with temperate nights IF2 (Table 3).
The real heliothermic index (IHr) had a value of 2.43, falling within the limits described in the specialized literature (1.35 and 2.70) and indicating a year with rich heliothermic resources.
The hydrothermal coefficient (CH) obtained through the correlation between temperature and precipitation, which indirectly characterizes the degree of humidity or dryness of a region, had a value of 1.07, indicating that the year 2023 is a dry year, with insufficient water regime for optimal requirements of the vine. For the year 2023, the viticultural bioclimatic index (Ibcv) had the value of 8.30, the heliothermic resources being sufficient for the development in good conditions of the phenophases of the vines in the Copou Iași viticultural centre.
The value of the oenoclimatic suitability index (IAOe) (4592.0) recorded in 2023 places the Copou Iași vine-growing centre in the area with medium favourability for the production of red wines, in which favourable conditions are met only in certain years (Dumitriu, 2008).
Physico-chemical determinations
The leaf is the main vegetative organ of the plant, with multiple physiological functions, having an essential contribution in achieving an adequate production (Burzo et al., 2005). For grapevine, the leaf shows different characters from one variety to another, thus constituting basic elements in the practical identification of the varieties.
The multiple characteristic elements of morphology and anatomy of the leaves (the size of the limb, the degree of sectioning, the thickness of the mesophyll, the epidermis and the cuticle) generates particularities in the photosynthetic activity of the plants. In order to determine how the main biometric characteristics of the leaves influence the assimilatory pigment content of the leaves, their size and shape was analysed during the vegetation period (Aruani et al., 2015).
The area of the leaves showed a positive evolution during the vegetative phases, with important values recorded with the growth of shoots and flowering (Table 4).
Thus, the leaf area varied between 2247±67 mm2 (‘Transilvania’ grape variety) after bud burst and 19916±159 mm2 (‘Milcov’) during veraison. Until the leaves fall, there is a gradual decrease in the area of the leaves (12683±117 mm2 for ‘Purpuriu’ variety), fact correlated with the reduction of the leaf moisture.
The data previously reported by the specialized literature show a sigmoidal type of leaf growth dynamics, confirming the data recorded in the case of the varieties studied.
Thus, according to Burzo et al. (2005), in the first days after formation, the rate of growth is low, it intensifies after about 10 days from the appearance, subsequently a decrease in the rate of leaf growth was observed. According to Burzo (2014) the observed changes are attributed to changes in the hormonal balance, the auxin/gibberellin ratio being monitored in particular.
The physico-mechanical characteristics of the grapes are determined by those of the elements that compose them. The qualitative value of the grapes is appreciated both according to the chemical composition of the berries and the physical composition. The main physico-mechanical characteristics of the grapes determined at full maturity are showed in Table 5.
The size of the grape, the size of the berry, the presence and number of seeds, the ratio between the morphological parts of the berry, the condition of the rachis, all are criteria for assessing the quality of table grapes and which, as a result, are of particular importance in the process of clonal selection and the creation of new varieties that capitalize on the valuable characters of the autochthonous genotypes.
From the analysis of the recorded data, it is found that the ‘Purpuriu’ variety had the smallest grapes (117.15±7.57 g), while the highest values were recorded for the ‘Cetățuia’ variety (278.27±7.67 g).
The weight of 100 berries indicates that the varieties ‘Purpuriu’ (289.10±10.68 g), ‘Milcov’ (297.70±23.38 g) and ‘Radames’ (299.27±14.19 g) varieties had medium sized berries, the rest of the varieties showing large berries, excluding ‘Splendid’ (630.49±38.21 g) and ‘Transilvania’ (875.17±43.96 g) which had very large berries.
The weight of the rachis had the lowest values for the ‘Purpuriu’ variety (1.88±0.16 g), with the highest values in the ‘Coarnă neagră selecționată’ variety (7.51±0.49 g). Usually, rachis weight decreases from veraison and maturation, due to its dehydration, losing moisture through evaporation and transpiration, usually to the advantage of the berries.
As for the number of seeds in the berry, it is found that, being table grapes, there were between 1-3 seeds, with the exception of the ‘Cetățuia’ variety where the average number of seeds was 3.33±0.58.
It is desirable that seeds number to be as low as possible, their consumption being thus more hygienic and more appreciated by consumers.
The structural index, representing the ratio between the mass of the berries and the mass of the rachis, presented higher values the more the grapes of the analysed variety were better constituted, having a higher berry yield.
In the given conditions, at technological maturity, the value of this index fell between 25.93 g for ‘Coarnă neagră selecționată’, and the highest values were found for the two grapevine varieties with complex resistances Purpuriu (61.31) and ‘Radames’ (58.04), followed by ‘Cetățuia’ variety (56.73).
The berry index, calculated as the number of berries in 100 grams of grapes, showed the highest values in the case of ‘Purpuriu’ variety (34.72), followed by the ‘Radames’ (34.36) and ‘Milcov’ (33.11), varieties with medium, but more numerous berries. The lowest value was presented by the ‘Transilvania’ variety, which also has the largest berries, the value of this index being 11.57.
The composition index, as a ratio of the mass of the pulp to the sum of the mass of the skin and that of the seeds, was recorded with minimum values in varieties with medium sized berries and more seeds, such as ‘Milcov’ (4.30) and ‘Purpuriu’ (4.65), while in the varieties with large berries, consistent flesh and few seeds, the values were higher, as was the case of ‘Splendid’ (10.84) and ‘Transilvania’ (12.09).
During the period of vegetation (physiologically active), during which the process of chlorophyll assimilation is also carried out, the leaf apparatus goes through various transformations (appearance, development, partial or total loss, change in pigmentation) (Keller, 2010).
Chlorophyll pigments form the receiving antennae and reaction centres of the two photosynthetic systems, participating in the photosynthesis process (Davies, 2004; Delian et al., 2012).
In specialized literature, the content of chlorophyll pigments and carotenoids in leaves of the species V. vinifera L. was reported to be between 1.05 and 1.58 mg/g, respectively between 0.33 and 0.65 mg/g leaf (Acatrinei and Andor, 2006; Burzo et al., 2005).
Foliar carotenoids (carotenes and xanthophylls) are gradually accumulated during the vegetation period, with a more important increase in the flowering phenophase when there was a doubling of the amounts in most of the varieties studied (mean value 0.72±0.11 mg/g), a phenomenon followed by a slow biosynthesis of carotenoids until the phenophase of grape maturation (Table 6).
According to Burzo et al. (2005), the carotenoid content of the leaves has a particular dynamic, with lower values during the flowering period, subsequent growth until veraison and then a decrease in concentration during September. Also, previous research has reported that young leaves show reduced photosynthetic activity, with the concentration of photosynthetic pigments reaching a maximum in leaves of 20-30 days, and more than that, with a continuous accumulation until the grape veraison (Matile and Hortensteiner, 1999; Keller, 2010; Burzo, 2014).
In some varieties such as ‘Someşan’, ‘Napoca’ and ‘Splendid’, the carotenoid content was constant during the ripening of the grapes or even decreased, confirming the need to know these parameters through the prism of the high variability of the results obtained under the influence of the genetic factor.
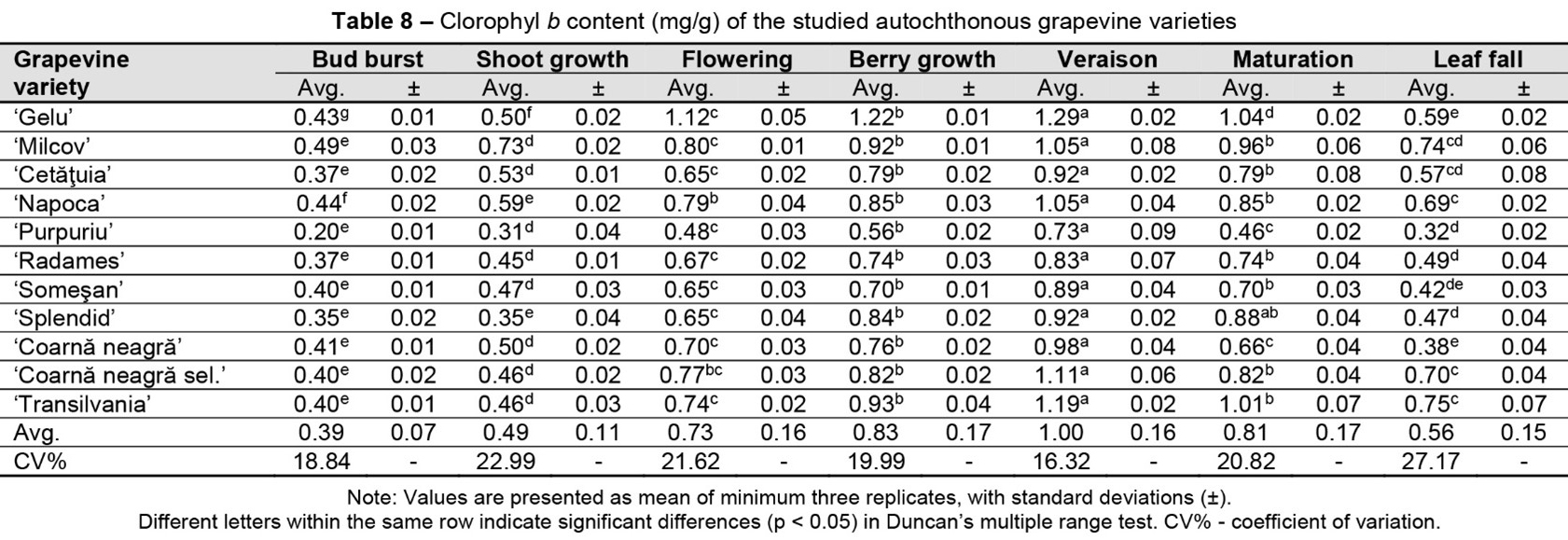
In the case of chlorophylls a (Table 7) and b (Table 8), the average values increased up to a maximum recorded at grape veraison, after which they showed a decrease in concentration.
‘Purpuriu’ variety presented the lowest concentrations of chlorophyll a and b, the values being included in the range of 0.2 (bud burst) – 0.73 mg/g (veraison).
As in the case of carotenoids, a significant increase in the total concentration of chlorophyll pigments was recorded in the interval between the phenophase of active growth of the shoots and flowering, the subsequent accumulations during the growth of the berries and up to veraison being lower.
Grapevine is a plant adapted to sun or semi-shade conditions (Warren, 2013). Mittal et al. (2011), specify that the ratio between chlorophyll a/b varies between 2.0 and 3.2 for plants adapted to shade conditions and 3.5-4.9 for plants adapted to insolation conditions.
According to Toma and Jităreanu (2007), the chlorophyll a/b ratio for Vitis vinifera L. varieties is maximum at the beginning of the vegetation period, reaching up to 3/1 and decreases during the ripening period of the grapes, while the chlorophyll/carotenoids ratio can register values of 4/1.
During the vegetation period, the values of the chlorophyll a/b ratio in the leaves of the analysed autochthonous table grape varieties were specific to each genotype and to each vegetative phase, the average values increasing continuously until the end of the analysed period, reaching values between 2.00 and 3.24 at grape maturation. The highest values of the chlorophyll a/b ratio were recorded during the veraison phase of ‘Someşan’ variety (3.28) and during grape maturation at the ‘Coarnă neagră’ variety (3.24) (Table 9).
According to Gross (1991) and Willows (2004), in mature leaves, chlorophyll a is the most abundant pigment, transforming light energy into chemical energy, while chlorophyll b is an accessory pigment, having the ability to absorb light, the two types coexisting in a ratio of approximately 3 to 1 (Neamţu, 1997). In specialized studies, it was often reported that the chlorophyll a/b ratio in leaves is at its maximum at the beginning of the vegetation period (Lichtenthaler et al., 1982; Toma and Jităreanu, 2007). In the studied Romanian varieties, this ratio generally had lower values than those presented in the international literature for this species, but comparable to the data reported through the research carried out in Romania and even in the study area (Iasi vineyard) (Acatrinei and Andor, 2006). Thus, it can be concluded that the low values of the chlorophyll a/chlorophyll b ratio could be a peculiarity of the autochthonous varieties, especially of those grown in the N-E area of the country, an area with a continental climate characterized by the alternation of hot and cold days, with a frequent lack of humidity, especially in hilly areas, which often causes blockages in the photosynthesis process (Cotea et al., 2000). Also, according to Hopkins and Hüner (2009), photosynthetic system (SF) 1 typically has a chlorophyll a/b ratio of about 4/1, and SF 2 contains 50 to 60% of the total chlorophyll, with a chlorophyll a/ b ratio of about 1/2, the majority being chlorophyll b and carotenoids (xanthophylls). These data, together with those reported by Jiang et al. (2006), who showed that the probability of energy transfer between SF 2 units in young leaves was significantly higher than in mature leaves, and that very few SF 1 develop in the initial phases of leaf growth may also explain the lower values of the chlorophyll a/b ratio of the leaves in the first phases of vegetation. The chlorophyll/carotenoid ratio showed specific values for each vegetative phase. The initial (bud burst) and final (grape maturation) phenophases presented the lowest values of this ratio, due to the lower content of chlorophylls and higher concentrations of carotenoids (Table 10).
It was observed that in the first phenophases the variability of the values was the lowest (CV%=6.9-7.3), the ratio of chlorophylls/carotenoids varying within very narrow limits between the varieties analysed. After veraison, the values of this ratio varied significantly in most varieties, as a result of the initiation of the gradual degradation of chlorophyll.
Table 9
Values of chlorophyll a / chlorophyll b ratio in leaves of the studied autochthonous grapevine varieties
|
Grapevine variety |
Bud burst |
Shoot growth |
Flowering |
Berry growth |
Veraison |
Maturation |
Leaf fall |
|
‘Gelu’ |
1.21 |
1.72 |
1.72 |
1.85 |
2.17 |
2.13 |
1.00 |
|
‘Milcov’ |
1.06 |
1.08 |
2.16 |
2.07 |
1.89 |
2.00 |
1.24 |
|
‘Cetăţuia’ |
1.41 |
1.36 |
2.77 |
2.35 |
2.91 |
2.46 |
1.70 |
|
‘Napoca’ |
1.18 |
1.29 |
2.11 |
2.35 |
2.09 |
2.34 |
1.09 |
|
‘Purpuriu’ |
2.35 |
2.23 |
2.65 |
2.36 |
2.03 |
2.98 |
1.72 |
|
‘Radames’ |
1.38 |
1.73 |
2.27 |
2.53 |
2.49 |
2.66 |
1.39 |
|
‘Someşan’ |
1.30 |
1.66 |
2.31 |
2.71 |
3.28 |
2.71 |
1.60 |
|
‘Splendid’ |
1.51 |
2.14 |
2.38 |
2.49 |
2.64 |
2.48 |
1.66 |
|
‘Coarnă neagră’ |
1.27 |
1.60 |
2.43 |
2.39 |
2.49 |
3.24 |
2.08 |
|
‘Coarnă neagră sel.’ |
1.28 |
1.74 |
2.19 |
2.50 |
2.14 |
2.68 |
1.20 |
|
‘Transilvania’ |
1.30 |
1.74 |
2.30 |
2.23 |
2.24 |
2.14 |
0.95 |
|
Avg. |
1.39 |
1.66 |
2.30 |
2.35 |
2.40 |
2.53 |
1.42 |
|
SD (±) |
0.34 |
0.34 |
0.28 |
0.23 |
0.42 |
0.38 |
0.36 |
|
CV% |
24.64 |
20.32 |
12.00 |
10.00 |
17.54 |
14.88 |
25.23 |
Note: Avg. – average values; SD (±) – standard deviation; CV% – coefficient of variation
Table 10
Values of the chlorophyll/carotenoid ratio in leaves of the studied autochthonous grapevine varieties
|
Grapevine variety |
Bud burst |
Shoot growth |
Flowering |
Berry growth |
Veraison |
Maturation |
Leaf fall |
|
‘Gelu’ |
3.17 |
3.16 |
3.35 |
3.59 |
4.05 |
2.99 |
2.07 |
|
‘Milcov’ |
3.16 |
3.53 |
3.42 |
3.71 |
3.33 |
2.74 |
2.16 |
|
‘Cetăţuia’ |
2.87 |
2.98 |
2.66 |
2.82 |
3.08 |
2.28 |
1.97 |
|
‘Napoca’ |
3.00 |
3.00 |
3.42 |
3.52 |
2.82 |
2.47 |
1.97 |
|
‘Purpuriu’ |
2.39 |
2.94 |
3.24 |
3.42 |
2.57 |
1.93 |
1.28 |
|
‘Radames’ |
3.03 |
2.86 |
3.17 |
3.43 |
3.30 |
2.91 |
1.92 |
|
‘Someşan’ |
2.88 |
2.98 |
3.52 |
3.42 |
3.40 |
2.32 |
1.38 |
|
‘Splendid’ |
2.84 |
2.75 |
3.49 |
3.45 |
3.76 |
3.48 |
2.12 |
|
‘Coarnă neagră’ |
2.82 |
3.17 |
3.24 |
3.27 |
3.56 |
2.83 |
1.92 |
|
‘Coarnă neagră sel.’ |
2.84 |
3.00 |
3.46 |
3.50 |
3.78 |
3.11 |
2.75 |
|
‘Transilvania’ |
2.88 |
2.86 |
3.39 |
3.19 |
3.50 |
2.83 |
1.95 |
|
Avg. |
2.90 |
3.02 |
3.31 |
3.39 |
3.38 |
2.72 |
1.95 |
|
SD (±) |
0.21 |
0.21 |
0.24 |
0.24 |
0.43 |
0.44 |
0.39 |
|
CV% |
7.22 |
6.96 |
7.29 |
6.96 |
12.84 |
16.10 |
19.85 |
Note: Avg. – average values; SD (±) – standard deviation; CV% – coefficient of variation.
In the usual evaluation of the chemical composition established by laboratory analysis, it always refers either to the dry substance or to the substance with a certain moisture. Secondly, the amount of water in the grapes depends on their stability and their ability to be stored for shorter or longer periods of time.
Grapes are among the sweetest fruits, with a content of sugars (carbohydrates) that can reach 20% of the fresh weight at full maturity (Teissedre and Chervin, 2011). The accumulation of sugars is higher with the duration and intensity of insolation (sugars are the result of the photosynthesis process), because the determining element is the light and not the temperature.
An excess of heat can, on the other hand, lead to a stagnation of sugar accumulation, thus influencing the normal evolution of grape ripening (Cotea et al., 2000).
Grape juice contains representatives from all groups of carbohydrates, the dominant class being the oses. Among the monosaccharides identified in grapes, hexoses are dominant, with glucose and fructose as the main representatives (over 95% of the total carbohydrates).
Carbohydrates (sugars) represent the compounds with the highest proportion in the soluble dry solids, at grapes maturity the content of glucose and fructose being approximately equal (Burzo et al., 2005).
Table 11 shows the content of soluble dry solids (°Bx) of the grapes during ripening, the positive change of the values towards ripening being observed, the berries of the ‘Radames’ variety showing significant differences in all phases of the experience and reaching up to 23.50 °Bx (>230 g/L sugars) when the grapes reach the maturation phase.
Table 11
The soluble dry solids content (°Bx) of the grapes during ripening
|
Grapevine variety |
Veraison |
After 15 days |
After 25 days |
Grape maturation |
|
‘Coarnă neagră’ |
11.25±0.12d |
12.97±0.16c |
14.36±0.30b |
18.52±0.16a |
|
‘Transilvania’ |
11.65±0.24d |
14.02±0.20c |
15.98±0.21b |
19.83±0.20a |
|
‘Coarnă neagră sel.’ |
11.98±0.08d |
13.56±0.42c |
14.75±0.28b |
18.12±0.14a |
|
‘Purpuriu’ |
12.05±0.42d |
15.46±0.18c |
16.80±0.12b |
19.34±0.30a |
|
‘Someşan’ |
12.07±0.20d |
13.58±0.10c |
14.60±0.08b |
18.64±0.24a |
|
‘Splendid’ |
12.09±0.19d |
14.64±0.44c |
17.09±0.11b |
18.38±0.12a |
|
‘Napoca’ |
12.28±0.22d |
15.26±0.26c |
17.84±0.18b |
18.70±0.40a |
|
‘Milcov’ |
12.38±0.28d |
14.92±0.20c |
17.48±0.40b |
19.18±0.28a |
|
‘Cetăţuia’ |
12.46±0.08d |
13.47±0.31c |
17.61±0.28b |
18.98±0.30a |
|
‘Gelu‘ |
13.03±0.12d |
13.69±0.18c |
17.01±0.12b |
20.33±0.17a |
|
‘Radames’ |
13.33±0.10d |
16.46±0.32c |
20.10±0.20b |
23.50±0.44a |
|
Avg. |
12.23d |
14.37c |
16.69b |
19.48a |
|
SD (±) |
0.58 |
1.07 |
1.70 |
1.57 |
|
CV% |
4.47 |
7.42 |
10.16 |
8.03 |
Note: Values are presented as mean of minimum three replicates, with standard deviations (±). Different letters within the same row indicate significant differences (p < 0.05) in Duncan’s multiple range test. CV% – coefficient of variation.
Table 12
Evolution of grapes acidity (g/L tartaric acid) during the maturation phenophase
|
Grapevine variety |
Veraison |
After 15 days |
After 25 days |
Grape maturation |
|
‘Coarnă neagră’ |
14.09±0.08a |
11.76±0.20b |
9.46±0.14c |
7.13±0.12d |
|
‘Transilvania’ |
20.79±0.31a |
10.66±0.20b |
8.66±0.14c |
6.50±0.28d |
|
‘Coarnă neagră sel.’ |
17.14±0.16a |
13.14±0.20b |
10.51±0.19c |
7.73±0.17d |
|
‘Purpuriu’ |
20.32±0.28a |
15.93±0.20b |
10.60±0.20c |
7.90±0.19d |
|
‘Someşan’ |
15.64±0.10a |
10.21±0.20b |
7.15±0.15c |
5.93±0.24d |
|
‘Splendid’ |
21.33±0.27a |
11.56±0.20b |
9.43±0.17c |
6.37±0.18d |
|
‘Napoca’ |
16.67±0.23a |
11.28±0.20b |
8.06±0.14c |
6.50±0.11d |
|
‘Milcov’ |
17.41±0.49a |
12.27±0.20b |
9.84±0.16c |
7.37±0.16d |
|
‘Cetăţuia’ |
21.74±0.36a |
14.59±0.20b |
9.16±0.14c |
5.20±0.10d |
|
‘Gelu’ |
14.04±0.16a |
9.14±0.20b |
6.58±0.12c |
4.94±0.12d |
|
‘Radames’ |
19.74±0.44a |
10.75±0.20b |
8.04±0.21c |
5.55±0.15d |
|
Avg. |
18.08a |
11.94b |
8.86c |
6.47d |
|
SD (±) |
2.84 |
1.97 |
1.30 |
1.00 |
|
CV% |
15.70 |
16.54 |
14.69 |
15.45 |
Note: Values are presented as mean of minimum three replicates, with standard deviations (±). Different letters within the same row indicate significant differences (p < 0.05) in Duncan’s multiple range test. CV% – coefficient of variation.
The lowest content in soluble dry solids at full maturity was recorded in the grapes of the ‘Coarnă neagră selecţionată’ variety of 18.12 °Bx (170 g/L sugars).
Determining the optimal moment of grape maturation by chemical methods is generally based on following the process of accumulation of sugars and decrease of acidity in the berries.
The accumulation of organic acids in the vacuoles of the berry’s cells took place in the early stages of grape maturation (veraison), when the maximum values were recorded for all the analysed varieties.
At ‘Gelu’ variety, the acidity of the juice was deficient, the values expressed in tartaric acid did not exceed 4.94 g/L when the grapes were ripe for consumption.
By comparison, varieties such as ‘Coarnă neagră’, ‘Coarnă neagră selecționată’, ‘Purpuriu’ or ‘Milcov’, maintained their high acidity at full maturity, with values of over 7.00 g/L tartaric acid (Table 12).
The acidity of the juice decreased considerably after 15 days from the starting of the veraison phenophase, by about 33%, and after 25 days by about 52%, so that upon grape ripening the decrease in the total acidity compared to veraison exceeded 64%.
CONCLUSIONS
The experimental data obtained indicate the special value of the autochthonous table grape genotypes analysed, through the morpho-structural characteristics and chemical composition, and justify their use as a source of germplasm in future breeding programes.
The area of the leaves showed important increases with the development of the shoots and flowering, a slight decrease of this parameter being recorded in the interval of grape veraison – maturation.
The group of varieties with large leaves was represented by ‘Gelu’ and ‘Milcov’ (>19 cm2), the varieties ‘Someşan’ and ‘Purpuriu’ presenting the smallest area of the leaf.
The average weight of a grape showed a continuous increasing trend until ripening, with a maximum in the ‘Transilvania’ and ‘Cetățuia’ varieties (>250 g), these varieties being able to be used as a source of autochthonous germplasm in order to improve the weight of the grapes.
The weight of 100 berries was the highest in the ‘Transilvania’ variety (>850 g), while the rachis weight was the lowest in the case of Purpuriu variety (< 2 g).
A high degree of non-uniformity of the berries, analysed on the basis of the standard deviation of the average weight of a berry, was recorded for ‘Transilvania’, ‘Splendid’ and ‘Someşan’ varieties.
In the interval between the phenophases of active shoot growth and flowering was registered a doubling of the concentrations of chlorophylls (a and b) and carotenoids (c and x) in most of the studied genotypes, a phenomenon followed by a slow biosynthesis until in the phenophase of grape maturation.
The concentration of foliar carotenoids was the highest in the ‘Cetățuia’ variety, at grape maturity (1.2 mg/g), while the chlorophyll a and b content was the highest in leaves of the ‘Gelu’ variety (2.22, respectively 1.3 mg/g).
The values of the chlorophyll a/chlorophyll b and chlorophyll/ carotenoids ratios showed differences regarding values, depending on the biological particularities of the investigated genotypes and phenophase, the average values gradually increasing until the end of the analysed period.
The total content of dry solids of grapes showed lower values in the veraison phenophase (11.25-13.33°Bx), following an upward trend in parallel with the advancement of the ripening phenomenon, at grape maturity the values being between 18.12 and 23.50 °Bx, respectively a concentration of sugars of 175 g/L for the ‘Coarnă neagră selecţionată’ variety and 235 g/L for the ‘Radames’ variety.
In the case of ‘Gelu’ variety, the acidity of ripened grapes was deficient (4.94 g/L tartaric acid), negatively affecting the glucoacidimetric index. By comparison, varieties such as ‘Purpuriu’, ‘Milcov’, ‘Coarnă neagră’, ‘Coarnă neagră selecționată’, maintained a high acidity in matured grapes (~7.0 g/L tartaric acid).
Considering the results obtained, is confirmed that the autochthonous varieties constitute an exceptional genetic treasure, and depending on the desired character, being the only resources capable of reacting favourably to the climatic adversities that are increasingly frequent in recent years in the vine-growing areas.
Author Contributions: Conceptualization: LR and VRF; methodology: LCC, VRF and RMF; analysis LR, RMF and VRF; investigation: RB and MM; writing: LR, LCC and VRF; review: VRC and MM. All authors declare that they have read and approved the publication of the manuscript in this present form.
Funding: This paper was published under the frame of ADER 6.1.7./20.07.2023 project, financed by the Ministry of Agriculture and Rural Development of Romania.
Conflicts of Interest: All authors declared no conflict of interest.
REFERENCES
Acatrinei, L.; Andor, I. Physiological researches at varieties of grapes in Cotnari vineyards under pesticides treatments. Scientific works USAMV Iaşi, Horticultură. 2006, 49, 317-322.
Amăriucăi, M.; Machidon, O. Characterization of some climatic parameters recorded at the Meteorological Stations Cotnari, Iasi and Barlad, during the period 1990-2011 (in Romanian). Internal project report POS-CCE 653, USAMV Iasi, 2012, 25-30.
Aruani, C.; Sotés, V.; Eibach, R.; De la Fuente, L.M.; Bois, B. Vine varieties: Origin, evolution and identification (in French). Oenologists’ Review. 2015, 157, 21-22.
Baggiolini, M. The benchmark stages in the annual development of the vine and their practical use (in French). Romande Review of Agriculture and Viticulture. 1952, 8, 4-6.
Bortoló, G. The landscape of vines and wine (in French). Wine Territories. 2012, 4, 1-17. https://doi.org/10.58335/territoiresduvin.1392
Bosoi, I.; Rotaru, L.; Pușcalău, M.; Colibaba, C. Vine varieties for white wines in the climate context of the Odobesti Vineyard, Romania. Journal of Applied Life Sciences and Environment. 2022, 55, 62-74. https://doi.org/10.46909/alse-551046
Brault, C. Grapevine breeding optimization with genomic and phenomic predictions (in French). PhD Thesis, University of Agricultural sciences, Montpellier SupAgro, France, 06/12/2021.
Burzo, I. Climatic changes and the effects on horticultural plants (in Romanian). Publishing House Sitech, Craiova, Romania, 2014, pp. 89-94.
Burzo, I.; Dejeu, L.; Serdinescu, A.; Bădulescu, L. Physiology of cultivated plants. Vol. III, Grapevine physiology (in Romanian). Publishing House Elisavaros, Bucharest, Romania, 2005, pp. 256-301.
Constantinescu, G. Ampelography of Romania (in Romanian), First edition. Publishing House Romanian Academy, Bucharest, Romania, 1970, pp. 33-66.
Cotea, D.V.; Barbu, N.; Grigorescu, C.; Cotea, V.V. Vineyards and wines of Romania (in Romanian). Romanian Academy Publishing House, Bucharest, Romania, 2000, pp. 255-270.
Davies, K.M. Plant pigments and their manipulation. In Annual plant reviews. Volume 14, CRC Press, Boca Raton, Florida, U.S.A., 2004, pp. 1-23.
Delian, E.; Bădulescu, L.; Dobrescu, A. Plant physiology. Practical work (in Romanian). Second edition. University Publishing House, Bucharest, Romania, 2012, pp. 147-158.
D’onofrio, C. Introgression Among Cultivated and Wild Grapevine in Tuscany. Frontiers in Plant Science. 2020, 11. https://doi.org/10.3389/fpls.2020.00202
Dumitriu, I.C. Viticulture (in Romanian). Publishing House Ceres, Bucharest, Romania, 2008, pp. 185-188.
Eichhorn, K.W.; Lorenz, D.H. Phenological Development Stages of the Grapevine (in German). Deutsche Pilanzenschutztagung. Braunschweig, Germany, 1977, 29, pp. 119-120.
Filimon, R.M.; Damian, D.; Nechita, A.; Filimon, V.R. Studies on the behavior of a new wine grape hybrid elite in the climatic conditions of the Copou-Iasi viticultural center. Romanian Journal of Horticulture. 2021, 2, 131-136. https://doi.org/10.51258/RJH.2021.17
Filimon, V.R.; Filimon, R.M.; Patraş, A.; Rotaru, L. Grape quality and ornamental potential of interspecific cultivars for temperate climate vineyards. Journal of Horticultural Science and Biotechnology. 2019, 95, 1-11. https://doi.org/10.1080/14620316.2019.1631127
Gross, J. Pigments in vegetables: chlorophylls and carotenoids. Van Nostrand Reinhold, New York, U.S.A., 1999, pp. 54-66.
Hopkins, W.G.; Hüner, P.A.N. Introduction to plant physiology. John Wiley & Sons, Inc., USA. 2009, pp. 69-75.
Jiang, C.D.; Shi, L.; Gao, H.-Y.; Schansker, G.; Tόth, S.Z.; Strasser, R.J. Development of photosystems 2 and 1 during leaf growth in grapevine seedlings probed by chlorophyll a fluorescence transient and 820 nm transmission in vivo. Photosynthetica. 2006, 44, 454-463. https://doi.org/10.1007/s11099-006-0050-5
Keller, M. The science of grapevines. In Anatomy and physiology, Second Edition, Academic Press, Elsevier Inc., 2010, pp. 23-44.
Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In Current Protocols in Food Analytical Chemistry (ed. Wrolstad R.E.). John Wiley & Sons, New York, U.S.A., Inc. 2001, F4.3.1-F4.3.8.
Matile, P.; Hortensteiner, S. Chlorophyll degradation. Annual Review of Plant Physiology and Plant Molecular Biology. 1999, 50, 67-95. https://doi.org/10.1146/annurev.arplant.50.1.67
Mittal, S.; Kumari, N.; Sharma, V. Differential responses of seven contrasting species to high light using pigment and chlorophyll a fluorescence. J. Stress Physiol. Biochem. 2011, 7(2), 20-33.
Mustea, M. Viticulture. Biological base, establishing and managing young vine plantations (in Romanian). Publishing House Ion Ionescu de la Brad, Iaşi, Romania, 2004, pp. 214-218.
Neamţu, G. Food biochemistry (in Romanian). Publishing house Ceres, Bucharest, Romania, 1997, pp.36-42.
International Organisation of Vine and Wine – OIV. Compendium of international methods of wine and must analysis. Vol. 1. International Organization of Vine and Wine (OIV), Paris, France, 2021.
Ollat, N.; Cookson, S.J.; Destrac-Irvine, A.; Lauvergeat, V.; Ouaked- Lecourieux, F.; Marguerit, E.; Barrieu, F.; Dai, Z.; Duchêne, E.; Gambetta, G.A. Grapevine adaptation to abiotic stress: An overview. Acta Horticulturae. 2019, 1248, 497-512. https://doi.org/10.17660/ActaHortic.2019.1248.68
Rotaru, L. Grapevine varieties for wine production (in Romanian). Publishing House Ion Ionescu de la Brad, Iaşi, Romania, 2009, pp. 59-62.
Sestraş, R. Breeding of horticultural species (in Romanian). Publishing house Academic Pres, Cluj-Napoca, Romania, 2004, pp. 129-135.
Teissedre, P.L.; Chervin, C. Grape. In Health-promoting properties of fruit and vegetables. Editor Terry, L.A. CAB International, USA, 2011, pp.145-155.
Toma, L.D.; Jităreanu, D. Plant physiology (in Romanian). Publishing House Ion Ionescu de la Brad, Iaşi, Romania, 2007, pp. 89-90.
Warren, P.L. Landscape vines for Southern Arizona. The University of Arizona – College of Agriculture and Life Sciences – Cooperative Extension, 2013. http://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1606.pdf
Willows, R.D. Chlorophylls. In Plant pigments and their manipulation. Annual plant reviews. Volume 14. Editor Kevin M. Davies, Crop & Food Research Palmerston North New Zealand. CRC Press, Boca Raton, Florida, USA, 2004, pp. 23-57.
Academic Editor: Dr. Mihaela Roșca
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Bernardis Roberto Renato, Colibaba Lucia Cintia, Filimon Roxana Mihaela, Filimon Vasile Răzvan, Mustea Mihai, Rotaru Liliana