S. Farouk, A.J. Al-Sanoussi
ABSTRACT. Two field experiments were done at a private farm in Kalabsho and Zayian district, Dakhlia Egypt, throughout 2014/2015 and 2015/2016 seasons, to evaluate the promotive role of chitosan (Chi, 250 and 500 mg/l) and/or sodium metasilicate (Si, 125 and 250 mg/l) foliar application on barley growth, yield, and some physiological attributes in newly reclaimed soil. Application of Si or Chi concentrations showed an improvement in plant growth as: plant height, tiller number per plant, flag leaf area and shoot dry weight; photosynthetic pigments; organic osmolytes; ion percentage, as well as yield and its quality in both growing seasons. Generally, the application of Si gave higher values in most cases than Chi application in the experimental year. It was concluded that application of 125 mg/l sodium metasilicate twice at 50 and 70 days from sowing is advan-tageous to improving plant growth and productivity under newly reclaimed soils.
Keywords: chitosan; Hordeum vulgare subsp. vulgare L.; silicon; yield.
View full article (HTML)
The role of biostimulants in increasing barley plant growth and yield under newly cultivated sandy soil
S. Farouk1*, A.J. Al-Sanoussi2
1Agric. Botany Dept., Fac. of Agric., Mansoura Univ., Egypt
2Plant Production Dept., Fac. of Agriculture, Sirte Univ., Libya
*E-mail: gadalla@mans.edu.eg
Received Mar. 18, 2019. Revised: June 19, 2019. Accepted: July 12, 2019. Published online: Oct. 18, 2019
ABSTRACT. Two field experiments were done at a private farm in Kalabsho and Zayian district, Dakhlia Egypt, throughout 2014/2015 and 2015/2016 seasons, to evaluate the promotive role of chitosan (Chi, 250 and 500 mg/l) and/or sodium metasilicate (Si, 125 and 250 mg/l) foliar application on barley growth, yield, and some physiological attributes in newly reclaimed soil. Application of Si or Chi concentrations showed an improvement in plant growth as: plant height, tiller number per plant, flag leaf area and shoot dry weight; photosynthetic pigments; organic osmolytes; ion percentage, as well as yield and its quality in both growing seasons. Generally, the application of Si gave higher values in most cases than Chi application in the experimental year. It was concluded that application of 125 mg/l sodium metasilicate twice at 50 and 70 days from sowing is advan-tageous to improving plant growth and productivity under newly reclaimed soils.
Keywords: chitosan; Hordeum vulgare subsp. vulgare L.; silicon; yield.
INTRODUCTION
The existing world population of 7.6 billion is expected to reach 11.2 billion in 2100 (Sustainable Development Goals, 2017). Con-sequently, rominence should be laid on the production of elevated quality food (Ghaly and Alkoaik, 2010). Barley (Hordeum vulgare subsp. vulgare L., Poaceae) is close to further cereal in terms of caloric value and protein content, conversely, contains elevated concentrations of tocols and β-glucans (Oscarsson et al., 1996), that decline coronary heart syndrome and is useful to type 2 diabetics (Jonnalagadda et al., 2011). In Egypt and Libya, it was prompt to utilize barley as a complementary cereal to satisfy this cereal scarcity due to barley’s capability, to grow healthy in new reclaimed soil.
Chitosan (Chi) has been shown to improve plant growth and productivity, of various crops (Helaly et al., 2018; Souza et al., 2018). Silicon (Si) is a second nutrient in the Earth’s crust, that served as a crucial physio-mechanical barrier in nearly all crops (Hobara et al., 2016), en-hancing enzymatic activity (Kim et al., 2017), and regulates gene expression (Merwad et al., 2018), enhanced pho-tosynthesis, and motivation of antioxi-dant system and increased the yield (Farouk et al., 2017; Merwad et al., 2018). The current study aimed to evaluate the role of Chi or Si on barley growth, some physiological parameters, and yield under newly reclaimed soil.
MATERIAL AND METHODS
Experimental layout
Two field experiments were carried out in the winter seasons of 2014/2015 and 2015/2016 on sandy soil at private farm in Kalabsho and Zayian districts, Dakhlia, Egypt. The experimental soil is known as sandy soil with a pH (1:2.5) 7.5-7.7, having 0.50-0.52% organic matter; and having 1.92-1.95 dS/m electrical conductivity. The irrigation water was coming from an irrigation channel under rotating irrigation wherever. A completely randomized design with five replicates, the area of the experimental plot was 15 m2. To avoid interface influence, levees 1.5 m wide were used to generate a safeguarded area among experimental plots.
Prior to sowing, the experimental field was deep plowed formerly and soil was brought to the good filth. The fertilizers (NPK 285:70:60 kg ha-1) were added in the form of ammonium nitrate, a mono-superphosphate, and potassium sulfate. Nitrogen fertilizer was added, as follows: 10% of the nitrogen was used at sowing as a basal dose, the remnants being applied previously to all irrigation in three portions till heading phase. Phosphorous fertilizer was added to the soil in two equivalent doses before planting and at tillering stage, meanwhile, a potassium fertilizer applied one time following one month from sowing. Pest management and cultural practices were performed following the instructions of local commercial crop production. Barley grains (Hordeum vulgare L., cv Giza129), at the rate of 160 kg ha-1, were sown on the 10th and 1st November in 15 cm rows to row distances using a single row hand drill. The biostimulants (sodium metasilicate ‘Si’ at 125 and 250 mg/l; chitosan ‘Chi’ at 250 and 500 mg/l; beside water spraying ‘control’) were sprayed two times at 50 and 70 days from sowing to an overflow at the rate of 20 liters per plot at early morning with a back sprayer once adding 0.1% (v/v) Tween 20 as a wetting agent to ensure optimal penetration into the leaves.
Data recorded
At 90 days from sowing, 15 plants from every experimental plot were randomly chosen and estimated plant growth trails (plant height, tillers number per plants; flag leaf area and shoot dry weight), and a number of physiological characteristics within the shoot and flag leaf.
Total chlorophyll and carotenoid in flag leaf were assessed with the protocol delineated by Lichtenthaler and Wellburn (1985). Leaf segments were extracted for 24 hrs at the lab, temperature with methanol supplemented with potassium carbonate, then spectrophotometrically determined at 470, 653 and 666 nm.
Proline concentration was estimated using D-proline as a standard (Magné and Larher, 1992), about 0.5 g shoot was homogenized with 3% (v/v) aqueous sulphosalicylic acid and centrifugation. To an aliquot of extract, 2 ml glacial acetic acid and 2 ml of acid ninhydrin was added and maintained at 1000C for 30 min. After cooling, 4 ml toluene was added, mixed strongly and the chromophore-containing toluene was read at 520 nm using toluene as blank. The proline concentration was deliberate by a standard curve of proline and was expressed as mg/g FW. The anthrone-sulfuric acid technique introduced by Sadasivam and Manickam (1996) was used to estimate total soluble carbohy-drates. The absorbance was measured spectrophotometrically at 620 nm against a blank. Ascorbic acid was extracted and determined, as delineating by Sadasivam and Manickam (1996). Total phenolic content in extracts was determined by Folin-Ciocalteu’s colorimetric routine, as documented by Julkunen-Tiitto (1985). Every solvent extracted solution (0.3 ml in triplicate) was mixed with 1.5 ml of 10% Folin-Ciocalteu’s reagent and 1.2 ml of 7.5% (W/V) sodium carbonate. The mixture was kept in the dark for 30 min and absorbance was read at 765 nm. Quantification was done on the basis of a standard curve of gallic acid and expressed as gallic acid equivalent (GAE), i.e. mg gallic acid/g FW.
Ion percentages were estimated in the dried shoot after wet digestion with HClO3/H2SO4. Total nitrogen, potassium and phosphorous were estimated by the micro-Kjeldahl method, flame photometer (Kalra, 1998), and reduced molybdo-phosphoric blue color technique (Cooper, 1977), respectively.
At harvest time, a random sample of one m2 was taken from each plot for determining grain yield per spike, grain index (1000-grain weight), as well as grain and straw, yields “ton ha-1”. Additionally, protein determination was determined by multiplying total nitrogen percentage in grains by 6.25 (Chapman and Pratt, 1978) and total carbohydrate percentage was estimated by the phenol-sulphuric acid protocol (Sadasivam and Manickam, 1996).
Statistical analysis
Data was subjected to one-way ANOVA (analysis of variance) with Costat software (Costat Institute, Cary, NC, USA). Where F tests were significant (p< 0.05), means were separated by Duncan’s multiple range test.
RESULTS AND DISCUSSION
Growth parameters
Commonly, data in Fig. 1 assess that foliar application of chitosan (Chi) or sodium metasilicate (Si) concentrations drastically raised plant growth corresponding to control plants; moreover, the data in the same figure verified that application of Si gave higher values than Chi. The maximum plant growth was recorded under foliar application with 125 mg/l Si, that increased plant height by 10% and 13%, tillers number per plant by 14% and 19%, flag leaf area by 18% and 27%, and shoot dry weight by 18% and 21% in the first and second season respectively above non-sprayed control plants.
The current research has docu-mentted that both Chi and Si concen-trations exhibit surprising impacts on plant growth comparative to control. The encouraging effect of Chi or Si on plant growth recorded in the existing investigation, possibly owing to: 1) improvement of water status (Alzahrani et al., 2018; Merwad et al., 2018), that reflected to boost ion content, as observed in the recent study; 2) increased the beginning of leaf primordial and decreased plasto-chron duration, that directs to raise leaf area and lastly plant biomass, throughout increased total soluble carbohydrates, as recorded earlier (Farouk et al., 2017; Alzahrani et al., 2018); 3) compensate the oxidative damage by modifiable antioxidant enzyme activities and declined the hydrogen peroxide concentration and oxidative damage of protein (Alzahrani et al., 2018; Merwad et al., 2018); 4) improved potassium content that may raise the chloroplasts per cell, the cell number per leaf and finaly leaf area (Taiz and Zeiger, 2010).
Photosynthetic pigment
Application of both biostimu-lants non-significantly increased total chlorophyll in flag leaf compared with untreated control plants; alternatively, application of biostimulants signify-cantly increased total carotenoids concentration in flag leaf (Fig. 2).
The highest concentration of total chlorophyll and total carotenoids were obtained by foliar application of 125 mg/l Si (Fig. 2).
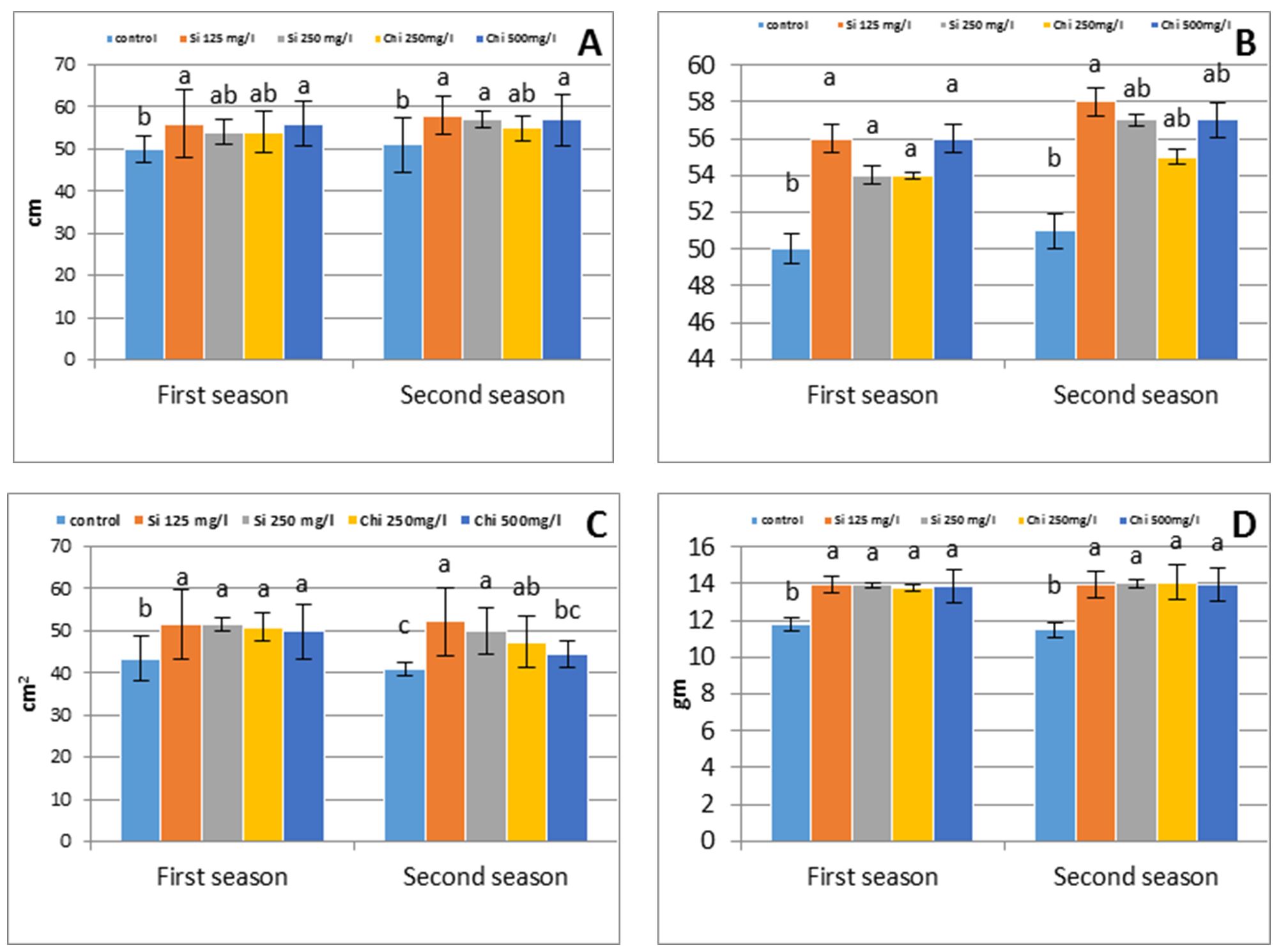
Figure 1 – Effect of silicon or chitosan concentration on barley plant height (A), tiller number per plant (B), flag leaf area (c) and shoot dry weight (D) at 90 days from sowing in the first and second season
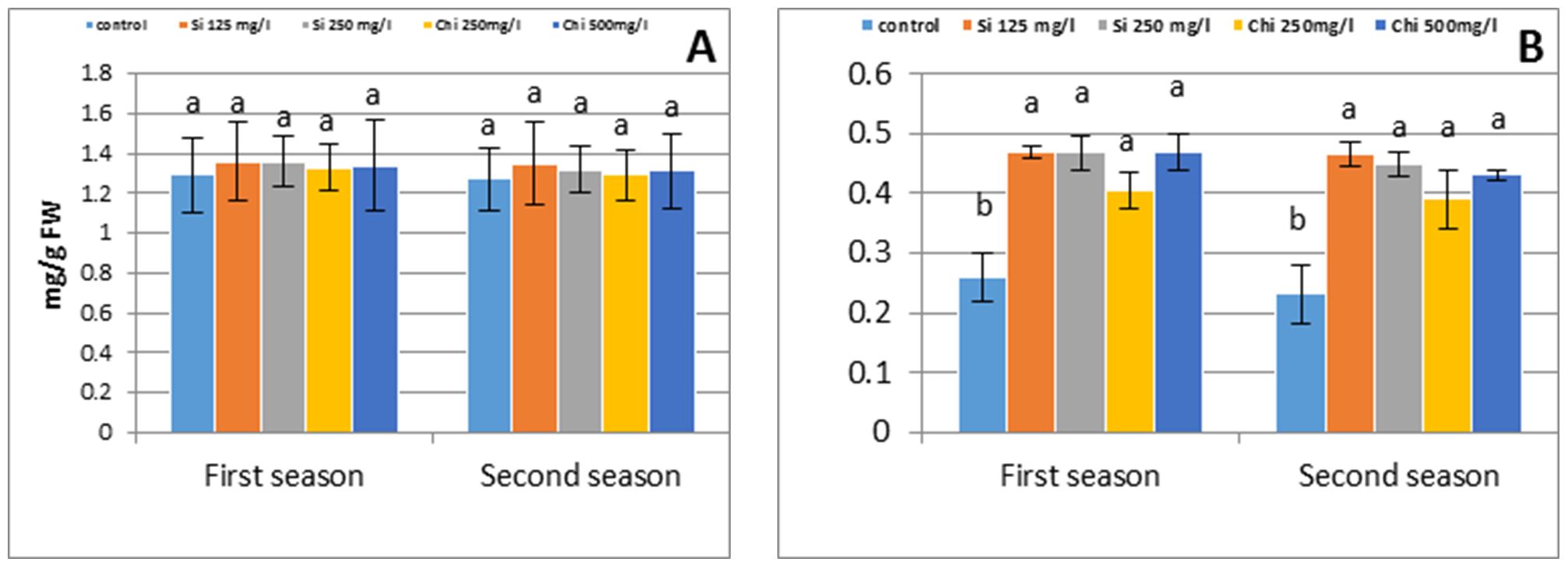
Figure 2 – Effect of silicon or chitosan concentration on total chlorophyll (A) and total carotenoid (B) concentration in barley flag leaf at 90 days from sowing in the first and second season
The advantage of Chi or Si on photosynthetic pigments are in harmo-ny with those of Farouk et al. (2017) for Si, Farouk and Amany (2012), Bistgani et al. (2017) for Chi. This rise may be recognized to: 1) Proficient scavenging of reactive oxygen species that will have otherwise damaged the chlorophyll (Farouk et al., 2017); 2) Increased the endogenous level of cytokinins that stimulates chlorophyll biosynthesis (Markovich et al., 2017); 3) Improved noticeably both nitrogen and potassium in the plant shoot, as indicated in the present study, that can be rising the number of chloroplast per cell, the cell size and number per unit area, as well as improved the synthesis of chlorophyll (Taiz and Zeiger, 2010).
Organic osmolytes
Data observed in Fig.3 recog-nized that barley plants under biostimulants application coupled with an increased ion influx in their cells by raising the accretion of solutes, like proline, total soluble carbohydrates, ascorbic and phenol as relative to the control. The maximum value of ascorbic and soluble carbohydrates was obtained due to the application of 125 mg/l Si, in the meantime, the maximum proline and phenol concentration were obtained by application of 500 mg/l Chi in both growing seasons.
Proline (Pro) is the mainly crucial solutes and signalling mole-cule to adjust mitochondrial functions, manipulate cell proliferation or cell death and trigger specific gene expression, which might be necessary for plant development under Si (Farouk et al., 2017; Alzahrani et al., 2018) and Chi (Bistgani et al., 2017) application.
In the plant tissues, the biosyn-thesis of Pro depends on upregulation of Pro biosynthesis genes, such as pyrroline-5-carboxylatesynthetase (P5CS) and pyrroline-5-carboxylate reductase (P5CR) (Bandurska et al., 2017), and glutamyl kinase activity or the low activity of degrading enzyme, proline oxidase (EC 1.5.99.8) and proline dehydrogenase (EC 1.5.1.2) (Zhang et al., 2017) to significant rate. Nevertheless, Pro metabolism in the incidence of Chi or Si is not recog-nized and requires extra investigation. Several types of research recognized that, the importance of soluble carbohydrates in accelerating Pro accumulation through an inhibition of the degradation enzymes and stimulated the synthesis enzymes. Consequently, Hare and Cress (1996) noticed that mRNA transcript encoding P5CR was exaggerated in phloem tissue in stress condition. Total soluble carbohydrate amount was increasingly increasing in plants under exogenous application of biostimulants. The outcome of the current study is in harmony with Alzahrani et al. (2018) for Si and Bistgani et al. (2017) for chitosan. The augmentation in soluble carbohy-drates attributable to Si or Chi supplementation might sequentially play a vital function in raising the osmotic pressure of the cytoplasm. Sugars are extensively established as an osmoprotectant, that regulates the osmotic adjustment, give membrane shield and scavenge noxious ROS against various stresses (Koyro et al., 2012).
Ions percentage
Data presented in Fig. 4 postula-ted that pronounces a tremendous rise in nitrogen, phosphorous and potassium percentages in the shoot attributable to the foliar application of biostimulants in growing seasons. The maximum values were observed due to foliar application of 125 mg/l Si that increased N, P and K by 12%, 11%, and 15% in the first season and by 15%, 10%, and 15% in the second season, respectively, relative to control.
The role of Chi or Si in accelerating ion buildup is not entirely understood, and there are few studies in this concern. In this concern, the application of Si or Chi raised the absorption and metabolism of nitrogen, phosphorous and potassium in plant tissues (Farouk et al., 2017; Alzahrani et al., 2018). Normally, it’s well noted that Si or Chi-mediated improvement of root growth, enhanced hydraulic conductance of roots and root activity could thus encourage nutrient absorption (Sonobe et al., 2011). Numerous physiological mechanisms seem to be occupied in the above-referenced positive impacts of biostimulants on mineral uptake and mobility into the plant tissues. A foremost mechanism arises from the efficiency of biosti-mulants to reduce plasma membrane permeability, thus maintaining membrane selectivity to ion influx and efflux. The plasma membrane is able to maintain the physiological balance in ion concentrations at intracellular level, while minimizing the influx of toxic ions as long as its selectivity to ion uptake is high, despite imbalanced concentrations in the root zone. A number of researchers found that biostimulants protect plasma membrane integrity and permeability in plants. The increase in P accumulation as results of Si and Chi application could also result from the avoidance of P fixation within the soil and the formation of humophospho complexes, which are simply assailable by the plants and this explains the more of P percentage of wheat plant in the present study.
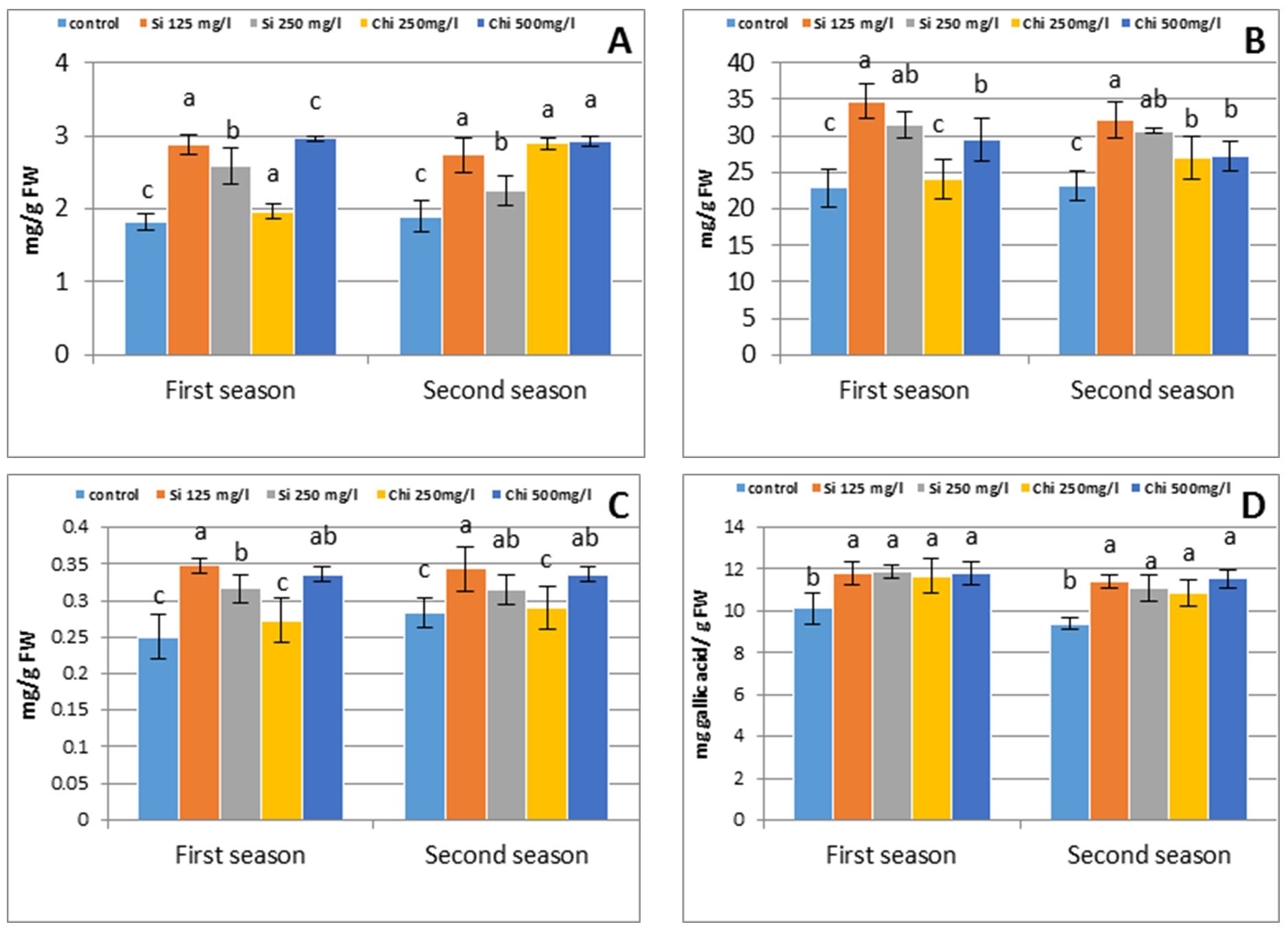
Figure 3 – Effect of silicon or chitosan concentration on barley shoot proline (A), soluble carbohydrates (B), flag ascorbic (c) and phenol (D) concentration at 90 days from sowing in the first and second season
Yield and its components
Data presented in Fig. 5 postu-lated that biostimulants application significantly (p≤ 0.05) improved the yield and its components. The greatest grain weight/spike (12% and 45%), grain index (22% and 43%), grain yield (12% and 33%), straw yield (41% and 34%) and grain protein percentage (17% and 9%) were obtained by exogenous application of 125 mg/l sodium metasilicate; meanwhile, the highest grain carbohydrates percentage (12% and 12%) was obtained by 500 mg/l chitosan foliar application in both seasons, relative to control.
The increase in barley yield attributable to Si or Chi may result from its effects in enhancing metabolic processes and anatomical alteration. Biostimulants increased yield and its components in several crop species (Farouk et al., 2017; Merwad et al., 2018). The yield enhancement by biostimulants corres-ponded to the maintenance of higher net photosynthesis rate and improving the source-sink relationship.
Consequently, Liang et al. (2013) assume that Si application enhance lodging resistance by strengthening the stem base, as well as leaves becomes additional straight, conse-quently dropping self-shading and improved photosynthesis ability, chiefly through the grain filling era, that connected with starch accretion in grains. Moreover, Si deposition on cell walls, provides mechanical strength and rigidity to plant tissue (Isa et al., 2010), that reduce lodging and enhance leaf thickness, erectness and rigidity, thereby improving leaf exposure to light leading to improved photosynthesis in plants by sus-taining net assimilate rate by in-creased the basal quantum yield and maximum quantum efficiency of the photosystem II, that leads to increased yield.
The increase in carbohydrate percentage of biostimulants pretreat-ment might be ascribed to the increase of photosynthetic pigment, leading to improvement of carbohydrate synthe-sis and accumulation.
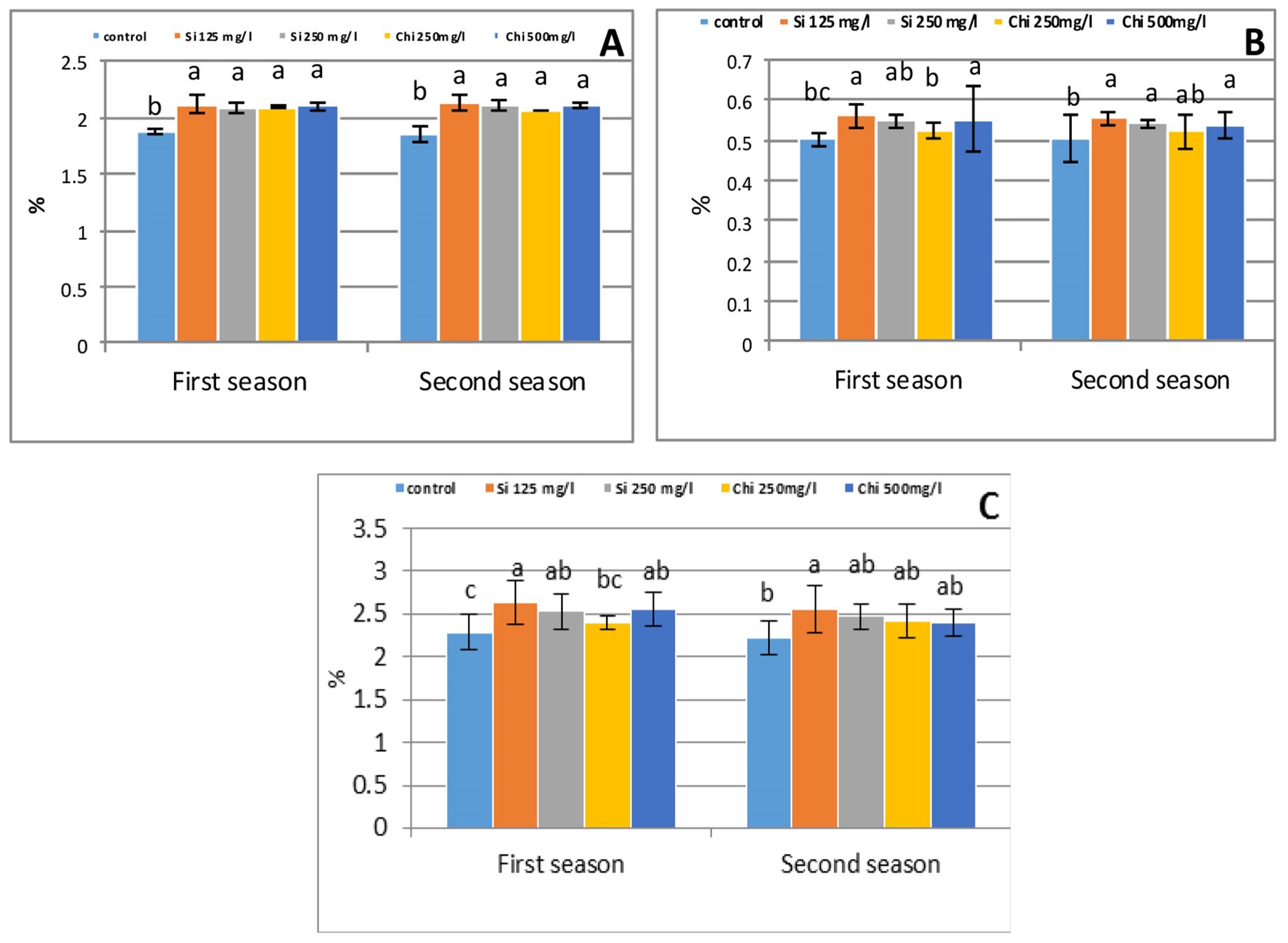
Figure 4 – Effect of silicon or chitosan concentration on nitrogen (A), phosphorous (B), potassium (c) percentage of barley shoot at 90 days from sowing in the first and second season
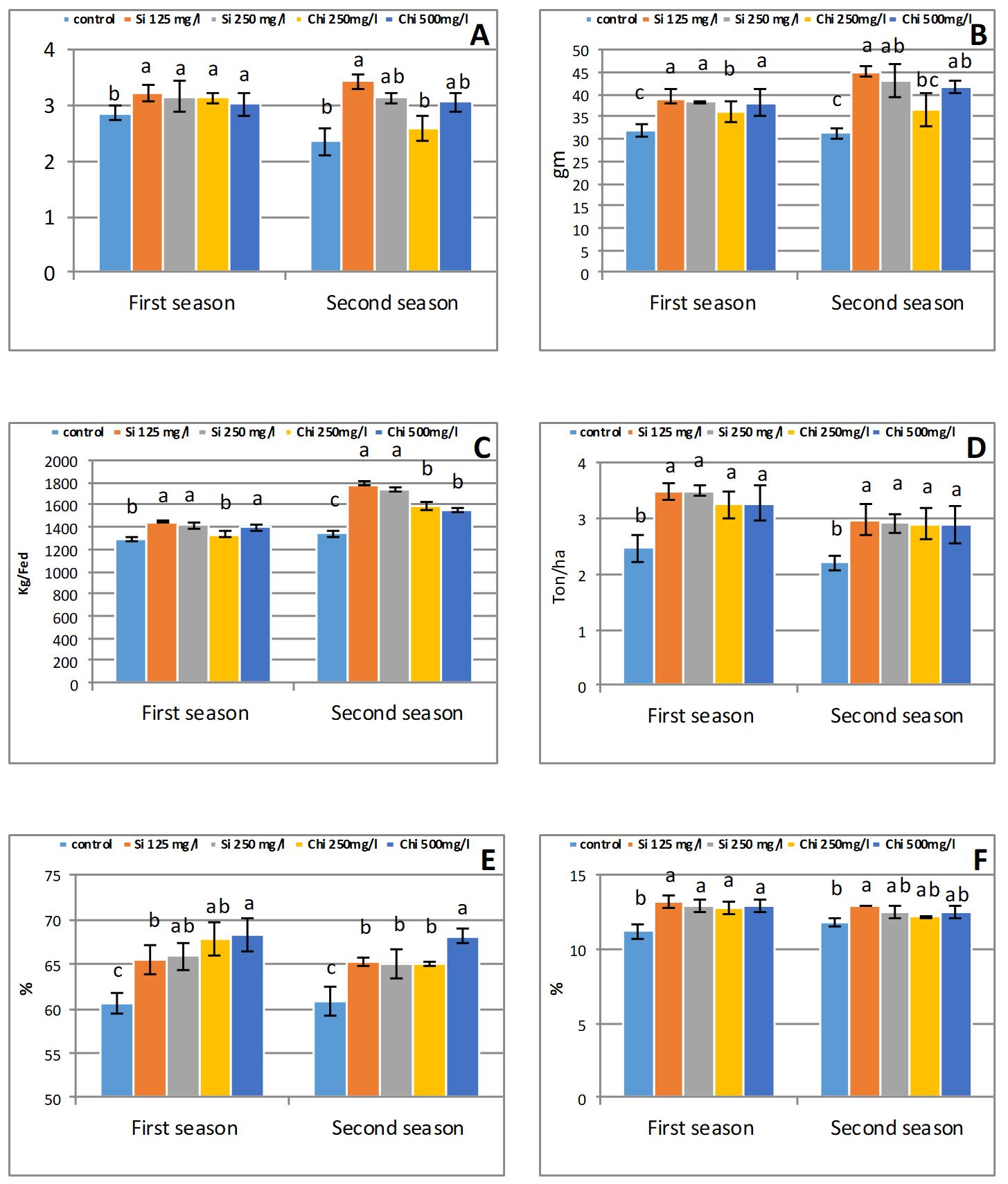
gure 5 – Effect of silicon or chitosan concentration on grain weight per spike (A), grain index (B), grain yield(c); straw yield (D); grain carbohydrates percentage (E); grain protein percentage (F) at harvesting time in the first and second season
CONCLUSION
There is more promise for the use of antitranspirant eco-friendly substances in crop production for minimizing irrigation water and chemical used in new reclaimed sandy soil. It was fulfilled that the application of 125 mg/l sodium metasilicate as a foliar application two times at 50 and 70 days from sowing is favorable to improve plant growth and productivity, as well as enhancing the grain quality.
REFERENCES
Alzahrani, Y., Kuşvuran, A., Alharby, H.F., Kuşvuran, S. & Rady, M.M. (2018). The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol.Environ.Saf., 154:187-196, DOI: 10.1016/j.ecoenv. 2018.02.057
Bandurska, H., Niedziela, J., Pietrowska-Borek, M., Nuc, K., Chadzinikolau, T. & Radzikowska, D. (2017). Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol. Biochem, 118: 427-437. DOI: 10.1016/j.plaphy.2017.07.006
Bistgani, Z.E., Siadat, S.A., Bakhshandeh, A., Pirbalouti, A.G. & Hashemi, M. (2017). Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop J., 5(5):407-415, DOI: 10.1016/j.cj. 2017.04.003
Chapman, H.D. & Pratt. R.F. (1978). Methods of analysis for soils, plants and waters. Univ. California Div. Agric. Sci. Priced Publication, Oakland.
Cooper, T.G. (1977). The tools of biochemistry (Wiley-Interscience Publication) 1st Edition, John Wiley and Sons, New York.
Farouk, S., Belal, B.E.A. & El-Sharkawy, H.H.A. (2017). The role of some elicitors on the management of Roumy Ahmar grapevines downy mildew disease and it’s related to inducing growth and yield characters. Sci.Hort., 225: 646-658, DOI: 10.1016/j.scienta.2017.07.054
Farouk, S. & Amany, A.R. (2012). Improving growth and yield of cowpea plant by foliar application of chitosan under water stress. Egypt.J.Biol., 7: 14-26, DOI: 10.4314/ejb.v14i1.2
Ghaly, A.E. & Alkoaik, F.N. (2010). Extraction of protein from common plant leaves for use as human food. Am.J.Appl.Sci., 7(3):331-342.
Hare, P.D. & Cress, W.A.(1996). Tissue-specific accumulation of transcript encoding Δ1-pyrroline-5- carboxylate reductase in Arabidopsis thaliana. Plant Growth Regul., 19(3): 249-256, DOI: 10.1007/BF00037798
Helaly, M.N., Farouk, S., Arafa, S.A. & Amhimmid Naema, B.I.A. (2018). Inducing salinity tolerance of rosemary (Rosmarinus officinalis L.) plants by chitosan or zeolite application. Asian J.Adv.Agric.Res., 5(4):1-20, DOI: 10.9734/AJAAR/ 2018/40051
Hobara, S., Fukunaga-Yoshida, S., Suzuki, T., Matsumoto, S., Matoh, T. & Ae, N. (2016). Plant silicon uptake increases active aluminum minerals in root-zone soil: implications for plant influence on soil carbon. Geoderma, 279(1): 45-52, DOI: 10.1016/j.geoderma.2016. 05.024
Isa, M., Bai, S., Yokoyama, T., Ma, J.F., Ishibashi, Y., Yuasa, T. & Iwaya-Inoue, M. (2010). Silicon enhances growth independent of silica deposition in a low-silica rice mutant, lsi1. Plant Soil, 331(1-2): 361-375, DOI: 10.1007/s11104-009-0258-9
Jonnalagadda, S.S., Harnack, L., Liu, RH., McKeown, N., Seal, C., Liu, S. & Fahey GC. (2011). Putting the whole grain puzzle together: health benefits associated with whole grains – summary of American Society for Nutrition 2010 Satellite Symposium. J.Nutr., 141(5): 1011S-1022S, DOI: 10.3945/jn.110.132944
Julkunen-Tiitto, R. (1985). Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J.Agric. Food Chem., 33(2): 213-217, DOI: 10.1021/jf00062a013
Kalra, Y.P. (1998). Handbook of reference method for plant analysis. CRC Press, Washington, DC.
Kim, Y.H., Khan, A.L., Waqas, M. & Lee, I.-J. (2017). Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: a review. Front. Plant Sci., 8: 510, DOI: 10.3389/fpls.2017.00510
Koyro, H.W., Ahmad, P. & Geissler, N. (2012). Abiotic stress responses in plants: an overview. In: Environmental adaptations and stress tolerance of plants in the era of climate change, Ahmad P. Prasad M.N.V. (Eds.), Springer, New York, DOI: 10.1007/978-1-4614-0815-4_1
Liang, X., Zhang, L., Natarajan, S.K. & Becker, D.F. (2013). Proline mechanisms of stress survival. Antioxid. Redox Signal, 19(9): 998-1011, DOI: 10.1089/ars.2012.5074
Lichtenthaler, H.K. & Wellburn, A.R. (1985). Determination of total carotenoids and chlorophylls A and B of leaf extracts in different solvents. Biol.Soc.Trans., 11(5): 591-592, DOI: 10.1042/bst0110591
Magné, C. & Larher, F. (1992). High sugar content of extracts interfers with colorimetric determination of amino acids and free proline. Anal.Biochem., 200(1): 358-362, DOI: 10.1016/0003-2697(92)90285-F
Markovich, O., Steiner, E., Kouřil, Š., Tarkowski, P., Aharoni, A. & Elbaum, R. (2017). Silicon promotes cytokinin biosynthesis and delays senescence in Arabidopsis and sorghum. Plant Cell.Environ., 40: 1189-1196, DOI: 10.1111/pce.12913
Merwad, A.M.A., Desoky, E.M. & Rady, M.M. (2018). Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci.Hortic., 228: 132–144. DOI: 10.1016/j.scienta.2017.10.008
Oscarsson, M., Andersson, R., Salomonsson, A.-C. & Åman, P. (1996). Chemical composition of barley samples focusing on dietary fibre components. J. Cereal Sci., 24(2): 161-169, DOI: 10.1006/ jcrs.1996.0049
Sadasivam, S. & Manickam A (1996). Biochemical methods. 2nd edition, New Age International (P) Limited Publishers, India.
Sonobe, K., Hattori, T., An, P., Tsuji, W., Eneji, A.E., Kobayashi, S., Kawamura, Y., Tanaka, K. & Inanaga, S. (2011). Effect of silicon application on sorghum root responses to water stress. J. Plant Nutr., 34(1): 71-82, DOI: 10.1080/01904167.2011.531360
Souza, P.U., Lima, L.K.S., Soares, T.L., de Jesus, O.N., Filho, M.A.C. & Girardi, E.A. (2018). Biometric, physiological and anatomical responses of Passiflora spp. to controlled water deficit. Sci.Hortic., 229: 77-90, DOI: 10.1016/j.scienta. 2017.10.019
Sustainable Development Goals (2017). (https://www.un.org/sustainabledevelopment/blog/2017/06/world-population-projected-to-reach-9-8-billion-in-2050-and-11-2-billion-in-2100-says-un/).
Taiz, I. & Zeiger, E. (2010). Plant physiology. 5th Edition, Sinauer Associates Inc., Massachusetts, USA.
Zhang, M., Wang, L.-F., Zhang, K., Liu, F.-Z. & Wan, Y.-S. (2017). Drought-induced responses of organic osmolytes and proline metabolism during pre-flowering stage in leaves of peanut (Arachis hypogaea L.). J.Integr.Agric., 16(10): 2197-2205, DOI: 10.1016/S2095-3119(16)615 15-0



