Christos Tsadilas, Eleftherios Evangelou, Thomai Nikoli, Miltiadis Tzioyvalekas
ABSTRACT. Phosphorus is considered as a basic essential element for plant growth which cannot be substituted or manufactured and is, therefore, a scarce resource to ensure food security; its sound management is considered important. One of the factors that play a significant role in its management is the determination of the critical available phosphorous (P) level in soil, in order to decide whether to apply P fertilizers or not. Since several soil and plant factors affect the value of the critical available soil P, it is considered necessary to carry out special experiments, in order to determine the soil critical P value for an area and a specific crop. The purpose of the present study was to establish critical soil and plant P values for wheat. A greenhouse pot experiment was performed on seven representative calcareous soils from the Thessaly plain in central Greece. The soils were deficient in available P and so they were fertilized with various rates of P. At appropriate stages, the above-ground plant parts were harvested and analysed for basic nutrients, including P. At the same time, soil samples were also taken and analysed for available P. The Cate and Nelson technique was applied, to determine critical values of both soil and wheat tissue P values. It was found that the critical soil P is 11 mg P kg-1 and the critical value for wheat tissue is 0.24% P dry matter.
Keywords: available P; critical values; wheat; calcareous soils; P fertilization.
Cite
ALSE and ACS Style
Tsadilas, C.; Evangelou, E.; Nikoli, T.; Tzioyvalekas, M. Determination of critical value of available soil phosphorus for wheat (Triticum aestivum L.) in calcareous soils from Greece. Journal of Applied Life Sciences and Environment 2021, 54(3), 322-332.
https://doi.org/10.46909/journalalse-2021-028
AMA Style
Tsadilas C, Evangelou E, Nikoli T, Tzioyvalekas M. Determination of critical value of available soil phosphorus for wheat (Triticum aestivum L.) in calcareous soils from Greece. Journal of Applied Life Sciences and Environment. 2021; 54(3): 322-332.
https://doi.org/10.46909/journalalse-2021-028
Chicago/Turabian Style
Tsadilas, Christos, Eleftherios Evangelou, Thomai Nikoli, and Miltiadis Tzioyvalekas. 2021. “Determination of critical value of available soil phosphorus for wheat (Triticum aestivum L.) in calcareous soils from Greece” Journal of Applied Life Sciences and Environment 54, no. 3: 322-332.
https://doi.org/10.46909/journalalse-2021-028
View full article (HTML)
Determination of Critical Value of Available Soil Phosphorus for Wheat (Triticum Aestivum L.) in Calcareous Soils from Greece
Christos Tsadilas1,*, Eleftherios Evangelou1, Thomai Nikoli1, Miltiadis Tzioyvalekas1
1Hellenic Agricultural Organization DEMETER, Institute of Industrial and Forage Crops
2Mediterranean Agronomic Institute of Chania, Department of Sustainable Agriculture, 73100 Chania, Greece
*E-mail: christotsadilas@gmail.com
Received: Jan. 11, 2022. Revised: Feb. 22, 2022. Accepted: Feb. 24, 2022. Published online: Mar. 02, 2022
ABSTRACT. Phosphorus is considered as a basic essential element for plant growth which cannot be substituted or manufactured and is, therefore, a scarce resource to ensure food security; its sound management is considered important. One of the factors that play a significant role in its management is the determination of the critical available phosphorous (P) level in soil, in order to decide whether to apply P fertilizers or not. Since several soil and plant factors affect the value of the critical available soil P, it is considered necessary to carry out special experiments, in order to determine the soil critical P value for an area and a specific crop. The purpose of the present study was to establish critical soil and plant P values for wheat. A greenhouse pot experiment was performed on seven representative calcareous soils from the Thessaly plain in central Greece. The soils were deficient in available P and so they were fertilized with various rates of P. At appropriate stages, the above-ground plant parts were harvested and analysed for basic nutrients, including P. At the same time, soil samples were also taken and analysed for available P. The Cate and Nelson technique was applied, to determine critical values of both soil and wheat tissue P values. It was found that the critical soil P is 11 mg P kg-1 and the critical value for wheat tissue is 0.24% P dry matter.
Keywords: available P; critical values; wheat; calcareous soils; P fertilization.
INTRODUCTION
Phosphorus (P) is one of the three basic essential macronutrients required for plant growth. To be substituted or manufactured it is not possible and, therefore, is a scarce resource for future food security. Before phosphate rocks discovery in the 19th century, phosphorus was largely supplied for crop production through manure, bone meal, human excreta and guano. Since the discovery of phosphate rocks, mined phosphate rocks have contributed the most to global fertilizer production. It is estimated that, today, about 90% of the world’s mined rocks are used in agriculture for food production and, to a lesser degree, for animal feed and food additives. In recent decades, this has lead to a decrease in quality and easily accessible phosphate reserves. There has also been a dramatic increase in the price of P fertilizers, which soared to an increase of about 800% during the 2008 food crisis (Neset and Cordel, 2012). It has been estimated that phosphate reserves and the availability of exploitable clean deposits are low and are expected to become exhausted soon, the sustainable management P has gained especially high importance. In 2014, rock phosphates were added to the list of Critical Raw Materials of the European Union with a substitutability index of 0.9 (where a maximum value of 1 is the least substitutable) (Ribarova et al., 2017). Some authors estimate that peak phosphorus consumption is expected to occur between 2030 and 2040 (Cordel et al., 2009), whereas others estimate that the available geological sources of phosphates will be adequate for more than 100 to 400 years (Vauren et al., 2010). Others put this figure at just 60 to 90 years (Lazaro et al., 2010) due to the lower quality, lower P content and greater concentration of heavy metals, particularly cadmium and uranium. On the other hand, it is estimated that out of the 90 to 95% of the P production which is used in agriculture, only 20 to 25% reaches the human food chain. This means that a serious modification of agricultural practices is needed to increase the efficiency of P use (Ribarova et al., 2017). In addition, all of the alternative sources with significant P content should be considered. One of these is municipal sewage sludge and this should be seriously investigated as an alternative source of P, since several studies have shown that P from municipal sewage sludge could be a P fertilizer substitute (Samaras et al., 2008). In order to face the P scarcity problem, which is globally recognized, a number of measures and practices are proposed, including the improvement of agricultural practices to increase P efficiency use in agriculture (Reijnders, 2014). Among the agricultural practices that may substantially help in improving efficient P use, are those referring to better guidance for P inputs in agriculture.
In soil, phosphorus occurs in several chemical forms, which are not all available to plants. Only some of them contribute to the available P pool, which also depends on soil properties, plant characteristics, and environmental conditions (Bollons and Barraclous, 1996; Fixen and Grove, 1990). Since phosphorous availability in soil is too high, monitoring of vegetation growth and analysing the available P in soil samples, which reflect the geographical heterogeneity of agricultural soils, is of critical importance. Phosphorus fertilization based on the critical values of available soil P, is significant. Another important factor in improving the efficient use of phosphorous is the balanced application of the basic nutrients, i.e. nitrogen (N), phosphorus (P) and potassium (K). Chuan et al. (2013) reported that estimating balanced nutrient requirements for wheat is essential to the management of more effective nutrient applications for increasing wheat yields and reducing the risk of environmental impacts. In a long term experiment using an appropriate model, it was found that, to produce 1 ton of grain, 22.8, 4.4 and 19.0 kg of N, P, and K were required, respectively; this corresponds to a N:P:K ratio of 5.18:1.00:4.32. The amounts of N, P, and K removed by the 1 ton of grain were 18.3, 3.6, and 3.5 kg, respectively, corresponding to an N:P:K ratio of 5.08:1.00:0.97. These two data sets should be considered when making balanced fertilizer recommendations for wheat, to avoid the excess or deficient nutrient supply. Another important factor affecting P-use efficiency of wheat is its variety. Akhtar et al. (2011) reported that one cultivar of wheat (MH-97), at an optimum N:P ratio of 1.5:1, provide a maximum response of 31.5%, while the other cultivar (Pasham-90) provide a maximum response of 25.9%, compared to the respective N-alone application. In general, it is accepted that lower crop yield may be attributed to non-balanced use of the basic nutrients N, P, and K (FAO, 2004). All these reasons, along with the dramatic increase of phosphate fertilizers, advocate the search for alternative strategies aimed at increasing efficiency of P-use in agriculture.
The classical way to determine the amount of fertilizers needed is through soil and plant testing. Concerning the testing of available soil P, several laboratory methods have been developed, including AB-DTPA, Bray P1 and P2, Mehlich I, II, III, Morgan, Truog, and Olsen, each performing with different accuracies depending on soil types. The Olsen method is the most widely used, especially in calcareous soils. The results of these laboratory methods have been correlated with plant responses through greenhouse and field studies. For this purpose, the establishment of field experiments are the most effective and result in safer results. They are widely used, e.g., Lin-Lin et al. 2015. However, pot experiments may also help manage this serious problem and they have been used alongside the field experiments (e.g., Goloran et al., 2014). In fact, sometimes greenhouse studies are preferred because they offer the possibility of eliminating the uncontrolled variables introduced in field experiments (Fixen and Grove, 1990). Soil samples are taken and analysed for the available nutrient forms and compared to the critical levels already established. In addition, plant tissue samples are also taken at appropriate growth stages and are analysed for nutrient concentrations.
The Cate-Nelson graphical technique (Cate and Nelson, 1965) has been used by several researchers for the determination of nutrients’ critical levels, mainly macronutrients (Bilias and Barbayiannis, 2016; Sobulo, 2008) and micronutrients (Nikoli et al., 2016). In this technique, data regarding the available soil nutrients (x-axis) are plotted against a biological parameter (y-axis), such as the plant tissue concentration of the nutrient, the absolute or relative yield, or the uptake, in order to separate the soils into two groups. The two groups are: i) those which are adequately provided with the nutrient under study and are not likely to respond to fertilization and ii) those which are not adequately provided with the nutrient and so the response to fertilization will be high. The point of the x-axis where these two groups separate is the critical level of the nutrient. A statistical procedure was developed by Cate and Nelson, 1971, where sequential analysis of the variance are performed on groups of the experimental data until R2 reaches a maximum value. At that point, the critical level of the nutrient is set.
There is no adequate information on the available P critical concentrations in Greek soils and wheat crops, which is important for Greek agriculture. The purpose of the present study was to estimate the critical levels of available soil phosphorus for wheat, as well as critical P concentrations in wheat tissues. This is important in calcareous soils with relatively high pH and high P fixing capacity.
MATERIALS AND METHODS
Soils
Seven surface (0-30) soil samples, which differed in P content and texture, were collected from representative soils of the Thessaly plain in central Greece. The soils were selected to represent regions where wheat is one of the main crops and their characteristics do not favor P availability. These soils are found in several places on the Thessaly plain, both in flat areas with alluvial, calcareous parent materials, as well as in sloping areas with eroded soils, where calcareous horizons were revealed at the surface. The classification of these soils is provided in Table 1. After air-drying, the soil samples were ground to pass a 6.35 mm sieve. Part of this soil material was passed through a 2 mm sieve and analysed for its physical-chemical properties in triplicate, whereas the remaining material was retained and used for the pot experiment with winter wheat (Triticum aestivum L.).
Particle-size analysis was performed by the hydrometer method (Bouyoucos, 1962), pH and electrical conductivity (EC) were measured in 1:1 suspensions with water, and organic carbon was determined by the wet oxidation method (Walkley and Black, 1934). The method used for carbonate content was the Bernard method, by measuring the evolved CO2 after the addition of HCl (Nelson, 1982).
Exchangeable cations were determined after extraction with 1M CH3COONH4 at pH 7, with a Jenway PFP7 flame photometer used for measuring K. Ca and Mg were determined with a Thermo iCE 3000 atomic absorption spectrophotometer.
Soil-available P was extracted by employing the method suggested by Olsen and Sommers (1982) and determined using spectrophotometry.
Greenhouse experiment
Three kg of each soil were mixed with four rates of a composite fertilizer 15-15-15 (15% N, 15% P2O5, 15% K2O) (0-Control, 2.5 g P-Low corresponding to 55 mgP/kg soil, 5 g P-Medium corresponding to 110 mgP/kg soil, 10 g P-High corresponding to 220 mgP/kg soil) and placed in plastic pots in four replications.
The pots were placed in a non-heated greenhouse, of completely randomized design and randomization was repeated every 20 days. Wheat was sown using 10 g of wheat seed and ten days later the plants were thinned to 6-8 per pot. Plants grown took place under natural lighting conditions and irrigated with distilled water until 60% of the field capacity was reached. The experiment started in the middle of March and 40 days after sowing the above-ground biomass of wheat was harvested (Mills and Jones, 1996). The plant material was dried at 70oC for 48 h until a constant weight was reached, weighed and ground. For plant analysis were used duplicates of ~1.0 g sub-samples, involving dry ashing (heating at 450oC for 5 h and digestion with 1N HCl) (Jones and Case, 1990) and Plant N content (1 g samples) was determined by the Kjeldahl wet-oxidation method (Bremner and Mulovaney, 1982). Tissue P concentrations and biomass dry weight were used to calculate the plant uptake of P per pot.
Statistical analysis
A simple linear regression analysis was carried out between P concentration in the soil and P in plant tissue or P uptake by wheat. Variance analysis was carried out between treatments of P application. For the determination of the critical levels of P, the Cate-Nelson graphical technique and statistical procedure were applied to the biomass yield of wheat and soil P data.
RESULTS AND DISCUSSION
Soil properties
The soil samples used in this experiment varied in terms of their basic physical-chemical properties. Three out of the seven soils tested were clayey and the rest of them were moderately coarse to moderately fine. All of them were inorganic and calcareous in reaction. Mean values of the main properties varied among soils and are presented in Table 1.
Effect of the fertilizer on soil-available P and on the nutrient status of wheat
Initial soil-available phosphorous concentrations ranged from 2.4 to 19.0 mg kg-1 soil (Table 2). These values are considered to be low, according to Poulton et al. (2013), who evaluated the response of winter wheat to Olsen P on a silty clay loam soil. They suggested that the critical Olsen P for wheat and barley ranged between 7 and 18 mg kg-1 and recommended maintaining Olsen P to about 20 mg kg-1 in the plough layer, for cereals grown on similar soils, to minimize the risk of losing yield. Critical Olsen P limits were found to be related to soil properties. For example, Sanchez-Alcala et al. (2015) reported that critical Olsen P was lower in soils with medium pH and positively correlated with pH and calcium carbonate content.
Table 1
Selected properties of the soils studied
|
a/a |
pH |
Sand |
Clay |
Loam |
Texture |
Soil classification |
Electrical conducti-vity |
CaCO3 |
Organic matter |
|
(H2O 1:1) |
(%) |
|
(μS cm-1) |
(%) |
|||||
|
1 |
8,2 |
38 |
23 |
39 |
L |
Typic Xerofluents |
212 |
3,7 |
1,2 |
|
2 |
8,3 |
20 |
57 |
23 |
C |
Vertic Xerofluvents |
569 |
13 |
1,6 |
|
3 |
8,4 |
42 |
21 |
37 |
L |
Typic Xerochrept |
150 |
2,4 |
1,0 |
|
4 |
8,5 |
39 |
39 |
22 |
CL |
Typic Xerorthent |
340 |
1,8 |
0,3 |
|
5 |
8,4 |
27 |
51 |
22 |
C |
Vertic Xerofluvent |
503 |
14 |
1,6 |
|
6 |
8,5 |
49 |
31 |
20 |
SCL |
Typic Xerothent |
283 |
8,4 |
0,8 |
|
7 |
8,2 |
19 |
55 |
26 |
C |
Vertic Xerofluvent |
569 |
4,8 |
1,7 |
In the present study, the addition of the composite fertilizer containing 15% of all the basic nutrients N, P (as P2O5), and K as (K2O) (marked as 15-15-15) caused an increase in soil available P, wheat biomass yield and P concentration in wheat tissues (Fig. 1 and Fig. 2). Plant tissue P concentrations varied from deficient (<0.15-0.20%) to sufficient (0.20-0.50%) ranges, according to values reported by Sanchez (2007), while nitrogen, potassium, calcium, and magnesium contents in general, varied at sufficient levels. Analysis of variance (ANOVA) and a post hoc test were performed with SPSS. The results showed that wheat yield (Fig. 1) and concentration of P in wheat tissue (Fig. 2) differed significantly between treatments.
As shown in Fig. 3, a significant correlation (R2=0.56, P<0.05) was obtained for the relationship between soil available P and P uptake by wheat.
Table 2
Soil available P (POlsen) and K, wheat yield, and nutrient content
|
POlsen |
Kexch |
Wheat Yield |
Ν |
P |
K |
Ca |
Mg |
|
mg kg-1 |
mg kg-1 |
g pot-1 |
% |
% |
% |
% |
% |
|
2,4∂ |
55 |
0,62 |
1,3 |
0,05 |
1,6 |
0,42 |
0,08 |
|
29ℓ |
793 |
9 |
4 |
0,39 |
4,6 |
3,4 |
0,85 |
|
11,5ᶴ (6.6) |
81 (180) |
4,9 (2.2) |
2,3 (0.6) |
0,21 (0.06) |
2,9 (0.61) |
1,2 (0.49) |
0,33 (0.14) |
Minimum ∂, maximum ℓ and average ᶴ values. Standard deviation is reported in parenthesis
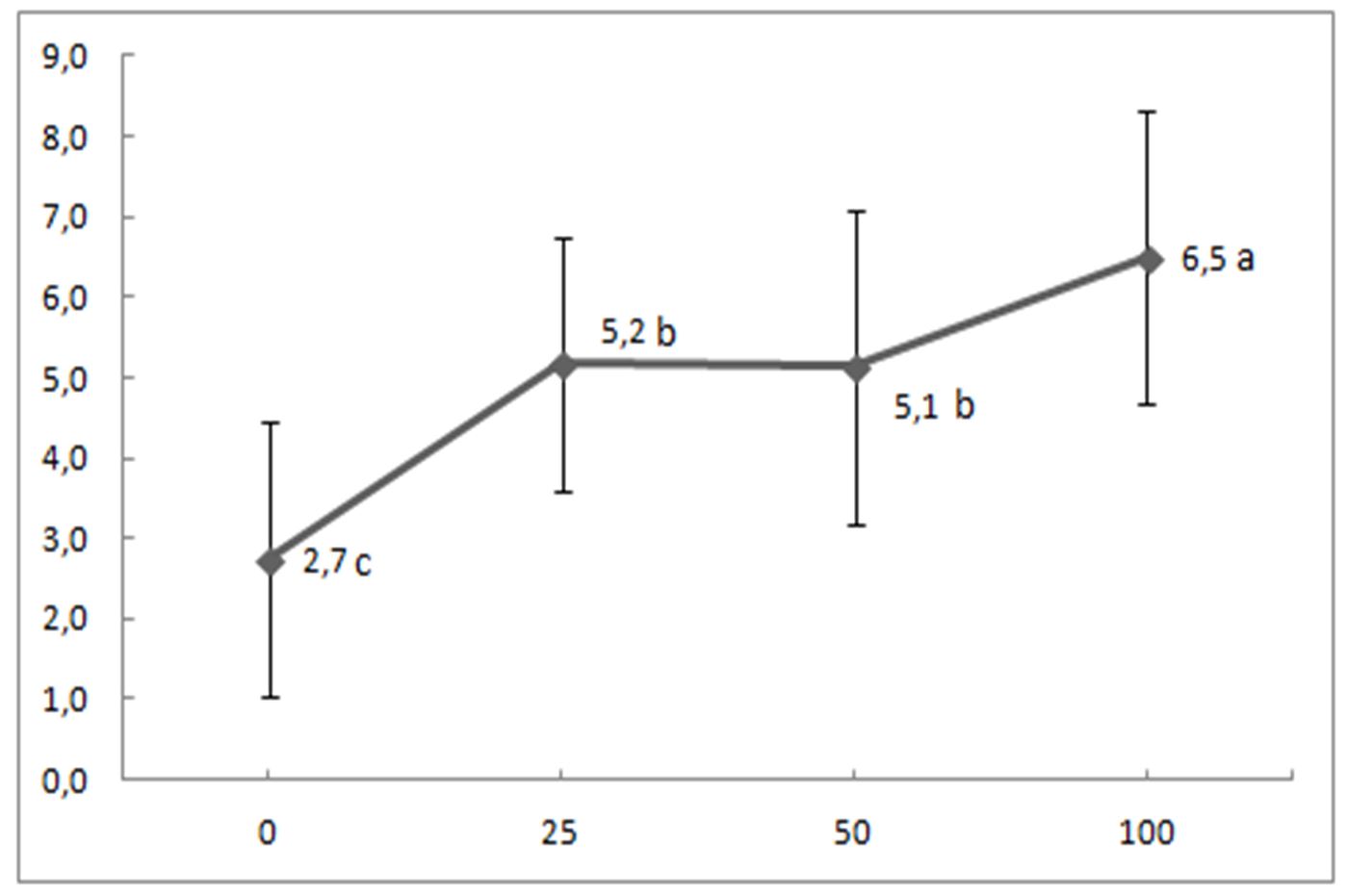
Figure 1 – Relationship between P application rate (mg P ha-1) and wheat yield (g pot-1). Treatments with different letters differ significantly according to the LSD test (P<0. 05).
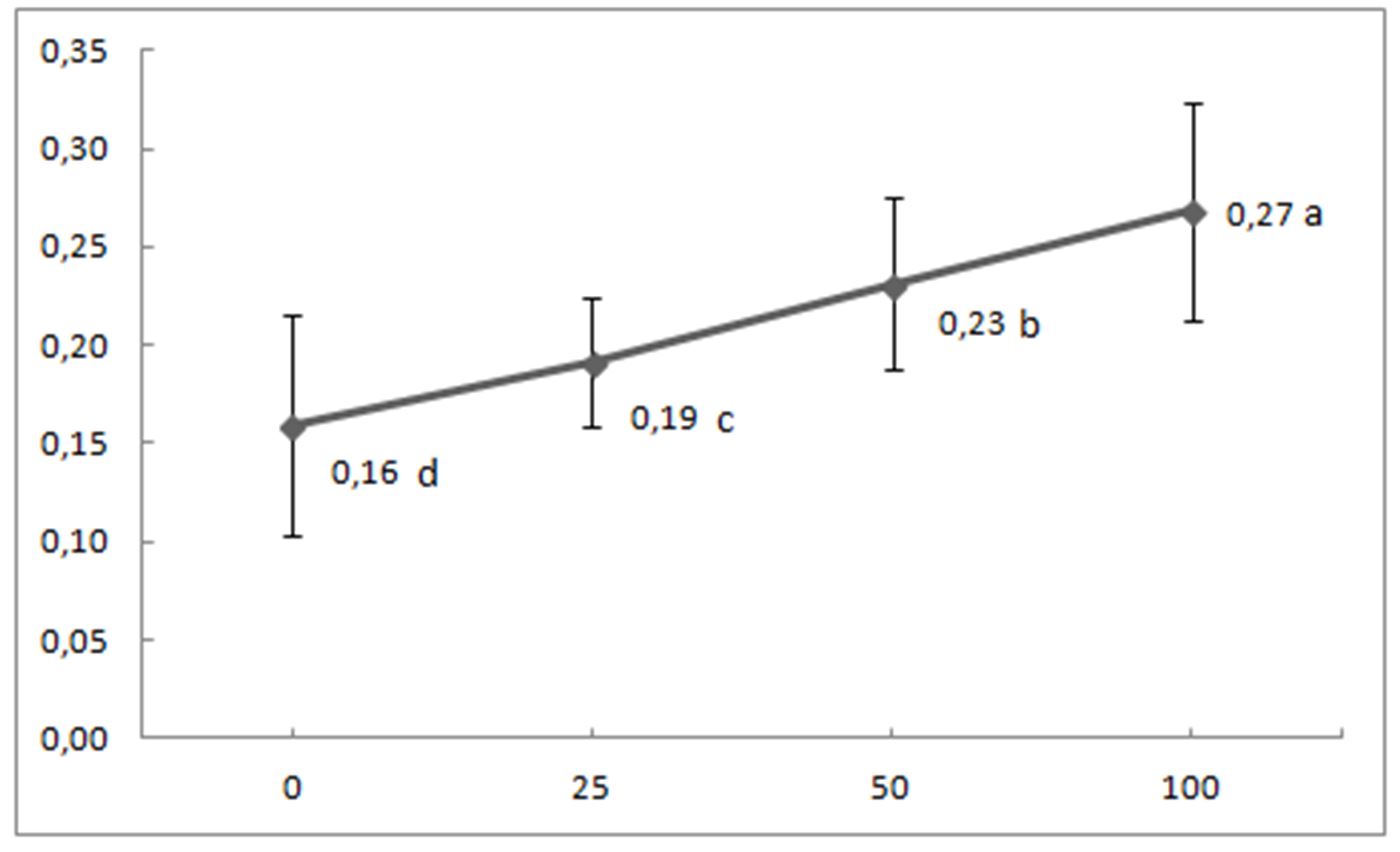
Figure 2 – Relationship between P application rate (mg P ha-1) and P in wheat foliage (P %). Treatments with different letters differ significantly according to the LSD test (P<0. 05).
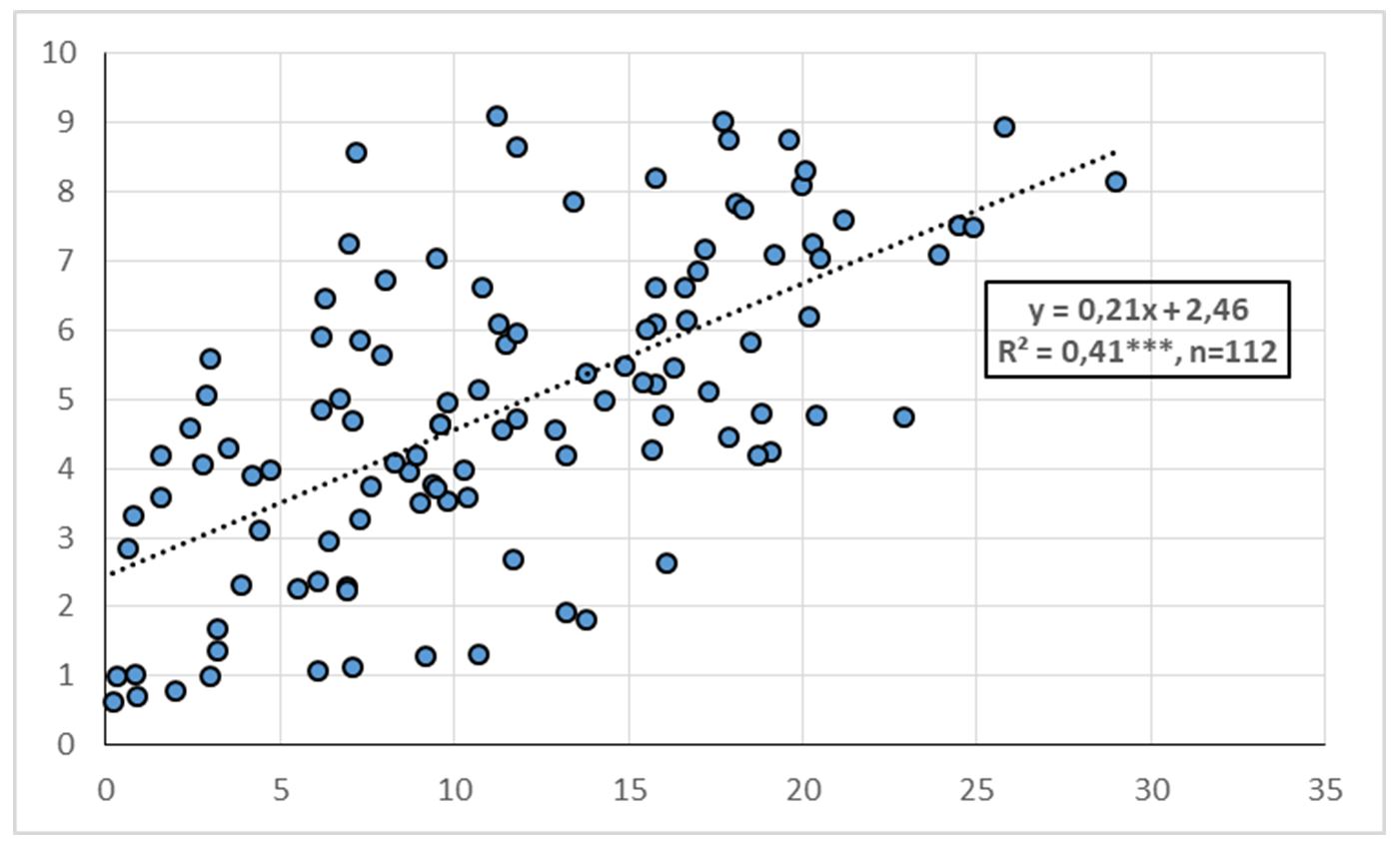
Figure 3 – Relationship between available soil P (POlsen) (X-axis, mgP/kg) and P uptake by wheat (Y-axis, g/pot)
Phosphorus critical levels
The graphical technique developed by Cate and Nelson (1965) was used for the determination of critical levels of P. Data for soil-available P, extracted by the Olsen method (Olsen and Sommers, 1982), were plotted against either wheat above-ground biomass yield or P in wheat foliage. As shown in Fig. 4 and Fig. 5, the soils under study were successfully divided into two groups: those that are insufficiently supplied with P (where the probability of responding to P application is high) and those that are sufficiently supplied with P (and not likely to respond to P application). The results were verified by employing the statistical procedure suggested by the same authors (Cate and Nelson, 1971). The coefficients of determination (R2) reached maximum values of 11 mg P kg-1 soil and 0.24 mg P kg-1 dry matter of wheat plant tissue (R2 = 0.36 and 0.34, respectively).
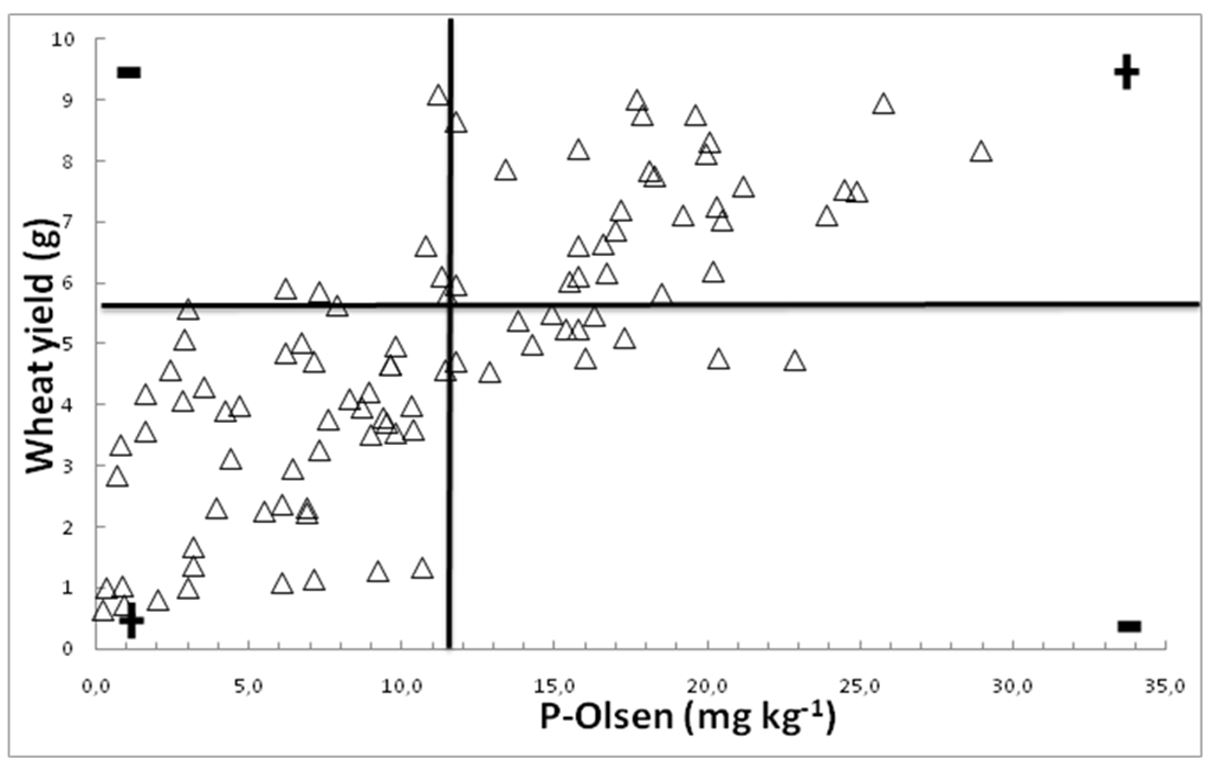
Figure 4 – Critical levels of soil available P (mg P kg-1 soil), extracted with the Olsen method, determined with the Cate-Nelson technique
Therefore, according to this analysis, the critical value of soil-available P for wheat in the soils is 11 P mg kg-1 (Fig. 4 and Fig. 5). The respective critical value for wheat tissue P is 0.24% (Fig. 5), similar to that referred to above. The differences recorded for the critical values reflect the influence of both the soil and plant factors that affect the availability of phosphorus to the plants. It may be concluded that there is no constant value for available soil P that could be used universally, but special experimental trials have to be carried out in order to establish critical values of available soil P in an area and for a special crop.
CONCLUSIONS
The critical values of soil phosphorous are seriously influenced by several factors, both soil and plants. Consequently, in order to recommend fertilization rates, special experimentation is needed for the soils and crops in an area. In this study, a short-term pot experiment was conducted, with the aim of establishing critical available soil P values and plant tissue P concentrations.
It has been shown that, in representative Greek calcareous soils, the critical value of available soil P for wheat was found to be 11 mg P kg-1 while the respective critical value for wheat tissue P concentration is 0.24% dry matter. Further field experimentation is needed to create safe results, which can be used as guidance values for the estimation of the adequate available soil P for a wheat crop.
Funding. This research received no external funding.
Conflicts of Interest. The authors declare no conflict of interest.
REFERENCES
Akhtar, M., Tahir, S., Asraf, M.Y., Akhter, J. & Alam, S.M. (2011). Influence of different rates of phosphorus on growth, yield and phosphorus use efficiency in two wheat cultivars. J. Plant Nutr., 34: 1223-1235, DOI: 10.1080/01904167.20 11.558163.
Bilias, F. & Barbayiannis, N. (2016). Evaluation of sodium tetraphenylboron (NaBPh4) as a soil test of potassium availability. Arch. Agron. Soil Sci., 63(4): 468-476, DOI: 10.1080/03650 340.2016.1218479.
Bolland, M.D.A. & Paynter, B.H. (1944). Critical phosphorus concentrations for Burr medic, Yellow sarradella, Subterranean clover and wheat. Commun. Soil Sci. Plant Anal., 25(3&4): 385-394, DOI: 10.1080/0010 3629409369045.
Bollons, H.M. & Barraclough, P.B. (1999). Assessing the phosphorus status of winter wheat crops: inorganic orthophosphate in whole shoots. J. Agric. Sci., Cambridge, 133: 285-295, DOI: 10.1017/S0021859699007 066.
Bouyoucos, G.J. (1962). Hydrometer method improved for making particle size analysis of soils. Agron. J., 54: 464-465, DOI: 10.2134/agronj1962.00 021962005400050028x.
Bremner, J.M. & Mulvaney, C.S. (1982). Nitrogen-total. In: A.L. Page et al. (Eds.) Methods of soil analysis. Part 2, 2nd ed., pp. 595-624. Agron. Monogr., 9. ASA and SSSA, Madison, WI.
Cate, R.B., Jr. & Nelson, L.A. (1965). A rapid method for correlation of soil test analysis with plant response data. N.C. State Univ. Agric. Exp. Stn., Int. Soil Testing series, Tech. Bull., no. 1.
Cate, R.B., Jr. & Nelson, L.A. (1971). A simple statistical procedure for partitioning soil test correlation data into two classes. Soil Sci. Soc. Am. Proc., 35: 658-660, DOI: 10.2136/sssaj1971. 03615995003500040048x.
Chuan, L., He, P., Jin, J., Li, S., Grant, C., Xu, X., Qiu, S., Zhao, S. & Zhou, W. (2013). Estimating nutrient uptake requirements for wheat in China. Field Crops Res., 146: 96-104, DOI: 10.10 16/j.fcr.2013.02.015.
Cordell, D., Drangert, J.O. & White, S. (2009). The story of phosphorus: global food security and food for thought. Glob. Environ. Change, 19: 292-305, DOI: 10.1016/j.gloenvcha.2008.10.009.
Fixen, P.E. & Grove, J.H. (1990). Testing soils for phosphorus. In: R.L. Westerman (Ed.), Soil testing and plant analysis, 3nd ed., pp. 141-180. SSSA, Inc, Mad. WI., USA.
F.A.O. (2006). Plant nutrition for food security. A guide for integrated nutrient management. Bull. 16, Rome, p. 366.
Goloran, J.B., Phillips, I.R., Xu, Z.H., Condron, L.M. & Chen, C.R. (2014). Effects of amendments and fertilization on plant growth, nitrogen and phosphorus availability in rehabilitated highly alkaline bauxite-processing residue sand. Soil Use Manag., 30:198-208, DOI: 10.1111/sum.12109.
Jackson, G.D., Kushnak, G.D., Carlson, G.R., Wichman, D.M. & Jacobsen, J.S. (1991). Correlation of the Olsen phosphorus soil test: Winter wheat response. Commun. Soil Sci. Plant Anal., 22(9&10): 907-918, DOI: 10.1080/00103629109368463.
Jones, J.B., Jr. & Case, V.W. (1990). Sampling, handling, and analyzing plant tissue samples. In: R.L. Westerman (Ed.), Soil testing and plant analysis. 3nd ed., pp 389-427. SSSA Inc., Madison, WI.
Lazaro, L., Abbate, P.E., Cogliatt, D.H. & Andrade, F.H. (2010). Relationship between yield, growth and spike weight in wheat under phosphorus deficiency and shading. J. Agric. Sci., 148: 83-93, DOI: 10.1017/S0021859609990402.
Mills, H.A. & Jones, J.B., Jr. (1996). Plant analysis handbook II. A practical sampling, preparation, analysis, and interpretation guide. MicroMacro Publishing, Inc., Athens, Georgia, USA, p. 422.
Nelson, R.E. (1982). Carbonate and gypsum. In: A.L. Page (Ed.), Methods of soil analysis, Part 2, 2nd ed., pp. 181-197. Agron. Monogr., 9. ASA and SSSA, Madison, WI.
Neset, T-S. S. & Cordell, D. (2012). Global phosphorus scarcity: identifying synergies for a sustainable future. J. Sci. Food Agric., 92: 2-6, DOI: 10.1002/ jsfa.4650.
Nikoli, T., Matsi, T. & Barbayiannis, N. (2016). Assessment of nickel’s sufficiency critical levels in cultivated soils, employing commonly used calibration techniques. J. Plant Nutr. Soil Sci., 179: 566-573, DOI: 10.1002/ jpln.201600173.
Olsen, S.R., Cole, C.V., Watanabe, F.S. & Dean, L.A. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U.S. Dept. of Agriculture, Serie Circular, no. 939.
Olsen, S.R., & Sommers, L.E. (1982). Phosphorus. In: Page A.L. et al. (Eds.) Methods of soil analysis, Part 2, 2nd ed., pp. 403-430. Agron Monogr., 9. ASA and ASSA, Madison WI.
Poulton, P.R., Johnston, A.E. & White, R.P. (2013). Plant-available soil phosphorus. Part I: the response of winter wheat and spring barley to Olsen P on silty clay loam. Soil Use Manag., 29: 4-11, DOI: 10.1111/j.1475-2743.20 12.00450.x.
Rashid, A., Awan, Z.I. & Ryan, J. (2005). Diagnosing phosphorus deficiency in spring wheat by plant analysis: Proposed critical concentration ranges. Commun. Soil Sci. Plant Anal., 36: 609-622, DOI: 10.1081/CSS-200043299.
Reijnders, L. (2014). Phosphorus resources, their depletion and conservation, a review. Resour., Conser. Recyc., 93: 32-49, DOI: 10.1016/j.resconrec.2014.09.006.
Ribarova, I., Dimitrova, S., Lambeva, R., Wintgens, T., Stemann, J. & Remmen, K. (2017). Phosphorus recovery potential in Sofia WWTP in view of the national sludge. Resour., Conser. Recyc., 116, 152-159. DOI: 10.1016/ j.resconrec.2016.10.003
Samaras, V., Tsadilas, C.D. & Stamatiadis, S. (2008). Effects of repeated application of municipal sewage sludge on soil fertility, cotton yield, and nitrate leaching. Agron. J., 100(3): 477-483, DOI: 10.2134/agronj2007.0162.
Sanchez, C.A. (2007). Phosphorus. Ιn: A.V. Barker and D.J. Pilbeam (Eds.), Handbook of plant nutrition, pp. 51-90. CRC Press, Boca Raton.
Sanchez-Alcala, I., Campillo, M.C. & Torrent, J. (2015). Critical Olsen P and CaCl2-P levels as related to soil properties: results from micropot experiments. Soil Use Manag., 31: 233-240, DOI: 10.1111/sum.12184.
Shi, L., Shen, M., Lu, C., Wang, H., Zhou, X., Jin, M. & Wu, T. (2015). Soil phosphorus dynamic, balance and critical P values in long-term fertilization experiment in Taihu Lake region, China. J. Integr. Agric., 14(12): 2446-2455, DOI: 10.1016/S2095-3119(15) 61183-2.
Sobulo, R.A. (1983). Critical soil and plant potassium for maize. Commun. Soil Sci. Plant Anal., 14(8): 727-737, DOI: 10.1080/00103628309367403.
Van Vuuren, D.P., Bouwman, A.F. & Beusen, A.H.W. (2010). Phosphorus demand for the 1970-2100 period: a scenario analysis of resource depletion. Glob. Environ. Change, 20: 428-439, DOI: 10.1016/j.gloenvcha.2010.04.004.
Walkley, A., & Black, I.A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci,. 37(1): 29-38, DOI: 10.1097/00010694-19340 1000-00003.
Evangelou Eleftherios, Nikoli Thomai, Tsadilas Christos, Tzioyvalekas Miltiadis




