E.L. Baideng, J.J. Pelealu, B.H. Assa, H.A.W. Lengkey
ABSTRACT. Along with the awareness to obtain quality plant products, the use of plant-based insecticides is increasingly being used. One of the plants used as a plant-based insecticide is Jatropha curcas L. (Jarak pagar) because it contains toxic ingredients to kill cabbage caterpillar pests (Crocidolomia binotalis). This study aims to determine the effectiveness of Jatropha curcas L. on the mortality of Crocidolomia binotalis cabbage caterpillars. The research method used was a Completely Randomized Design (CRD) 7 × 3, consisting of seven treatment concentrations (0,000 ppm, 10,000 ppm, 20,000 ppm, 30,000 ppm, 40,000 ppm, 50,000 ppm, 60,000 ppm), with three replications. Observations were made at 24, 48, 72, 96, 120, 144 hours after application (HAA) of Jatropha. The research activities were carried out in two stages, namely 1) the extraction stage of Jatropha curcas L. seeds and the breeding of the Crocidolomia binotalis test larvae and 2) the testing stage with seven concentration levels of Jatropha curcas L. extract as a plant-based insecticide. ANOVA test showed that the treatments of Jatropha curcas L. extract gave the death effect on larvae [F-count > F-table (116.8 > 2.37)]. Dead larvae change color to black and their body shape will curve. The fastest larval death occurs 24 HAA, with a concentration of 40,000 ppm, which is 50%. At lower concentrations (30,000 ppm), which can kill larvae up to 50%, occurs 120 HAA. With a concentration of 50,000 ppm, 90% of larvae can be killed occurring 96 HAA.
Keywords: plant-based insecticide; Jatropha curcas L., Crocidolomia binotalis, mortality; concentration.
View full article (HTML)
Efficacy of Jatropha Curcas L. Seed Extract on Mortality of Cabbage Crop Larvae (Crocidolomia Binotalis Zeller: Lepidoptera: Pyralidae)
E.L. Baideng1,*, J.J. Pelealu1, B.H. Assa2, H.A.W. Lengkey3
1Department of Biology, Faculty of Mathematics and Natural Sciences, Sam Ratulangi University, Manado, Indonesia
2Department of Plant Pests and Diseases, Faculty of Agriculture, Sam Ratulangi University, Manado, Indonesia
3Faculty of Animal Husbandry, Universitas Padjadjaran, Jatinangor, Indonesia
*E-mail: evabaideng@unsrat.ac.id
Received: July 10, 2020. Revised: July 24, 2020. Accepted: Aug. 10, 2020. Published online: Oct. 16, 2020
ABSTRACT. Along with the awareness to obtain quality plant products, the use of plant-based insecticides is increasingly being used. One of the plants used as a plant-based insecticide is Jatropha curcas L. (Jarak pagar) because it contains toxic ingredients to kill cabbage caterpillar pests (Crocidolomia binotalis). This study aims to determine the effectiveness of Jatropha curcas L. on the mortality of Crocidolomia binotalis cabbage caterpillars. The research method used was a Completely Randomized Design (CRD) 7 × 3, consisting of seven treatment concentrations (0,000 ppm, 10,000 ppm, 20,000 ppm, 30,000 ppm, 40,000 ppm, 50,000 ppm, 60,000 ppm), with three replications. Observations were made at 24, 48, 72, 96, 120, 144 hours after application (HAA) of Jatropha. The research activities were carried out in two stages, namely 1) the extraction stage of Jatropha curcas L. seeds and the breeding of the Crocidolomia binotalis test larvae and 2) the testing stage with seven concentration levels of Jatropha curcas L. extract as a plant-based insecticide. ANOVA test showed that the treatments of Jatropha curcas L. extract gave the death effect on larvae [F-count > F-table (116.8 > 2.37)]. Dead larvae change color to black and their body shape will curve. The fastest larval death occurs 24 HAA, with a concentration of 40,000 ppm, which is 50%. At lower concentrations (30,000 ppm), which can kill larvae up to 50%, occurs 120 HAA. With a concentration of 50,000 ppm, 90% of larvae can be killed occurring 96 HAA.
Keywords: plant-based insecticide; Jatropha curcas L., Crocidolomia binotalis, mortality; concentration.
INTRODUCTION
In the cultivation of cabbage (Brassica oleraceae L.) in the province North Sulawesi, Indonesia, the use of chemical insecticides is still widely used in the cultivation of food crops, as well as excessive use due to the lack of information about the use of natural ingredients as insecticides and the lack of public knowledge.
The low production of cabbage, one of which is due to the attack of Plutella xylostella and Crocidolomia binotalis, and on the other hand, the population continues to increase, causing consumer needs for nutritious food and safe against pesticide residues, also continues to increase (Sembel et al., 2014). In addition, with increasing consumer knowledge and awareness about consuming food that is safe for health, thus, it is necessary to utilize natural ingredients as insecticides so that the agricultural products produced do not contain harmful chemical residues.
One of the plants that have insecticide properties is the Jatropha curcas L. plant. This plant also has many benefits, for example as biodiesel feedstock, in addition to treating wounds and bruises. Jatropha curcas L. is also useful as larvicide in controlling Aedes aegypti LC 50 1507 ppm mosquitoes at 24 hrs (Iswantini et al., 2011). Within the scope of agriculture it is also used as a molluscicide in controlling snail pests in rice plants. Nurwidayati et al. (2014) reported Jatropha curcas L. seed extract with a concentration of 32 ml/L and 64 ml/L of water within 24 hrs after application, can cause 100% of snail deaths. Banjarnahor et al. (2016) reported the use of Jatropha curcas L. seed extract with a concentration of 15 g/L and 20 g/L can cause 100% snail death on the third day. Jatropha curcas L. seeds contain phorbolester and curcin, which are very toxic in killing dead cells (Wina et al., 2008); also, contains alkaloids, saponins, cardenolins and bufadienols, flavonoids (Nurwidayati et al., 2014). Alkaloids are the compounds most commonly found and are found in leaves, stems, roots and seeds of plants and has toxic activity.
MATERIALS AND METHODS
The research was carried out in the Ecology and Biology Laboratory of the Faculty of Mathematics and Natural Sciences, Sam Ratulangi University, Indonesia, conducted in two stages, namely the first stage of preparation for extraction of Jatropha curcas L. seeds and Crocidolomia binotalis test larvae, and the second stage is the testing stage of Jatropha curcas L. extract. This study used a Completely Randomized Design (CRD), which consisted of seven treatments (0,000 ppm, 10,000 ppm, 20,000 ppm, 30,000 ppm, 40,000 ppm, 50,000 ppm, 60,000 ppm) of Jatropha curcas L. extract concentration, with three replications. Observations were made at 24, 48, 72, 96, 120, 144 HAA. Each treatment used ten Crocidolomia binotalis larvae.
Preparation of Jatropha curcas L. extraction
Jatropha curcas L. fruit is separated from the skin of the fruit to obtain the seeds, then the seeds are crushed and mashed using a disk mill and sifted using a sieve + 0.5 mm in diameter to obtain a powder that is ready for extraction. Extraction was carried out by immersion in methanol at a ratio of 1:10 (w/v) in an Erlenmeyer flask and shaken with a magnetic stirrer for + 24 hrs and counter-current distribution partitions (Dadang and Nugroho, 1999), the submersion results are filtered in stages using two Buchner funnels, the upper funnel using coarse filter paper, and for the lower funnel using Whatman # 41 fine filter paper. Then, the extract is collected in another Erlenmeyer flask. The filter results are rinsed repeatedly until the filter results become clear. After weighing, the filtered liquid is put into a vaporizer flask, then the methanol is evaporated with a rotary evaporator at a temperature of 45-500C, a rotational speed of 50-60 rpm, and a pressure of 150-200 mmHg. Containers containing extracts are weighed again, after evaporation is complete, to obtain the extract weight, i.e. the difference between the weighing before and after extraction.
The crude extract of the methanol fraction obtained from evaporation is partitioned in a hexane-methanol system (95%) in a ratio of 1:10:10 (w/v/v) in a separating flask for + 6 hrs, then the hexane phase is removed, after washing with methanol 95%. Then the 95% methanol phase is evaporated with a rotary evaporator, then repartitioned in the ethyl acetate-water system as described above. Then the water phase is discharged and the ethyl acetate phase obtained is stored in the refrigerator (400C), and ready for use.
Propagation of Crocidolomia binotalis larvae
Crocidolomia binotalis insects were obtained from the cabbage farmland of Rurukan – Tomohon village, North Sulawesi Province, and each larvae was kept in the laboratory in a separate plastic box that was given a stencil of paper base, until it became a pupa. During maintenance, larvae are given cabbage leaves as feed. The pupa is then taken from a plastic box, and then put into a maintenance jar, until it becomes an imago, then transferred to a container in which eight pots of cabbage plants have been placed, so the imago can lay its eggs for one day. As feed, imago is given a 30% honey solution that is applied to a plastic sheet hung in the container. After Crocidolomia binotalis eggs hatch into larvae, the larvae are ready to be used for testing. The third larval instar used, because in this stage, the instar has the active larvae damage. These larvae are then transferred into a Petri dish and fasted for 12 hrs, then immediately applied as a plant-based insecticide.
Mortality testing and monitoring
Testing of Jatropha curcas L. extract used seven treatment doses, namely 0,000 ppm, 10,000 ppm, 20,000 ppm, 30,000 ppm, 40,000 ppm, 50,000 ppm, 60,000 ppm, with three replications.
The prepared feed is dipped in the insecticide test solution for a few moments and dried for 15 min. The feed is then put into a test bottle containing 10 larval Crocidolomia binotalis. At this stage, Crocidolomia binotalis mortality was observed and a mortality count was calculated at 24, 48, 72, 96, 120 HAA. The data obtained were then analyzed by Analysis of Variance (ANOVA), and followed by the Least Significant Difference test (LSD) at the 5% level.
RESULTS AND DISCUSSION
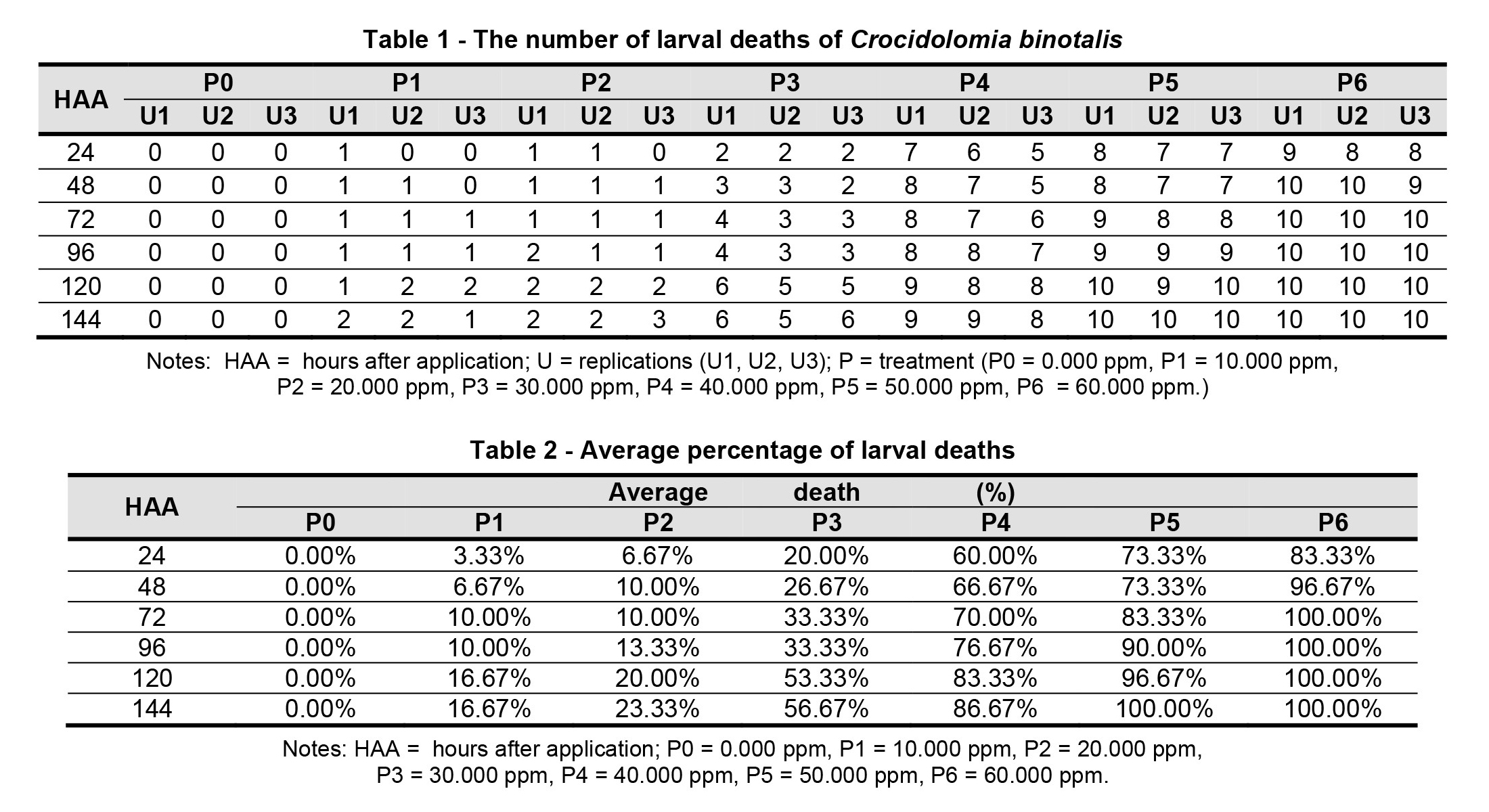
Observation data on the number of death of Crocidolomia binotalis larvae after the treatment of Jatropha curcas L. seed extract are shown in Table 1.
In Table 2, there is an average percentage of Crocidolomia binotalis larval death after treatment.
Giving Jatropha curcas L. seed extract on several concentrates gives the effect of death on larvae. Dead larvae change color to black and curved body shape. The most rapid death of larvae is more than 50%, occurring at 24 HAA, at a treatment concentrate of 40,000 ppm (P4). Meanwhile, for a smaller dosage of 30,000 ppm, it can cause more than 50% mortality, at a longer time after 120 HAA. The best concentration is 50,000 ppm (P5), which can kill up to 90% of larvae, after 96 HAA. From the percentage of larval mortality data, an analysis of variance (ANOVA) test of the mortality of larval Crocidolomia binotalis was conducted, due to the extraction of Jatropha curcas L., found in Table 3.
From the data in Table 3, the calculated F-value (116.88) is greater (>) than the F-table (2.37), this shows that the giving of Jatropha curcas L. seed extract significantly affected the mortality rate of the Crocidolomia binotalis larvae, then the Least Significant Difference (LSD) test contained in Table 4.
Based on LSD test data, the mortality rate of Crocidolomia binotalis larvae in the treatments P0, P1, P2, P3, P4, P5 and P6 obtained results that between treatments P0-P1, P2-P3, P3-P4, P4-P5 and P5-P6 have values greater than LSD values (0.1052), which means that they are significantly different at the 0.05 test level, but between P1-P2 has a value that is smaller than the LSD value that is only 0.0917, which means that it is not significantly different at the 0.05 test level. Based on LSD test, the best concentration to kill larvae is at the treatment of 40,000 ppm, which is 73.89%.
Sayuthi et al. (2014) states that the castorite seed fraction with a concentration of 60 ml/L at 120 HAA caused the mortality of C. pavonana caterpillars by 46.67%. Furthermore, Pebriansyah et al. (2016) stated that Jatropha curcas L. seed extract concentration of 10,000 ppm, at 96 HAA, caused mortality of C. pavonana more than 50%. The different effectiveness of the concentration that causes the death of larvae is influenced by several things including differences in the extraction method and the solvent used to extract.
From these observations it can be seen that Jatropha curcas L. can be used as an alternative insecticide to suppress the population of the Crocidolomia binotalis pest that attacks cabbage plants.
Tabel 3
Analysis of variance mortality of Crocidolomia binotalis larvae after treatment of Jatropha curcas L. extract
|
Source of variation |
SS |
df |
MS |
F-value |
P-value |
F-table |
|
Between groups |
5.66 |
6 |
0.94 |
116.88 |
1.16E-21 |
2.37 |
|
Within groups |
0.28 |
35 |
0.01 |
|
|
|
|
Total |
5.94 |
41 |
|
|
|
|
Table 4
Least Significant Difference test results (LSD) mortality rate of Crocidolomia binotalis larvae due to giving Jatropha curcas L. seed extract
|
Treatments |
Average |
LSD (0.05) = 0.1052 |
|
P0 |
0.0000 |
a |
|
P1 |
0.1056 |
b |
|
P2 |
0.1389 |
b |
|
P3 |
0.3722 |
c |
|
P4 |
0.7389 |
d |
|
P5 |
0.8611 |
e |
|
P6 |
0.9667 |
e |
Notes: The similar superscript in the same column has no significant difference (p> 0.05).
CONCLUSION
The mortality percentage of Crocidolomia binotalis larvae increased with increasing concentrations of Jatropha curcas L. extract, which was applied. The fastest death of larvae by 50% occurred at 24 hours after application (HAA), at a treatment concentrate of 40,000 ppm, while for a smaller dose of 30,000 ppm, to cause death by 50% occurs at a longer time, that is 120 HAA. The best concentration that can kill larvae up to 90%, at 96 HAA, at a dose of 50,000 ppm.
REFERENCES
Banjarnahor, I., Wibowo, L., Hariri, A.M. dan Hasibuan, R. (2006). Pengaruh pemberian ekstrak biji jarak pagar (Jatropha curcas L.) terhadap mortalitas keong mas (Pomacea sp.) dirumah kaca (Effect of Jatropha curcas L. extract on mortality of golden snail (Pomacea sp.) in greenhouses. J.Agrotek. Tropika, 4(2): 130-134.
Dadang dan Nugroho, B.W. (1999). Bahan pelatihan pengembangan dan pemanfaatan insektisida alami (Training materials for the development and utilization of natural insecticides), Bogor, 9-13 August 1999, Bogor, Pusat kajian Pengendalian Hama Terpadu Institut Pertanian Bogor, pp. 8-20.
Iswantini, D., Riyadhi, A., Kusumawati, U., Rosihan, R., Mangunwidjaja, D. dan Rahminiwati, M. (2011). Potensi jarak pagar (Jatropha curcas) sebagai larvasida hayati pencegah penyakit demam berdarah dengue (Potential of Jatropha (Jatropha curcas) as a biological larvicide to prevent dengue fever). J. IImu Pertanian Indonesia, 16(1): 7-13.
Nurwidayati, A., Srikandi, Y. dan Risti, R. (2014). Skrining fitokimia ekstrak jarak pagar (Jatropha curcas) dan ekstrak Jarak kastor (Riccinus communis) Famili Euphorbiaceae (Phytochemical screening of Jatropha curcas extract and Jatropha castor (Riccinus communis) family Euphorbiaceae). J. Vector Borne Dis., 8(1), 2014:15-20.
Pebriansyah, R., Yasin, N., Subeki dan Sudarsono, H. (2016). Toksisitas ekstrak biji jarak pagar (Jatropha curcas L.) terhadap ulat krop kubis (Crocidolomia pavonana F.) (Toxicity of jatropha seed extract (Jatropha curcas L.) against cabbage head caterpillar (Crocidolomia pavonana F.) J.Agrotek. 4(3): 211-216.
Sayuthi, M., Hasnah dan Jannah, S. (2014). Ekstrak daun pepaya dan biji jarak kepyar berpotensi sebagai insektisida terhadap hama Crocidolomia pavonana (Lepidoptera: Pyralidae) pada tanaman brokoli (Papaya leaf extract and castor bean seed potential as an insecticide against the Crocidolomia pavonana pest (Lepidoptera: Pyralidae) on broccoli plants). Jurnal Biologi Edukasi, Ed. 13, 6(2): 78-82.
Sembel, D.T. (2014). Serangga-serangga hama tanaman pangan umbi dan sayur (Insects – pest of tuber and vegetable food plants). Bayu Media Publishing, Malang, Indonesia.
Wina, E., Susana, I.W.R. dan Pasaribu, T. (2008). Pemanfaatan bungkil jarak pagar (Jatropha curcas) dan kendalanya sebagai bahan pakan ternak. (Utilization of Jatropha curcas and its constraints as animal feed ingredients). Wartazoa, 18(1): 1-8.