I. Kareem, E.A. Akinrinde, Y. Oladosu, S.Y. Abdulmaliq, E.K. Eifediyi, S.Y. Alasinrin, S.A. Kareem
ABSTRACT. To guard against soil phosphorus (P) toxicity in tuber production and have optimum tuber yield at lesser cost of P-fertilization, better understanding of the dynamics of phosphorus release in sandy loamy soil is inevitable. Therefore, this work was carried out to investigate the trend of P-release from time of application to its optimum release and its effect on sweet potato growth and tuber production. To achieve this, a 5-week incubation study under laboratory conditions was carried out to study P-release dynamics using different P sources. Similar experiment was conducted on the field using the same P sources and application rate to monitor the influence P-release rate on sweet potato production. Data on number of leaves, vine length, tuber yield, soil extractable phosphorus and phosphorus uptake of the plants were taken. Relationships between P-uptake and tuber yield, number of leaves, vine length were also established. It was found that the trend of phosphorus release was a sigmoid shape. Leaf production and vine length were improved by P-application, while yield was suppressed. It is recommended that P-fertilizer should not be applied to the soil at short intervals to avoid nutrient toxicity.
Keywords: incubation; phosphorus sources; phosphorus uptake; P-release dynamics; tuber yield.
View full article (HTML)
Soil phosphorus dynamics of sweet potato-based cropping system in a rainforest region of Nigeria
I. Kareem1*, E.A. Akinrinde2, Y. Oladosu3, S.Y. Abdulmaliq4, E.K. Eifediyi1, S.Y. Alasinrin1, S.A. Kareem5
1Department of Agronomy, University of Ilorin, Ilorin, Nigeria
2Department of Agronomy, University of Ibadan, Ibadan, Nigeria
3Institute of Tropical Agriculture, Universiti Putra Malaysia, Serdang, Selangor, Malaysia
4Department of Agronomy, Ibrahim Badamasi Babangida University, Lapai, Niger State, Nigeria
5Department of Biology, School of Secondary Education (Science Programme), Federal College of Education (Special), Oyo, Nigeria
*E-mail: abdulkareemishaaq@gmail.com
Received: Oct. 11, 2019. Revised: Dec. 14, 2019. Accepted: Dec. 28, 2019. Published online: Mar. 06, 2020
ABSTRACT. To guard against soil phosphorus (P) toxicity in tuber production and have optimum tuber yield at lesser cost of P-fertilization, better understanding of the dynamics of phosphorus release in sandy loamy soil is inevitable. Therefore, this work was carried out to investigate the trend of P-release from time of application to its optimum release and its effect on sweet potato growth and tuber production. To achieve this, a 5-week incubation study under laboratory conditions was carried out to study P-release dynamics using different P sources. Similar experiment was conducted on the field using the same P sources and application rate to monitor the influence P-release rate on sweet potato production. Data on number of leaves, vine length, tuber yield, soil extractable phosphorus and phosphorus uptake of the plants were taken. Relationships between P-uptake and tuber yield, number of leaves, vine length were also established. It was found that the trend of phosphorus release was a sigmoid shape. Leaf production and vine length were improved by P-application, while yield was suppressed. It is recommended that P-fertilizer should not be applied to the soil at short intervals to avoid nutrient toxicity.
Keywords: incubation; phosphorus sources; phosphorus uptake; P-release dynamics; tuber yield.
INTRODUCTION
Phosphorus is an essential nutrient element for plants. The key to increased agricultural production and sustainability is optimum application of essential nutrients in their right proportion with the use of appropriate method of application (Cisse and Amar, 2000). The necessity of phosphorus as a plant nutrient is emphasized by the fact that it is an essential constituent of many organic compounds that are very important for metabolic processes, blooming and root development (Purekar, 1992). In the same vein, neither plants nor animals can grow without phosphorus because it is an essential component of high energy yielding compounds, like adenosine triphosphate (ATP). It is also a component of nucleic acid like deoxyribonucleic acid (DNA), which is the seat of genetic inheritance and ribonucleic acid (RNA) which is responsible for directing protein synthesis in both plants and animals. As essential as this nutrient element is, its optimal application to the soil in many occasions does not guarantee sufficient availability of the nutrient in the soil pool. This can be the result of release rate of the element from the soil. When this happens, there is need for phosphorus addition for the maintenance of the required critical level to realize optimal yield of the target crop in agricultural production (FAO, 2008). This is so because phosphorus limits growth and yield in unfertilized soils and most especially when it is laden with calcium carbonate which makes P accessibility a difficulty by reducing its availability (Ibrikci et al., 2005). The fact that phosphorus is less available in the soil solution as a result of its slow diffusion and high degree of fixation has made it a major limiting factor for crop production (Rehman et al.,2013). This is true because uptake of phosphorus and its utilization are vital determinants of final yield of agricultural crops.
Furthermore, the use efficiency of applied P is generally very low. It ranges from 10 to 30% in the year of application. In most soils, in spite of the considerable addition of P-fertilizers, the amount available for plants is usually low since it is converted to unavailable form by its reaction with the soil constituents (Hassan et al., 2005). To elucidate how applied P behaves in the soil, Grotz and Guerinot (2002) explained that when P-fertilizer is applied, 80% of the total P available is made inaccessible through fixation as a result complexity involved in soil phosphorus chemistry while only 20% is left for plant use. Beside fixation, phosphorus is not as mobile as nitrogen and potassium and, therefore, some available phosphorus, which is not used up by sweet potato plant, may not experience leaching (Fixen and Vivekananda, 1990).
Fortified organic fertilizer sources also help in P uptake by plants. For instance when cow dung is fortified with NPK fertilizer and applied to sweet potato, there will be higher nutrient uptake of which phosphorus is part of the component. This consequently leads to resultant increase in crop yield which is attributed to significant increase in phosphorus, potassium and calcium uptake by sweet potato (Forbes and Watson, 1994). In addition to that, P-fertilizer application positively increased sweet potato productivity (Hassan et al., 2005). These increments were attributed to the beneficial effect of P-element on the activation of photosynthesis and metabolic processes of organic compounds in plants and hence increase in plant growth (Purekar, 1992). They also attributed the important role of phosphorus to its being an essential component of many organic compounds in plants, such as phospholipids, nucleic acids and nucleotides, which may indirectly reflect positively on yield (Mc Laughlin et al., 1992). Similarly, fertilization of sweet potato plants with P-fertilizer leads to significant increase in total and marketable yield (Hassan et al.,2005).
There have been efforts on determining P-release in different soil types to have full grasp of the amount of additional P required for crop production and avoid toxicity of the soil, but there is virtually none on sandy loamy soil in the rainforest region of Nigeria. Furthermore, the studies carried out were on cereals like rice and sorghum and none on root or tuber crops like sweet potato. To extend the research to root crops, this study was, therefore, conducted to determine soil phosphorus dynamics of sweet potato-based cropping system in a rain forest region of Nigeria.
MATERIAL AND METHODS
Experimental site, soil sampling and soil sample preparation
The site of this experiment was the Teaching and Research Farm of Agronomy Department, University of Ibadan, Parry Road, Ibadan, Oyo State (7.27oN 3.54oE). The temperature range was between 22oC and 28oC with the annual rainfall between1000 mm and 1600 mm. The phosphorus content of the soil used was 6.80 mg/kg. Soil samples were collected using systemic soil sampling method. Incubation study was carried on the composite sample. Part of the sample was also analysed to determine the soil nutrient composition before planting. The samples got from incubation study were air dried using sheets of paper and 2 mm sieve was used in sieving the samples.
Treatments and experimental design
The treatments used in this study were pacesetter organic fertilizer (POF) at the rate of 5 t/ha, single super phosphate (SSP) at the rate of 500 kg/ha, crystallizer at the rate of 500 kg/ha and the control. The design of this experiment was randomized complete block (RCB), replicated three times.
Field preparation
Clearing and stumping of the used field were respectively done with a cutlass and hoe to make the soil workable. Then, manual ridging was done using a hoe. This was done to make the soil conducive to tuber development. The field was finally divided into twelve plots to accommodate four treatments replicated three times in RCBD.
Planting and cultural practices
Shaba variety of sweet potato was used in this experiment. 25 cm long sweet potato vine cuttings were used. The slanting angle for placement of the vines in the soil was 45o. For proper establishment after sprouting, two parts of the length of the vines used were put under the soil. The spacing of the plants was 30 cm by 100 cm to give a population of 18 plants per plot and 33,333 plants per hectare. Fertilizer application was done at four weeks after planting using hill placement method. Weeding of the plots was done according to necessity using hand hoeing and a weed-free field was maintained throughout the experiment. In addition to hand hoeing, hand pulling was used in controlling weeds after the plant vines had covered the field. Cypermetrin was used in chasing away the grasshopper that invaded the field.
Soil incubation study
Soil incubation study was conducted to study the trend of phosphorus release in the field. To achieve this objective, new bower vessels were used in keeping well-prepared soil samples from the used field before planting. A total of twelve new bower vessels were used. One vessel was for the soil of one plot. The study was carried out in the plant physiology laboratory of the Department of Agronomy, University of Ibadan. The same treatment given to the plots on the field was applied to each of the bower vessels that represent the field plots. Each bower vessel was given the same treatment as it was applied on the represented field plot. The bower vessels were watered using 60% of the soil field capacity to ease nutrient (phosphorus) release and avoid leaching. The layout of the experiment in the laboratory was completely randomized design (CRD) because of homogeneity of the environment. Sampling started after completion of the first week and was consecutively done for five weeks.
Phosphorus extraction
After the soil samples taken had been air-dried on sheets of paper, Bray-1 method was used in phosphorus extraction while molybdenum blue procedure (Kuo, 1996) was used in determining its concentration.
Data collection
From each plot, three representative plants were randomly selected and tagged. The border rows were excluded to avoid border effect. From the representative plants, number of leaves was counted and vine length was measured from the base of the plant to the apex of the topmost leaf on the vine using a meter rule. This data collection lasted for five weeks starting from the first week after fertilizer application. At harvest, leaves of the sampled plants were collected and prepared for laboratory analysis to extract their phosphorous content using Bray-1 method while molybdenum blue procedure (Kuo, 1996) was used in determining its concentration. Phosphorus uptake was calculated as follows:
Phosphorus uptake = Plant P concentration × dry matter used (Moustakas et al., 2011).
Statistical analysis
All the data collected were statistically analysed using analysis of variance (ANOVA) with the aid of GENSTAT statistical package and significant means were separated using least significant difference (LSD) at 5% probability level.
RESULTS
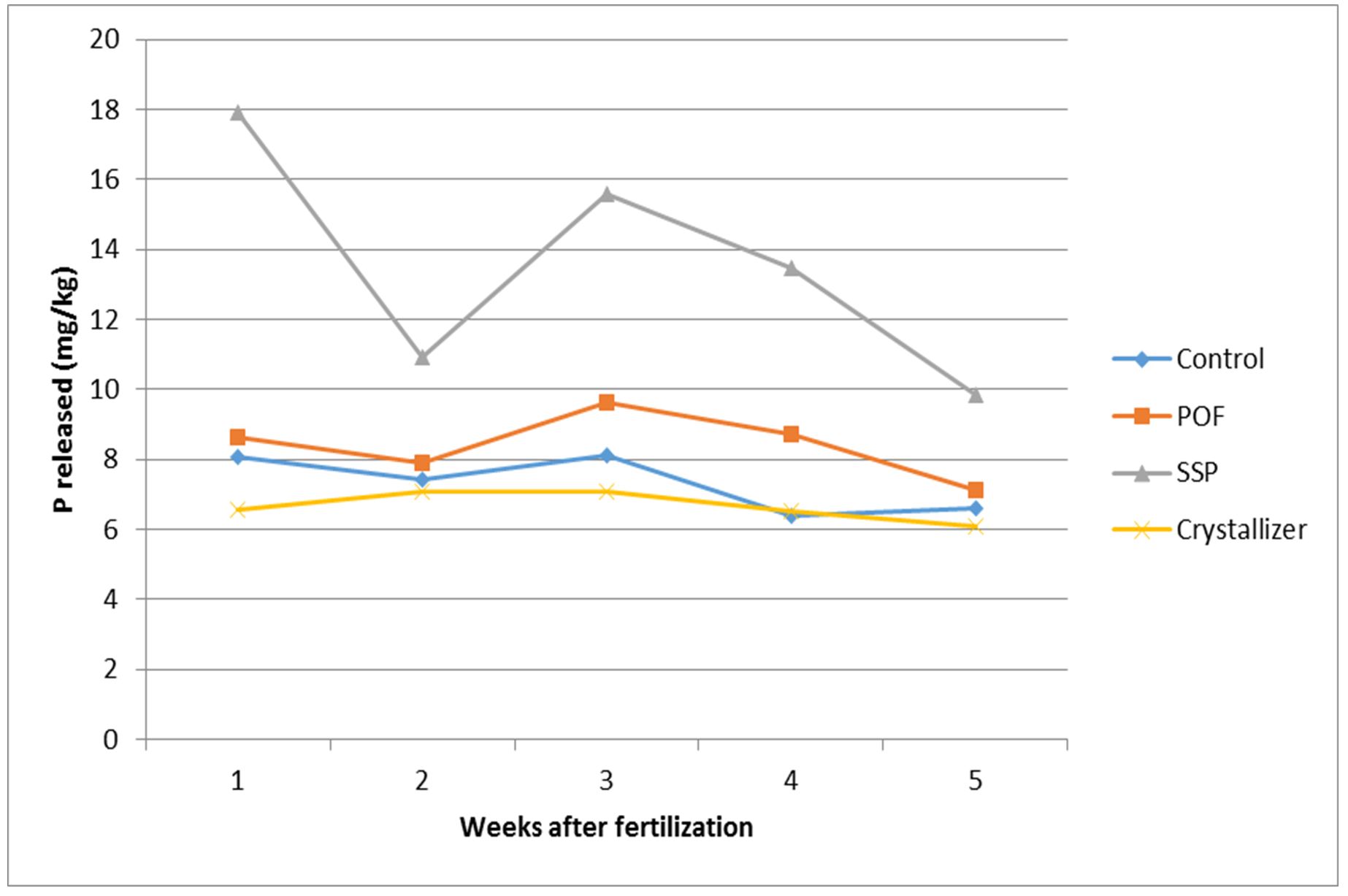
It was found that passage of time dictated the level of phosphorus found in the soil at any instance after application of phosphorus fertilizers. The trend of P-release in all the P-fertilizers used had higher P-release a week after the treatment application. After the second week, all the treatments had lower P-release than the first week.
The third week witnessed a rise again from all the treatments except crystallizer that maintained what it had in the second week. After this, the fourth and fifth weeks had a progressive decrease in P release.
The highest P-release was from SSP while the lowest P release was from crystallizer in all the treatments (Fig. 1).
Effect of P-release kinetics on sweet potato vine
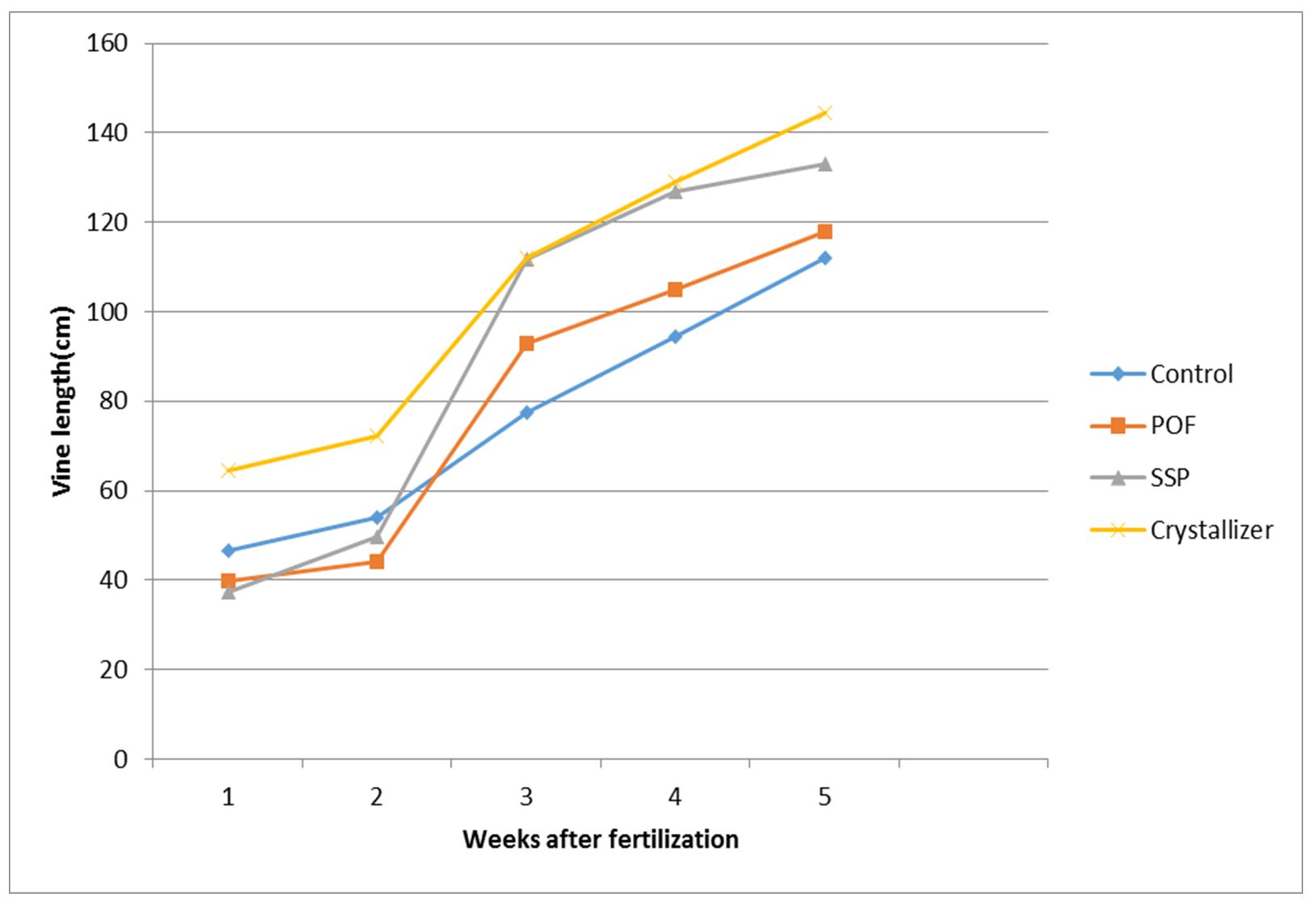
It was obvious from this experiment that the rate of P-release had impact on vine production. All the fertilizer treatments were better than the control except during the first two weeks of data taking where only crystallizer performed better than the control. The order of vine increase among the treatments in the first week after fertilization (WAF) was crystallizer > control > POF > SSP. For the second week after fertilization, the order was crystallizer > control > SSP > POF. For the third, fourth and fifth weeks after fertilization a single trend was followed. The trend was crystallizer > SSP > POF > control (Fig. 2).
Effect of P-release kinetics on number of leaves
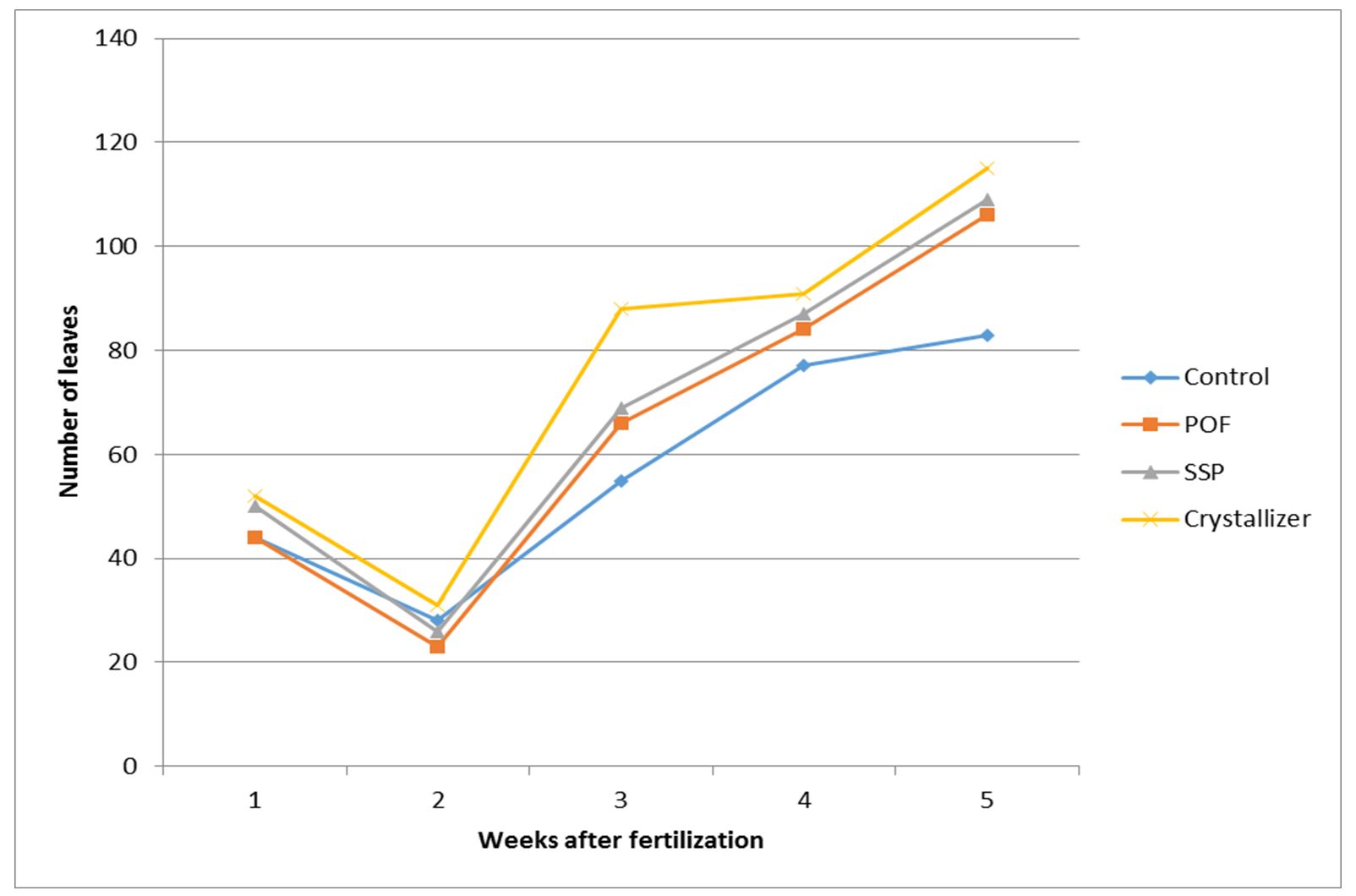
The progressive release dynamics of P aided with P-fertilizers positively influenced leaf production in sweet potato, as it did for vine growth. A week and two weeks after fertilization, only POF could not produce higher number of leaves than the control. But for rest periods of observation and data collection, all the P-fertilizer treatments produced higher number of leaves than the control. Crystallizer was the best throughout, followed by SSP. POF was next to SSP in performance except for the first week after fertilization when it was the same with the control and second week when it had less than the control (Fig. 3).
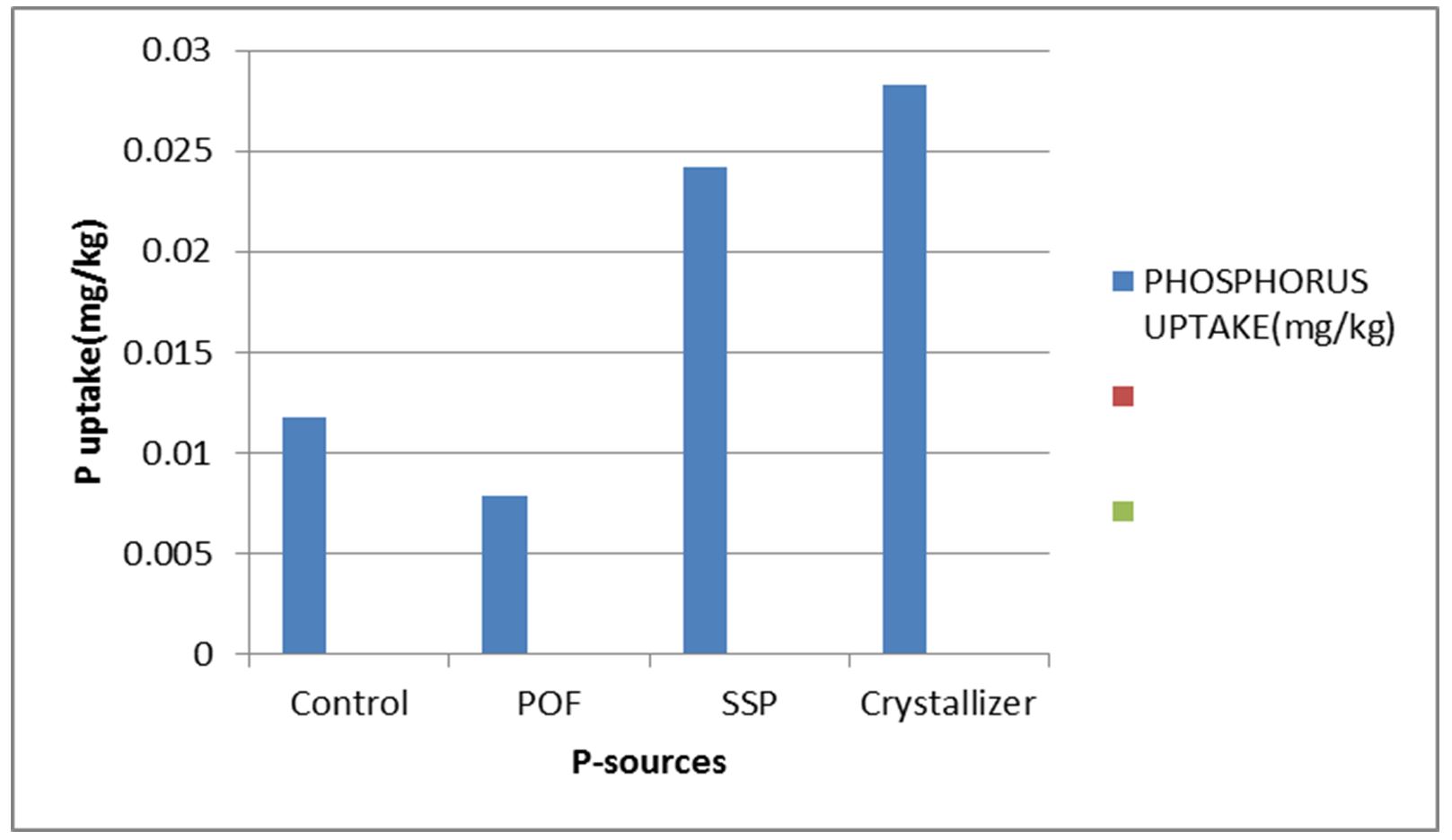
Effect of P-release kinetics on leaf P uptake
All the P-fertilizers enhanced leaf P uptake above the control except POF. The highest P-uptake per plant was observed in crystallizer treated plants while the least was from POF treatment (Fig. 4). The P-uptake highly correlated with vine length and number of leaves produced per plant (Table 1).
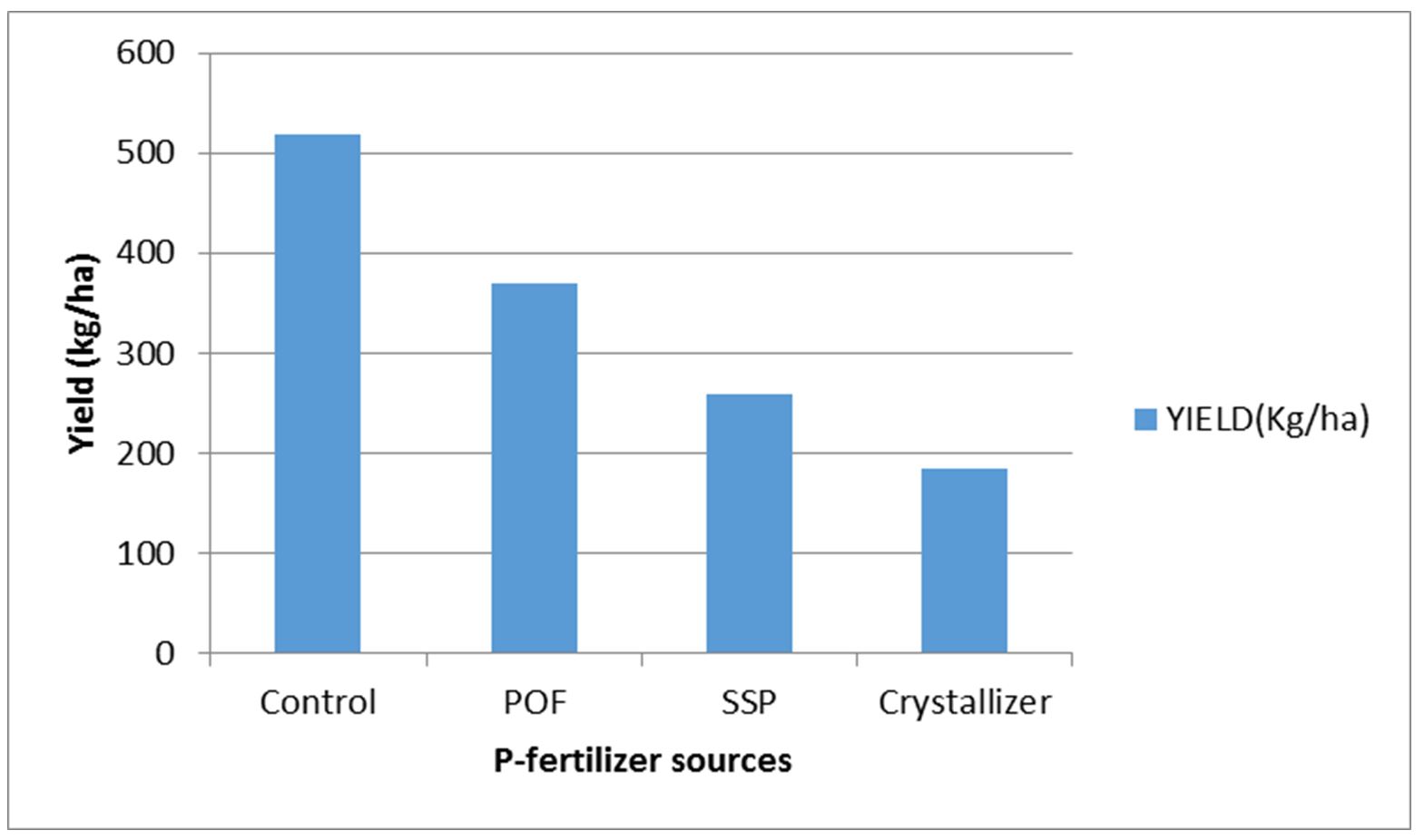
Effect of P-release kinetics on sweet potato yield
Tuber production was generally decreased by all the P-fertilizers applied. POF was next to the control in tuber production while crystallizer had the lowest yield (Fig. 5). Tuber yield indirectly related to both soil P and P-uptake (Table 1). Contrarily, leaf and vine productions had direct relationships with both soil P and P-uptake (Table 1).
Table 1
Correlation between different measured parameters
|
Yield |
versus |
Phosphorus uptake |
-0.93 |
|
Phosphorus released |
versus |
Phosphorus uptake |
0.95 |
|
Number of leaves |
versus |
Phosphorus uptake |
0.85 |
|
Number of leaves |
versus |
Soil phosphorus |
1 |
|
Vine length |
versus |
Phosphorus uptake |
0.99 |
|
Vine length |
versus |
Soil phosphorus |
0.99 |
DISCUSSION
The passage of time dictated the level of phosphorus found in the soil at any instance after application of phosphorus fertilizers. The release of P is based on its desorption from the soil. The soil nutrient, like P that adheres to the clay mineral, gets detached (desorbed) with availability of moisture. This implies that desiccation could cause locking up of nutrient that should have been desorbed or released. The observed low P in soil treated with crystallizer could be as a result of strong adherence of nutrients to the clay minerals. It could also be that crystallizer had suppressive power which prevented the release of innate P and that made the result from crystallizer to be lower than that of the control. The implication is that crystallizer could be successfully used to treat soil with extremely high phosphorus level if our target crop requires very little P as against high innate P detected through soil testing.
The amount of P-released by each of the P-fertilizers did not commensurate with the applied P in the soil except for the control in which the native P was 6.80 mg/kg as at the commencement of the experiment. In the control, it was realized that when water was available for P–dissolution, the P availability increased and followed the trend found in the other P treated soils. All the P-fertilizers used (POF, SSP and Crystallizer) did not release P to measure up with the concentration applied. This is because the soil P is converted to unavailable form by its reaction with the constituent parts of the soil (Marschner, 1995). Furthermore, when P-fertilizer is applied, 80% of the total P available is made inaccessible through fixation as a result of complexity involved in soil phosphorus chemistry (Grotz and Guerinot, 2002). So, only 20% is left for plant use. Beside fixation, phosphorus is not as mobile as nitrogen and potassium and, therefore, some available phosphorus, which is not used up by sweet potato may not experience leaching (Vivekananda and Fixen, 1990).
The vines and leaves which represent the vegetative life of sweet potato responded positively to phosphorus nutrient application right from the third to the fifth week of observation. The increase in vine length, as well as the number of leaves, followed the trend of phosphorus uptake. In the light of this, it is evident that the role played by P in vegetative life of sweet potato corresponds with the expected role of nitrogen. So, it could be said that P-fertilization can be used to enhance vegetative life of sweet potato. This is because when P is applied at the optimum level, the energy required for plant metabolism is released and chemically stored in an organic form as adenosine triphosphate (ATP) which is made available and released as required so that important growth chemical processes will be steadily driven (Adams, 1986). In addition to that, P-fertilizer application can positively increase sweet potato productivity (Hassan et al., 2005). The increments in vine length and number of leaves could be attributed to the beneficial effect of P-element on the activation of photosynthesis and metabolic processes of organic compounds in plants which leads to increase in plant growth (Purekar et al., 1992). The increments might equally be attributed to the important role of phosphorus as an essential component of many organic compounds in plants such as phospholipids, nucleic acids and nucleotides which may indirectly reflect positively on the plant (McLaughlin, 1992). In this experiment, the increments were recorded but they did not commensurate with the applied nutrient. However, it has been discovered that leaves and other vegetative parameters could be commensurately increased by high phosphorus nutrition (Rashid and Waithaka (2009).
The phosphorus uptake followed the trend of vegetative growth of sweet potato. Since P has been discovered to aid vegetative yield of sweet potato, P-uptake was, therefore, higher whenever vegetative growth was equally higher. The available soil P can explain more than 90% of the uptake (Table 1). Crystallizer treated plants produced the highest vegetative yield which resulted in highest P-uptake per plant. The least P-uptake was from the control because of low leaf production which is an index of nutrient uptake according to the formula used (Moustakas et al., 2011). Despite high fixation experienced by crystallizer, luxuriant growth of the crop was not disturbed and such resulted in higher P-uptake. The fixation is because when higher amount of phosphorus is released, P-recovery by crop uptake is about 15% – 30% while about 60% of the P-fertilizer is adsorbed or fixed by the soil and so a certain amount of P is added every year to top the amount already present in the soil (Olusola, 2009). Furthermore, despite the considerable addition of P-fertilizers, the amount available for plants is usually low since it is converted to unavailable form by its reaction with the constituent parts of the soil (Marschner,1995).
Tuber production had inverse relationship with leaf P-uptake (Table 1). This implies that for higher tuber yield, phosphorus uptake from the soil must be very low. It also depicts that phosphorus has suppressive power on tuber production in general with inclusion of sweet potato. For successful sweet potato production, phosphorus has to be kept to the bare minimum and more importantly avoidance of additional P to soil should be avoided to achieve better tuber production. The innate phosphorus content of the soil is enough to produce substantial yield without ado. The introduction of more phosphorus into the soil may be detrimental to the life of sweet potato as it would result in nutrient imbalance.
For proper development of root and tuber economic parts, the addition of nutrient should be potassium>nitrogen>phosphorus. In the same vein, if nitrogen is to be in the soil, only the vegetative life of the crop will be enhanced at the expense of its reproductive life and this is contrary to the objective of sweet potato production.
The effect of high P-releasing fertilizers on tuberous yield of sweet potato was completely different from the expected result (i.e. the more the phosphorus in the soil, the more the tuberous yield). This can be attributed to nutrient imbalance resulting from additional phosphorus nutrition through fertilizer application. This also established the fact that bulking of the tuberous root as well as grain filling in cereals requires lots of potassium nutrition and not high level of phosphorus which is required at a minimal level. This is because high phosphorus level in the soil suppresses tuber development of sweet potato as well as other root and tuber crops (FAO, 1994). So, elimination of phosphorus from the nutrition of sweet potato would not affect its yield in the least (FAO, 2005).
CONCLUSIONS
It was found that the trend of phosphorus release was a sigmoid shape. Leaf production and vine growth were improved by P-application while tuber yield was suppressed. It could be said that P-fertilizer should not be applied at close intervals even if its effects are yet to be felt on plants because its release into the soil is over a period of time. Otherwise, such application will lead to soil nutrient toxicity.
REFERENCES
Adams, P. (1986). Mineral nutrition. In: Atherton, J.G. and Rudick, J. (Eds). The tomato crop. Chapman and Hall Publishers, New York, pp. 281-324.
Cisse, L. & Amar, B. (2000). The importance of phosphatic fertilizer for increased crop production in developing countries. In: Proceedings of the AFA 6th International Annual Conference, 31 Jan. – 2 Feb., 2000, Cairo, Egypt.
FAO (1994). Tropical root and tuber crops: Production, perspective and future prospect. Food and Agricultural Organization (FAO). Plant Production and Protection Paper, pp. 126-228.
FAO (2005). Fertilizer use by crop in Ghana. Food and Agriculture Organization of the United Nations (FAO). Food and Nutrition Series, No 28, 190 p.
FAO (2008). Efficiency of soil and fertilizer phosphorus use. FAO Fertilizer and Plant Nutrition Bulletin 18, Rome.
Forbes, J.C. & Watson, D. (1994). Plant in agriculture. Cambridge University Press. First Published in 1992, Reprinted in 1994.
Grotz, N. & Guerinot, M.L. (2002). Limiting nutrients: an old problem with new solutions? Curr.Opin. Plant Biol., 5(2): 158-163, DOI: 10.1016/S 1369-5266(02)00247-9.
Hassan, M.A., El-Seifi, S.K., Omar, E.A. & Saif EI-Deen, U.M. (2005). Effect of mineral and bio-phosphate fertilization and foliar application of some micronutrients on growth, yield and quality of sweet potato (Ipomoea batata, L). 1- Vegetative growth, yield and tuber characteristics. J.Agric.Sci. Mansoura Univ., 30 (10): 6149-6166.
Ibrikci, H., Ryan, J., Ulger, A.C., Buyuk, G., Cakir, B., Korkmaz, K., Karnez, E., Comertpay, G. & Konuskan, O. (2005). Maintenance of P fertilizer and residual P effect on corn production. Nutr.Cycl.Agroecosyst., 72(3): 279-286, DOI: 10.1007/s10 705-005-3367-8.
Kuo, S. (1996). Phosphorus. In: Sparks, D.L. (Ed.), Methods of soil analysis, Part 3: Chemical Methods, SSSA Book Series 5, Soil Science Society of America, Madison, Wisconsin, pp. 869-920.
Marschner, H. (1995). Mineral nutrition of higher plants. 2nd Ed., Academic Press, Harcourt Brace and Company, Publishers. London, New York, Tokyo, 864 p.
McLaughlin, M.J., Fillery, I.R.P. & Till, A.R. (1992). Operation of the phosphorus, sulphur and nitrogen cycles. In: Australia’s Renewable Resources: Sustainability in Global Change, Ed. R.M. Gifford and M.M. Barson, pp. 67-116. Canberra, Australia: Bureau of Rural Resources Proceedings, No.14.
Moustakas, N.K., Akoumianakis, K.A. & Passam, H.C. (2011). Patterns of dry biomass accumulation and nutrient uptake by okra (Abelmoschus esculentus (L.) Moench.) under different rates of nitrogen application. Aus.J. Crop Sci., 5 (8): 993-1000
Olusola, O.O. (2009). Understanding soil and plant nutrition. Salman Press & Co. Nig. Ltd, Keffi, Nassarawa State, Nigeria.
Purekar, P.N., Singh, R.R. & Deshmukh, R.D. (1992). Plant physiology and ecology. 2nd Ed., Chand, S. and Company, New Delhi, India.
Rashid, K. & Waithaka, K. (2009). The effect of phosphorus fertilization on growth and tuberization of sweet potato, Ipomoea batatas L. ISHS, Acta Hortic., 153: IX African Symposium on Horticultural Crops DOI: 10.17660/ActaHortic.1985.153. 47.
Rehman, O.U., Mehdi, S.M., Sarfraz, M., Shakir, M.A. & Shabir, G. (2013). Phosphorus dynamics in Rasulpur soil series (Typic camborthid) under rice based cropping system. J.Anim. Plant Sci., 23(2):480-486.
Vivekananda, M. & Fixen, P.E. (1990). Effect of large manure application on soil P intensity. Comun.Sci. Soil Plant.Anal., 21(3-4): 287-293, DOI: 10.1080/00103629009368231.
Abdulmaliq S.Y., Akinrinde E.A., Alasinrin S.Y., Eifediyi E.K., Kareem I., Kareem S.A., Oladosu Y.