Gabriela Buema, Nicoleta Lupu, Horia Chiriac, Dumitru Daniel Herea, Lidia Favier, Gabriela Ciobanu, Loredana Forminte (Litu), Maria Harja
ABSTRACT. The fly ash generated from a Romanian power plant was used as a starting material in this study. The aim of the study was to obtain a low cost material based on the treatment of fly ash with Fe3O4 for utilization as an adsorbent for cadmium ion removal. The adsorbent that was synthesized was characterized using different techniques. The adsorption process was investigated by the batch technique at room temperature. The quantity of cadmium ion adsorbed was measured spectrophotometrically. The experimental data showed that the material can remove cadmium ions at all three working concentrations. The adsorption capacity increased with an increase in concentration, respectively contact time. The results were analyzed through two kinetic models: pseudo first order and pseudo second order. The kinetics results of cadmium adsorption onto a magnetic material are in good agreement with a pseudo second order model, with a maximum adsorption capacity of 4.03 mg/g, 6.73 mg/g, and 9.65 mg/g. Additionally, the pseudo second order model was linearized into its four types. The results indicated that the material obtained show the ability to remove cadmium ions from an aqueous solution.
Keywords: magnetic adsorbent; adsorption; cadmium ions; kinetic study.
Cite
ALSE and ACS Style
Buema, G.; Lupu, N.; Chiriac, H.; Herea, D.D.; Favier, L.; Ciobanu, G.; Forminte (Litu), L.; Harja, M. Fly ash magnetic adsorbent for cadmium ion removal from an aqueous solution. Journal of Applied Life Sciences and Environment 2021, 54(1), 42-50.
https://doi.org/10.46909/journalalse-2021-004
AMA Style
Buema G, Lupu N, Chiriac H, Herea DD, Favier L, Ciobanu G, Forminte (Litu) L, Harja M. Fly ash magnetic adsorbent for cadmium ion removal from an aqueous solution. Journal of Applied Life Sciences and Environment. 2021; 54(1): 42-50.
https://doi.org/10.46909/journalalse-2021-004
Chicago/Turabian Style
Buema, Gabriela, Nicoleta Lupu, Horia Chiriac, Dumitru Daniel Herea, Lidia Favier, Gabriela Ciobanu, Loredana Forminte (Litu), and Maria Harja. 2021. “Fly ash magnetic adsorbent for cadmium ion removal from an aqueous solution” Journal of Applied Life Sciences and Environment 54, no. 1: 42-50.
https://doi.org/10.46909/journalalse-2021-004
View full article (HTML)
Fly Ash Magnetic Adsorbent for Cadmium Ion Removal from an Aqueous Solution
Gabriela Buema1, Nicoleta Lupu1, Horia Chiriac1, Dumitru Daniel Herea1, Lidia Favier2, Gabriela Ciobanu3, Loredana Forminte (Litu)3, Maria Harja3,*
1National Institute of Research and Development for Technical Physics, Iasi, Romania
2Université de Rennes, École Nationale Supérieure de Chimie de Rennes, CNRS, ISCR-UMR6226, F-35000 Rennes, France
3“Gheorghe Asachi” Technical University of Iasi, Faculty of Chemical Engineering and Environmental Protection, Iasi, Romania
*E-mail: mharja@tuiasi.ro
Received: Mar. 02, 2021. Revised: Mar. 17, 2021 Accepted: Mar. 26, 2021. Published online: Mar. 31, 2021
ABSTRACT. The fly ash generated from a Romanian power plant was used as a starting material in this study. The aim of the study was to obtain a low cost material based on the treatment of fly ash with Fe3O4 for utilization as an adsorbent for cadmium ion removal. The adsorbent that was synthesized was characterized using different techniques. The adsorption process was investigated by the batch technique at room temperature. The quantity of cadmium ion adsorbed was measured spectrophotometrically. The experimental data showed that the material can remove cadmium ions at all three working concentrations. The adsorption capacity increased with an increase in concentration, respectively contact time. The results were analyzed through two kinetic models: pseudo first order and pseudo second order. The kinetics results of cadmium adsorption onto a magnetic material are in good agreement with a pseudo second order model, with a maximum adsorption capacity of 4.03 mg/g, 6.73 mg/g, and 9.65 mg/g. Additionally, the pseudo second order model was linearized into its four types. The results indicated that the material obtained show the ability to remove cadmium ions from an aqueous solution.
Keywords: magnetic adsorbent; adsorption; cadmium ions; kinetic study.
INTRODUCTION
Cadmium ions have a negative effect on human health because of its toxicity (Abbasi et al., 2020; Buema et al., 2020). Many researchers have investigated the possibility of eliminating cadmium ions from an aqueous solution (Bagheri et al., 2019; Es-Sahbany et al., 2021). One of the most applied methods to remove cadmium ions is the adsorption process (Sun et al., 2019; Yang et al., 2018; Yilmaz et al., 2020; Zhang et al., 2021). For example, hydroxyapatite porous materials were used as adsorbents for cadmium removal (Ramdania et al., 2020). Yaacoubi et al. studied the removal of cadmium from water using a natural phosphate as an adsorbent (Yaacoubi et al., 2014). Biochars Produced from Agro-Residues adsorbents were studied by the research group of López et al. (2020). Also, the literature shows studies about recycling fly ash in adsorption domain, taking into account that the unused fly ash presents a problem (specifically, the modified fly ashes as adsorbent were investigated (Harja et al., 2015; Mushtaq et al., 2019; Buema et al., 2020; Huang et al., 2020).
In the last period, of particular interest among the adsorbents presented in the literature are magnetic materials based on fly ash. An advantage of the synthesized adsorbent is that it is easily separated from the aqueous solution using an external magnet.
The properties of fly ash were combined with the properties of Fe3O4 in order to obtain a good magnetic adsorbent. Therefore, the aim of this study was to evaluate the ability of one material obtained from fly ash treated with Fe3O4 to be used in the cadmium ion adsorption process. The obtained product was analysed through morphological, chemical, physical, and magnetic properties.
The capacity of this material for cadmium adsorption was discussed as a function of the initial concentration and contact time. Kinetic data were evaluated with the pseudo first order model and pseudo second order model (four types of its linearization).
MATERIALS AND METHODS
The fly ash used in this research was collected from CET II Holboca, located in Iasi, North-East Romania. Fe3O4 was purchased from Alfa Aesar and used without any pre-treatment. All chemical reagents were analytical grade and used as received.
Material synthesis
The adsorbent was prepared as described in a previous work (Harja et al., 2021). Fly ash was used as the starting material. The adsorbent was prepared by mixing the fly ash with Fe3O4; a ratio of 9/1 Fly ash/ Fe3O4 was used. The contact time of the synthesis was 4 h.
It should be noted that the adsorbent synthesized is quickly separated from the aqueous solution by an external magnetic field.
Material characterization
The obtained adsorbent was characterized before the adsorption study. Thus, several techniques were applied for the characterization: SEM, EDAX, XRD, FTIR, BET surface area, and VSM.
Adsorption experiments
The initial cadmium solutions were prepared by diluting a stock cadmium nitrate tetrahydrate solution (500 mg/L) with distilled water.
Two adsorption conditions, such as initial cadmium concentration and contact time, were used in order to study the cadmium adsorption capacity and cadmium removal efficiency, respectively.
The experiments were performed at laboratory room temperature, a pH of 5 in the Erlenmeyer flasks containing 25 mL of solution with an initial cadmium concentration of 50 mg/L, 70 mg/L, and 100 mg/L, and 20 mg of the adsorbent. The prepared material was withdrawn from the aqueous solutions, and the cadmium concentration in the supernatant was analyzed using a UV–vis spectrophotometer at 576 nm using xylenolorange (C31H28N2Na4O13S). The experiments were performed with intermittent stirring.
The parameters used in this experiment are presented in Table 1.
Table 1
Parameters used in the batch experiments
|
Parameter |
Value |
|
pH |
5 |
|
Adsorbent dose |
20 mg/25 mL |
|
Initial Concentration, mg/L |
50, 70, 100 |
|
Contact time, min |
5-120 |
|
Temperature, oC |
25 |
The cadmium adsorption capacity, q (mg/g), was calculated using Eq. (1):
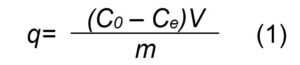
The cadmium removal efficiency, R (%), was calculated using Eq. (2):
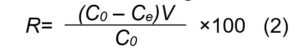
where, C0 and Ce are the initial and equilibrium cadmium concentrations (mg/L), q is the amount of cadmium adsorbed onto the adsorbent (mg/g), V represents the volume of cadmium solution (L), m is the quantity of adsorbent (g).
RESULTS AND DISCUSSION
Characterization of adsorbent
The comprehensive characteriza-tion of the adsorbent can be found in the literature (Harja et al., 2021). An overview of the material characteriza-tion is presented below (Fig. 1).
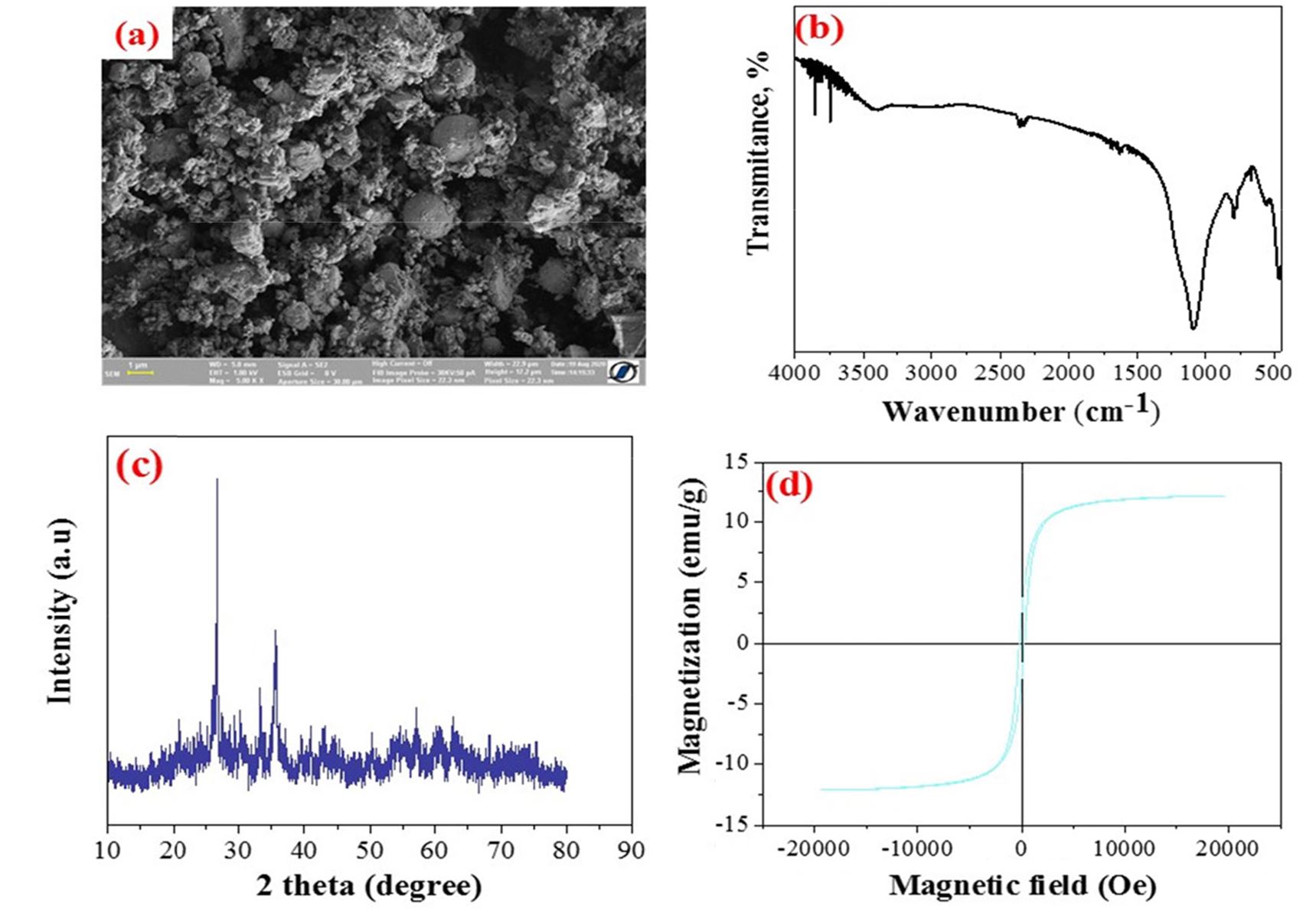
Figure 1 – Adsorbent characterization (a) SEM analysis; (b) FTIR analysis; (c) XRD analysis; (d) VSM analysis
The surface area of the adsorbent is 6.153 m2/g, while the chemical composition obtained through EDAX analysis revealed that the adsorbent contains C: 18.25%, O: 46.72%, Si: 13.95%, Al: 10.22%, Ca: 1.7%, Fe: 7.74%, K: 0.41%, Mg: 0.34%, Ti: 0.67%.
Kinetic study
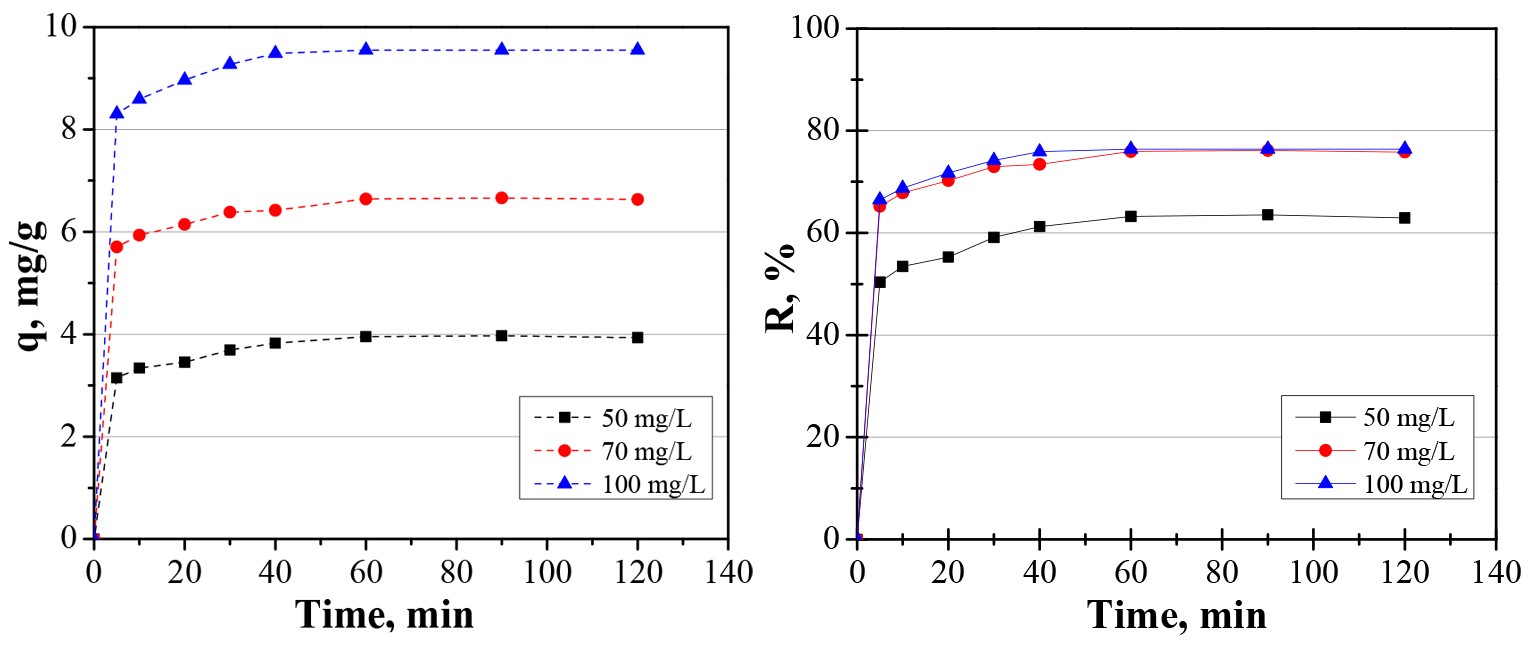
The establishment of the time needed to reach equilibrium in the adsorption process is essential in wastewater treatment applications. The parameters for the design of adsorption devices can be established through the results of the kinetic study. The influence of contact time was studied at three different concentrations. The study was conducted with 20 mg/25 mL dose of adsorbent, a pH value of 5, 120 min contact time, and a temperature of 25oC with intermittent stirring. The results are presented in Fig. 2. This shows that there is an increase in adsorption capacity when the initial cadmium concentration was increased from 50 mg/L to 70 mg/L and 100 mg/L. On the other hand, the adsorption capacity was increased by increasing the contact time from 5 to 120 min. Also, the results show that the equilibrium is reached quickly (in approx. 45 min of contact time). This fact can be attributed to the active sites of the adsorbent surface.
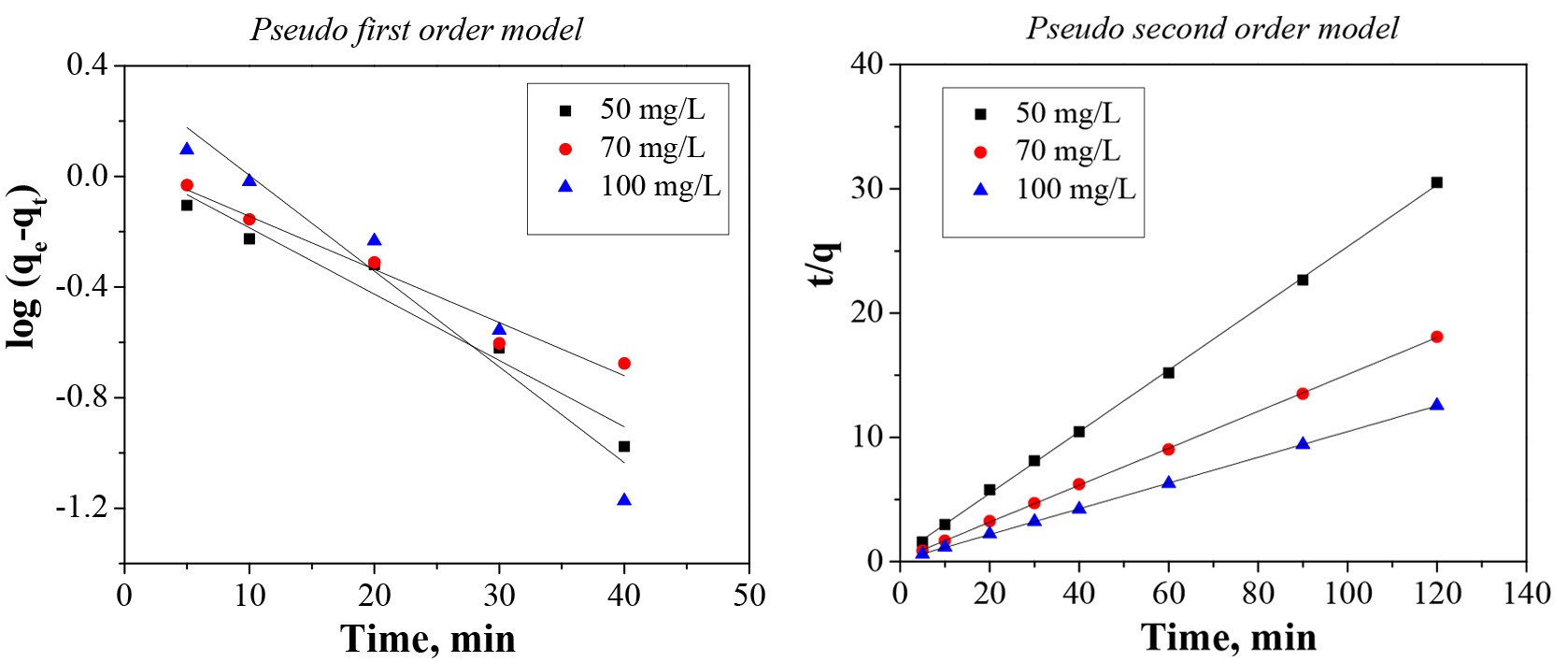
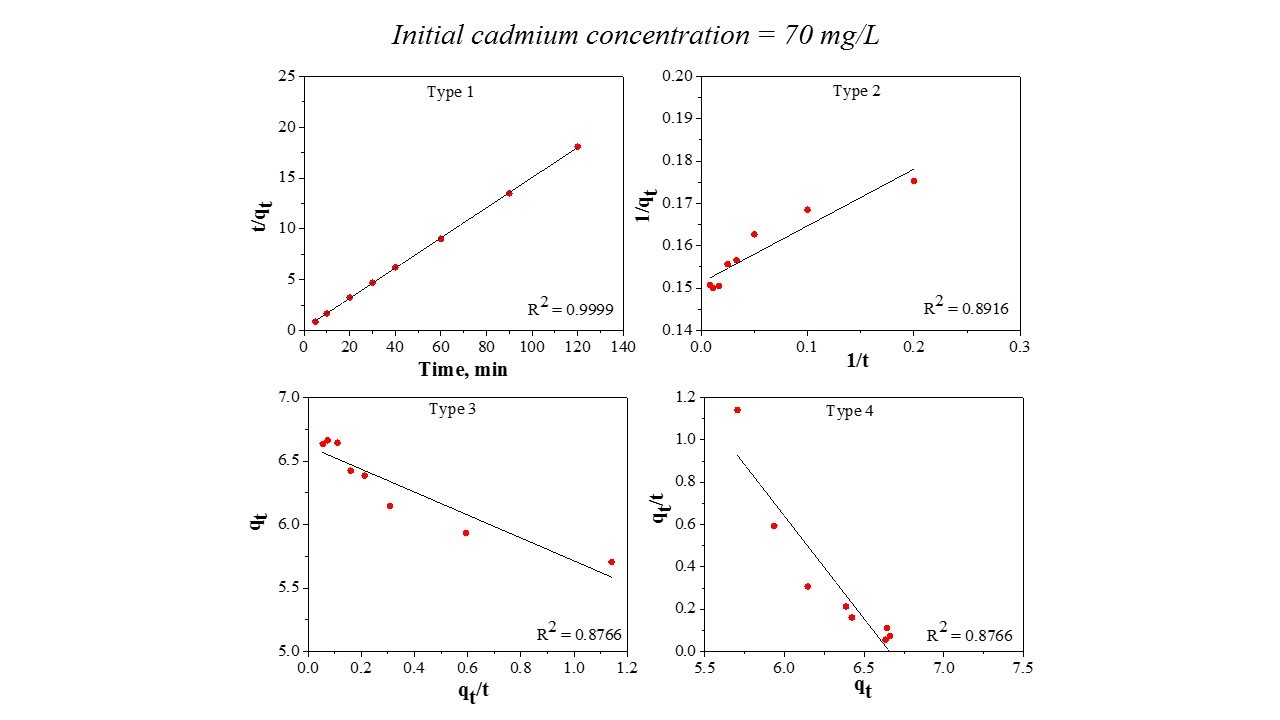
The data obtained in the batch experiments at 50 mg/L, 70 mg/L, and 100 mg/L were analyzed for the best fit through two kinetic models: pseudo first order and pseudo second order (Buema et al., 2020), which are graphically represented in Fig. 3. The calculated parameters are presented in Table 2. From Table 2, it can be seen that the k1 values calculated from the pseudo first order model for cadmium adsorption onto the prepared material were 0.0552, 0.0442, and 0.0796. The k2 values were 0.1168, 0.1064, and 0.0976. The calculated adsorption capacity is consistent with the experimental data. Based on the correlation coefficient, R2, it can be stated that the kinetic data are better fit through the pseudo second order model (Pashai Gatabi et al., 2016; Kahrizi et al., 2018), with a maximum adsorption capacity of 4.03 mg/g, 6.73 mg/g, and 9.65 mg/g. Additionally, the pseudo second order model was linearized in its four linearization types, Fig. 4 (a-c).
Table 2
Kinetic parameters of cadmium adsorption
|
Initial cadmium concentration |
Pseudo first order model |
Pseudo second order model |
||||
|
|
k1, 1/min |
R2 |
qe exp, mg/g |
qe cal, mg/g |
k2, g/mg min |
R2 |
|
50 mg/L |
0.0552 |
0.9563 |
3.93 |
4.03 |
0.1168 |
0.9996 |
|
70 mg/L |
0.0442 |
0.9722 |
6.63 |
6.73 |
0.1064 |
0.9999 |
|
100 mg/L |
0.0796 |
0.9463 |
9.55 |
9.65 |
0.0976 |
0.9999 |
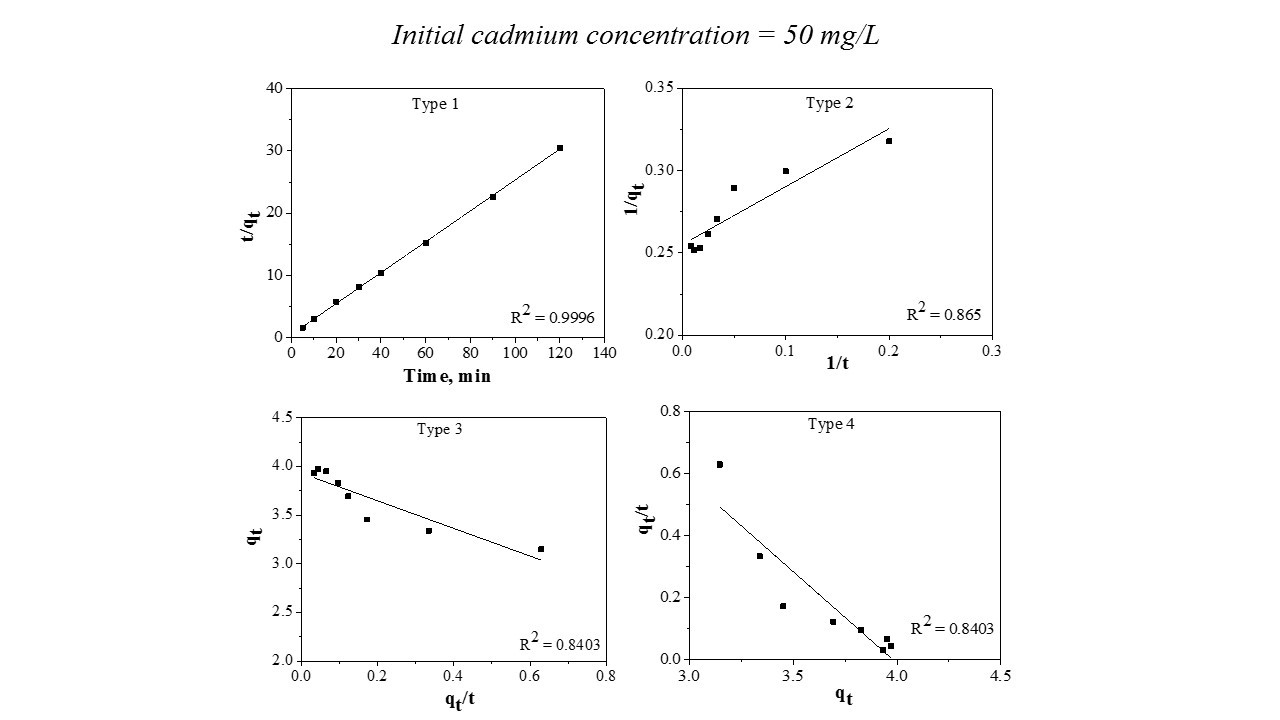
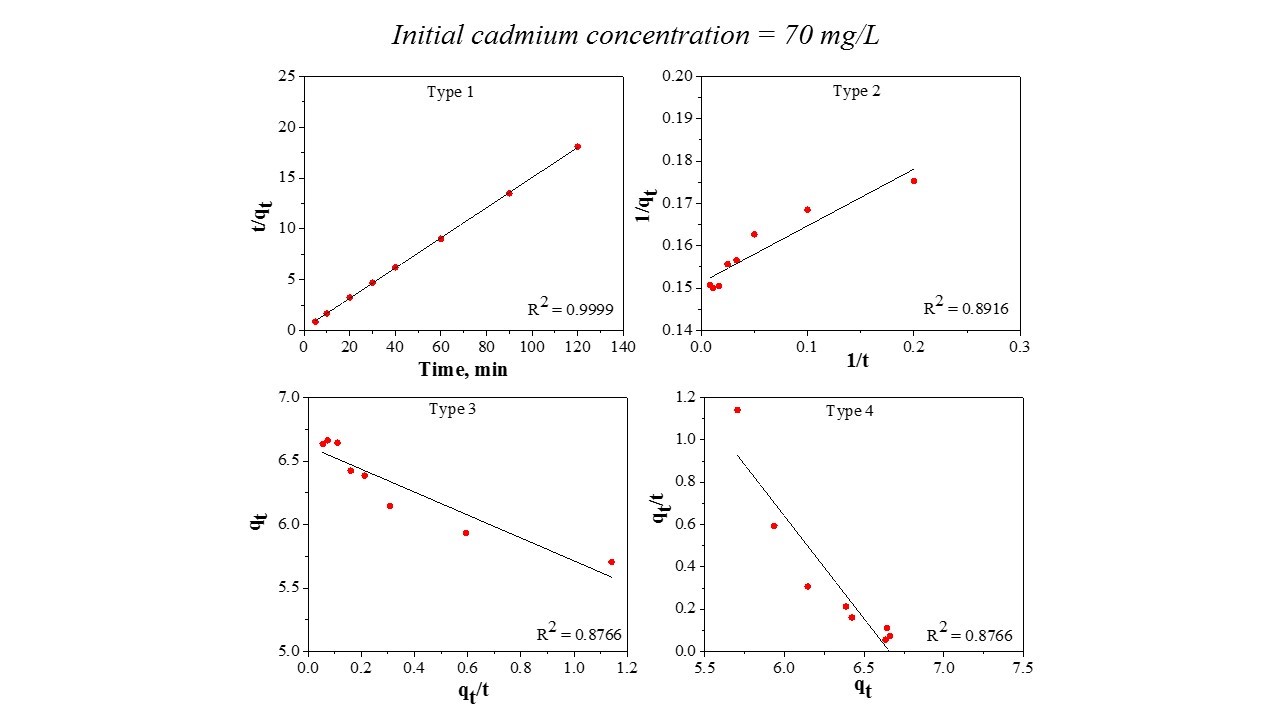
Figure 4 – Pseudo second order model Type 1 – Type 4
This model was linearized into four forms (Type 1 – Type 4) in order to make a comparison between the differences that occur from the application of the linear regression method to kinetic data. It should be noted that the most popular linear form used in the specialized literature is Type 1. Table 3 reports the data.
From Table 3, it can be seen that the values of the k parameter obtained from the four linear forms are different. The correlation coefficient value, R2, of Linear Type 1 shows the highest result. The other three types of the pseudo second order model exhibited an unfavorable fit between linearized versions with the experimental data, the R2 values being lower compared with Type 1. Based on the results obtained, it can be stated that is not recommended to estimate the pseudo second order parameters by Type 2, 3, and 4. Consequently, Type 1 is able to represent the cadmium adsorption onto the prepared material.
Table 4 shows a comparison of the cadmium adsorption capacities reported in the literature and those obtained for the magnetic material prepared in this study.
Table 3
Pseudo second order kinetic parameters obtained from the linear forms
|
Kinetic model |
Parameters |
Values |
||
|
50 mg/L |
70 mg/L |
100 mg/L |
||
|
Type I |
qe (mg/g) |
4.03 |
6.73 |
9.65 |
|
k (g/mg min) |
0.1168 |
0.1064 |
0.0976 |
|
|
R2 |
0.9996 |
0.9999 |
0.9999 |
|
|
Type II
|
qe (mg/g) |
3.92 |
6.61 |
9.57 |
|
k (g/mg min) |
0.1852 |
0.1707 |
0.1234 |
|
|
R2 |
0.865 |
0.8916 |
0.9094 |
|
|
Type III |
qe (mg/g) |
3.93 |
6.62 |
9.57 |
|
k (g/mg min) |
0.3614 |
0.1365 |
0.0897 |
|
|
R2 |
0.8403 |
0.8766 |
0.8995 |
|
|
Type IV |
qe (mg/g) |
3.98 |
6.66 |
9.62 |
|
k (g/mg min) |
0.1485 |
0.1457 |
0.1089 |
|
|
R2 |
0.8403 |
0.8766 |
0.8995 |
|
Table 4
Summary of cadmium adsorption capacity from selected studies
|
Adsorbent |
q, mg/g |
References |
|
Magnetic biochar composite |
1.67; 2.74; 2.95 |
Reddy and Lee, 2014 |
|
Iron oxide nanoparticles with tangerine peel extract |
10.9 |
Ehrampoush et al., 2015 |
|
BC600, BC800, MBC600-0.6300, MBC800-0.6300 |
10.82; 13.02; 24.32; 39.26 |
Khan et al., 2020 |
|
Imogolite, Magnetite, Imo-Fe25, Imo-Fe50 |
9.1; 13.1; 17.4; 22.7 |
Arancibia-Miranda et al., 2020 |
|
Fe3O4-chitosan composite |
4.78; 9.34; 13.69; 17.85 |
Rai et al., 2021 |
|
Deposited silt |
0.0453 |
Korake and Jadhao, 2021 |
|
FA/Fe3O4 |
4.03; 6.73; 9.65 |
Current study |
It must be emphasized that the adsorption conditions were different. From Table 4, it can be seen that the magnetic material obtained shows good adsorption capacity.
CONCLUSION
This research presents preliminary results of an ongoing study regarding cadmium adsorption using a low cost magnetic material based on fly ash. On the basis of the results, the following conclusions can be drawn:
The adsorbent synthesized was characterized using six basic techniques.
The influence of two parameters, initial cadmium concentration and contact time, were investigated for the cadmium adsorption experiments performed in this study. The results show that the adsorption of cadmium ion on the adsorbent surface is dependent on the contact time and initial concentration.
The study reported that using the proposed working conditions, cadmium ions were removed with an adsorption capacity of 4.03 mg/g, 6.73 mg/g, and 9.65 mg/g, respectively.
The results were fitted using two kinetic models. The R2 values for the pseudo second order model were higher compared to the pseudo first order model for all three working concentrations. Consequently, it can be stated that the chemical adsorption process is predominant.
The overall results suggest that a magnetic material can be used as a low cost adsorbent for treating aqueous solutions polluted with cadmium ions.
Acknowledgment. This work is funded by the UEFISCDI Agency through Project PN-III-P1-1.2-PCCDI-2017-0152 (Contract No. 75PCCDI/2018).
REFERENCES
Abbasi, H., Salimi, F. & Golmohammadi, F. (2020). Removal of cadmium from aqueous solution by nano composites of bentonite/TiO2 and bentonite/ZnO using photocatalysis adsorption process. Silicon, 12: 2721-2731. https://doi.org/10.1007/ s12633-019-00372-6
Bagheri, S., Amini, M.M., Behbahani, M. & Rabiee, G. (2019). Low cost thiol-functionalized mesoporous silica, KIT-6-SH, as a useful adsorbent for cadmium ions removal: A study on the adsorption isotherms and kinetics of KIT-6-SH. Microchem.J., 145: 460-469. https://doi.org/10.10 16/j.microc.2018.11.006
Buema, G., Lupu, N., Chiriac, H., Roman, T., Porcescu, M., Ciobanu, G., Burghila, D.V. & Harja, M. (2020). Eco-Friendly materials obtained by Ffy ash sulphuric activation for cadmium ions removal. Materials, 13(16): 3584. https://doi.org/10.3390/ma13 163584
Buema, G., Harja, M., Lupu, N., Chiriac, H., Forminte, L., Ciobanu, G., Bucur, D. & Bucur, R.D. (2021). Adsorption performance of modified fly ash for copper ion removal from aqueous solution. Water, 13: 207. https://doi.org/10.3390/w13020207
Es-sahbany, H., El Hachimi, M.L., Hsissou, R., Belfaquir, M., Es-sahbany, K., Nkhili, S., Loutfi, M. & Elyoubi, M.S. (2021). Adsorption of heavy metal (Cadmium) in synthetic wastewater by the natural clay as a potential adsorbent (Tangier-Tetouan-Al Hoceima-Morocco region). Materials Today: Proceedings. https://doi.org/10.1016/ j.matpr.2020.12.1102
Harja, M., Buema, G., Bulgariu, L., Bulgariu, D., Sutiman, D.M. & Ciobanu, G. (2015). Removal of cadmium (II) from aqueous solution by adsorption onto modified algae and ash. Korean J.Chem.Eng., 32: 1804-1811. https://doi.org/10.1007/ s11814-015-0016-z
Harja, M., Buema, G., Lupu, N., Chiriac, H., Herea, D.D. & Ciobanu, G. (2021). Fly ash coated with magnetic materials with improved adsorption capacities. Materials, 14: 63. https://doi.org/10.3390/ma14010063
Huang, X., Zhao, H., Hu, X., Liu, F., Wang, L., Xin Zhao, X., Gao, P. & Ji, P. (2020). Optimization of preparation technology for modified coal fly ash and its adsorption properties for Cd2+. J.Hazard.Mater., 392: 12246. https://doi.org/10.1016/ j.jhazmat.2020.122461
Kahrizi, P., Mohseni-Shahri, F.S. & Moeinpour, F. (2018). Adsorptive removal of cadmium from aqueous solutions using NiFe2O4/ hydroxyapatite/graphene quantum dots as a novel nano-adsorbent. J. Nanostructure Chem., 8: 441-452. https://doi.org/10.1007/s40097-018-0284-3
López, J.E., Builes, S., Heredia Salgado, M.A., Tarelho, L.A.C., Arroyave, C., Aristizábal, A. & Chavez, E. (2020). Adsorption of cadmium using biochars produced from agro-residues. J.Phys.Chem. C, 124(27): 14592-14602.
Mushtaq, F., Zahid, M., Bhatti, I.A., Nasir, S. & Hussain, T. (2019). Possible applications of coal fly ash in wastewater treatment. J.Environ. Manage., 240: 27-46. https://doi.org/ 10.1016/j.jenvman.2019.03.054
Pashai Gatabi, M., Milani Moghaddam, H. & Ghorbani, M. (2016). Efficient removal of cadmium using magnetic multiwalled carbon nanotube nanoadsorbents: equilibrium, kinetic, and thermodynamic study. J. Nanoparticle Res., 18: 189. https:// doi.org/10.1007/s11051-016-3487-x
Ramdania, A., Kadechea, A., Adjdirb, M., Talebc, Z., Ikhoua, D., Talebc, S. & André Deratani, A. (2020). Lead and cadmium removal by adsorption process using hydroxyapatite porous materials. Water Pract.Technol., 15(1): 130. https://doi.org/10.2166/wpt.2020.003
Sun, H., Xia, N., Liu, Z., Kong, F. & Wang, S. (2019). Removal of copper and cadmium ions from alkaline solutions using chitosan-tannin functional paper materials as adsorbent. Chemosphere, 236: 124370. https://doi.org/10.1016/j.che mosphere.2019.124370
Yaacoubi, H., Zidani, O., Mouflih, M., Gourai, M. & Sebti, S. (2014). Removal of Cadmium from water using Natural phosphate as Adsorbent. Procedia Eng., 83: 386-393. https://doi.org/10.1016/j.proeng. 2014.09.039
Yang, T., Sheng, L., Wang, Y., Wyckoff, K.N., He, C. & He, Q. (2018). Characteristics of cadmium sorption by heat-activated red mud in aqueous solution. Sci.Rep., 8: 1355. https://doi. org/10.1038/s41598-018-31967-5
Yilmaz, E., Erenler, F.M. & Boztuğ, A. (2020). Synthesis and modification of amine-terminated maleic anhydride butyl acrylate copolymer and investigation of adsorption properties for cadmium (II) ions (Cd2+). J.Mol.Struct., 1222:128924. https://doi.org/10.1016/j.molstruc.2020.128924
Zhang, D., Zhang, K., Hu, X., He, Q., Yan, J. & Xue, Y. (2021). Cadmium removal by MgCl2 modified biochar derived from crayfish shell waste: Batch adsorption, response surface analysis and fixed bed filtration. J.Hazard.Mater., 408:124860. https://doi.org/10.1016/j.jhazmat.2020.124860.
Buema Gabriela, Chiriac Horia, Ciobanu Gabriela, Favier Lidia, Forminte (Litu) Loredana, Harja Maria, Herea Dumitru Daniel, Lupu Nicoleta