Bala Mohan, Sheela Priyadarshinee, Ramaswamy Kalpana, Periyakali Saravana Bhavan, Narasimman Manickam, Perumal Santhanam, Duraiswamy Prabha
ABSTRACT. The plankton communities are important source of food for the aquatic organisms, and if any undesirable changes in aquatic environment may affect plankton diversity and density. Therefore, assessment of planktonic communities in the freshwater ecosystems is essential because they serve as bio-indicators of water quality parameters. Hence, the present research was focused to evaluate the freshwater phytoplankton and zooplankton diversity and their abundance in Valankulam Lake (Lat. 10.59° N and Long. 76.57° E), at Coimbatore city, Tamil Nadu, India. Results from the study revealed that a total of 77 species of phytoplankton and zooplankton were recorded, under 37 families and 46 genera. In addition to that, a total of 43 phytoplankton species were recorded under 25 families and 30 genera, (which includes; 15 species of Cyanophyceae, 17 species of Chlorophyceae, 08 species of Bacillariophyceae, 03 species of Euglenophyceae). and a total of 34 species of zooplankton were recorded under 12 families and 17 genera, (which includes 13 species of Rotifera, 09 species of Cladocera, 08 species of Copepoda and 04 species of Ostracoda). The maximum plankton diversity was observed during the monsoon season and the minimum in the summer season. Results from study revealed the ecological status of the lake is categorized as moderately polluted due to the presence of municipal waste and industrial discharges into the lake water. Therefore, the assessment of planktonic communities in water bodies will be useful to monitor and maintain the water quality parameters and wealth of aquatic biota in the aquatic ecosystem.
Keywords: plankton; species composition; community structure; water quality.
Cite
ALSE and ACS Style
Mohan, B.; Priyadarshinee, S.; Kalpana, R.; Bhavan, P.S.; Manickam, N.; Santhanam, P.; Prabha, D. Impact of seasonal changes in freshwater phytoplankton and zooplankton biodiversity at Valankulam lake, Coimbatore district, Tamil Nadu, India. Journal of Applied Life Sciences and Environment 2022, 55 (3), 271-292.
https://doi.org/10.46909/alse-552063
AMA Style
Mohan B, Priyadarshinee S, Kalpana R, Bhavan PS, Manickam N, Santhanam P, Prabha D. Impact of seasonal changes in freshwater phytoplankton and zooplankton biodiversity at Valankulam lake, Coimbatore district, Tamil Nadu, India. Journal of Applied Life Sciences and Environment. 2022; 55 (3): 271-292.
https://doi.org/10.46909/alse-552063
Chicago/Turabian Style
Mohan, Bala, Sheela Priyadarshinee, Ramaswamy Kalpana, Periyakali Saravana Bhavan, Narasimman Manickam, Perumal Santhanam, and Duraiswamy Prabha. 2022. “Impact of seasonal changes in freshwater phytoplankton and zooplankton biodiversity at Valankulam lake, Coimbatore district, Tamil Nadu, India” Journal of Applied Life Sciences and Environment 55, no. 3: 271-292.
https://doi.org/10.46909/alse-552063
View full article (HTML)
Impact of Seasonal Changes in Freshwater Phytoplankton and Zooplankton Biodiversity at Valankulam Lake, Coimbatore District, Tamil Nadu, India
Bala MOHAN1a*, Sheela PRIYADARSHINEE2, Ramaswamy KALPANA1b, Periyakali Saravana BHAVAN1b, Narasimman MANICKAM3, Perumal SANTHANAM3 and Duraiswamy PRABHA1a
1 Bharathiar University, Coimbatore, 641 046, Tamil Nadu, India;
a Department of Environmental Sciences; b Department of Zoology
2 Government Arts College, PG & Research Department of Zoology, Coimbatore – 641 018, Tamil Nadu, India
3 Bharathidasan University, Department of Marine Science, Tiruchirappalli – 620 024, Tamil Nadu, India
*Correspondence: mohannethu300@gmail.com
Received: Dec. 10, 2022. Revised: Feb. 13, 2023. Accepted: Feb. 14, 2023. Published online: Feb. 27, 2023
ABSTRACT. The plankton communities are important source of food for the aquatic organisms, and if any undesirable changes in aquatic environment may affect plankton diversity and density. Therefore, assessment of planktonic communities in the freshwater ecosystems is essential because they serve as bio-indicators of water quality parameters. Hence, the present research was focused to evaluate the freshwater phytoplankton and zooplankton diversity and their abundance in Valankulam Lake (Lat. 10.59° N and Long. 76.57° E), at Coimbatore city, Tamil Nadu, India. Results from the study revealed that a total of 77 species of phytoplankton and zooplankton were recorded, under 37 families and 46 genera. In addition to that, a total of 43 phytoplankton species were recorded under 25 families and 30 genera, (which includes; 15 species of Cyanophyceae, 17 species of Chlorophyceae, 08 species of Bacillariophyceae, 03 species of Euglenophyceae). and a total of 34 species of zooplankton were recorded under 12 families and 17 genera, (which includes 13 species of Rotifera, 09 species of Cladocera, 08 species of Copepoda and 04 species of Ostracoda). The maximum plankton diversity was observed during the monsoon season and the minimum in the summer season. Results from study revealed the ecological status of the lake is categorized as moderately polluted due to the presence of municipal waste and industrial discharges into the lake water. Therefore, the assessment of planktonic communities in water bodies will be useful to monitor and maintain the water quality parameters and wealth of aquatic biota in the aquatic ecosystem.
Keywords: plankton; species composition; community structure; water quality.
INTRODUCTION
Water is a prime abiotic factor that supports life and major components in the natural environment. Any undesirable changes in the hydrographical profile can influence the life of aquatic biota, where different species of flora and fauna can show great variations in environmental ecosystem (Dey et al., 2021; Pinheiro et al., 2021). The aquatic ecosystems can be divided into types namely, freshwater and marine water ecosystems. The total availability of freshwater on the earth is hardly gently 0.3 to 0.5 % and they are very essential to aquatic biota and to the human beings (Maltby et al., 2011). The rivers, lakes, ponds, pools, estuaries, streams, and wetlands considered to be a part of freshwater ecosystems. Freshwater bodies provide valuable ecosystem services, such as drinking, irrigation purposes, and also they provide support to the production recreation to aesthetics (Alan Yeakley et al., 2016). The continuous monitoring of freshwater bodies is essential, to maintain the health and wealth of aquatic organisms. Nowadays aquatic stress is increasing due to various anthropogenic activities like the dumping of municipal waste, mixing of veterinary antibiotics, the release of sewage sludge, and industrial effluents into freshwater bodies. Moreover, deteriorate surface water can also impair the basic use freshwater bodies and they may cause pollution by releasing toxic substances into surface water which significantly affect the water quality (Li et al., 2019; Onyemesili et al., 2022).
The pollutant may be derivative from the point or non-point sources into the water bodies. Point source of pollution can be identified as originating from only one location in their environments such as the release of industrial effluents, spillage, urban sewage treatment plants and municipal waste (Beckers et al., 2018; Anju et al., 2010). Non-point source of pollution can be identified as originating from different locations in the environment and they are generally afforded from more diffuse sources like urban storm runoff, agriculture waste, eutrophication, and, etc., (Xue et al., 2019). These types of pollution (Point and Non-Point) will release substances that can alter the inherent hydrographical profile and biological properties in aquatic ecosystems (Ossai et al., 2020; Nilsson and Renöfält, 2008). Therefore, continuous monitoring of water quality is essential for controlling surface water pollution in aquatic ecosystems. In addition, plankton density and diversity are used to assess the status of aquatic pollution in the past few decades, because they are highly sensitive to small changes in the aquatic environment and have short life spans (Ghosh and Biswas, 2015). Hence, plankton diversity and density are considered to be important ecological parameters. The composition of each and every species will be represented morphologically as well as taxonomically different. A strong relationship exists between phytoplankton and zooplankton species, and these both are mainly filter feeders, and raptorial predators in aquatic ecosystems (Gołdyn and Kowalczewska-Madura, 2008).
Phytoplankton species will respond quickly to environmental changes and are very good bio-indicators of water quality at any type of water body in aquatic ecosystems and they used in the understanding pattern of lentic water bodies (Qureshi and Dube, 2022; Yusuf, 2020; Gökçe, 2016; Mohan and Priyadarshinee, 2023). The diversity, density, appearance, disappearance, and distribution pattern depend upon the biotic factors in aquatic ecosystems.
The basic trophic level of phytoplankton is followed by the next level of zooplankton (Frederiksen et al., 2006). The population density of zooplankton species is mainly influenced by the hydrographical characteristics of lake water. They play important role in the bio-monitoring process to assess the status of pollution in water bodies, especially Rotifer species are used as a bio-monitoring agent in all aquatic ecosystems (Xiong et al., 2020; Panikkar et al., 2022). In the past few decades, the bio-monitoring program has become an essential part, because water bodies are facing tremendous pollution due to various anthropogenic activities in their surrounding environment.
Therefore, assessment of plankton diversity and density in aquatic ecosystems is essential for the aquatic organisms. Hence, the present study was focused to evaluate the seasonal impact of seasonal in freshwater phytoplankton and zooplankton at Valankulam Lake, Coimbatore city, Tamil Nadu, India. In addition, Valankulam Lake has provided a habitat for various flora and fauna.
MATERIALS AND METHODS
Description of the Study Area
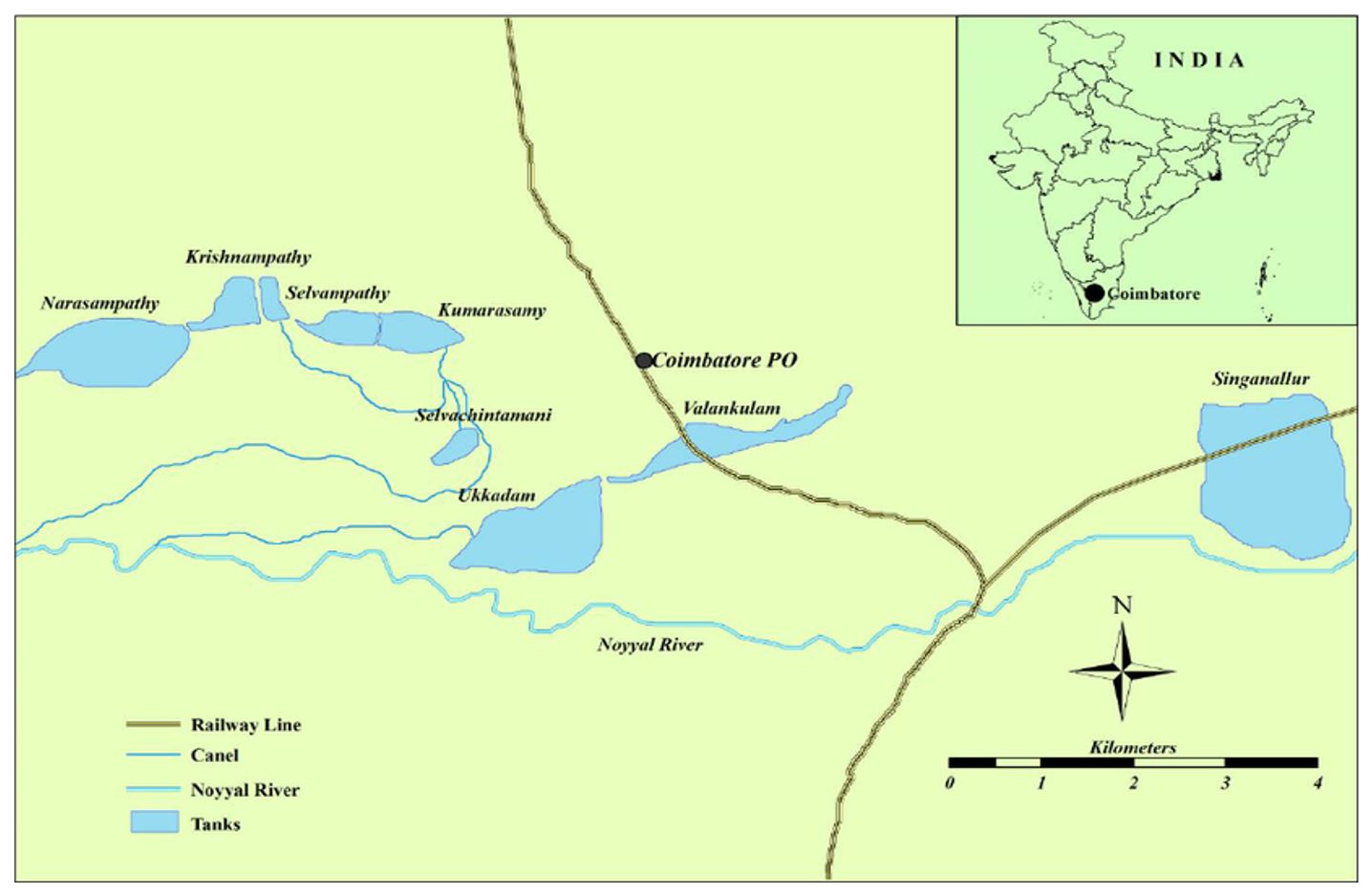
The Valankulam Lake located in Coimbatore city, Tamil Nadu, India, (Lat. 10.59° N and Long. 76.57° E) is fed by canals derived from Noyyal River.
The Valankulam Lake located upstream in the north (Figure 1).
This lake also receives drainage water from various sources like, industrial runoff, agricultural waste, and other anthropogenic activities. And it has an inlet connection with Ukkadam Lake. Valankulam Lake water is spread over an area of 64.75 ha with average depth of 4.5 Mtrs, a storage capacity of 27.88 Mcft, and a catchment area of 479.27 ha. The major activities carried out here are fishing by local fishermen.
Physico-chemical characteristics of lake water
The surface water samples were collected by using coracle bottles, during the early morning hours between 5.00 AM to 7.00 AM, once in a fortnight and period of nine months from September-2021 to May-2022 at five different sites and pooled to check the on-field physico-chemical parameters, such as water temperature, PH, salinity, dissolved oxygen, total dissolved solids, electrical conductivity, phosphate, chloride, total alkalinity, total hardness, calcium hardness, nitrate and ammonia by using “μP Based Water & Soil Analysis Kit” (Model 1160).
Qualitative and quantitative analyses of plankton
The phytoplankton and zooplankton samples were collected by using Towing-Henson’s standard plankton net (25 µm for phytoplankton and 150 μm mesh for zooplankton) by towing horizontally at surface of lake water at five different sites. The quantitative analysis of plankton species, was performed by filtering 100 litters of water through plankton net made up blotting silk (25 µm and 150 μm) using a 10 litter capacity plastic container. Immediately after filtering out the water, plankton biomass were transferred to specimen bottles containing 4 % of neutralized formalin and subjected to microscopic analysis. The sample (1 ml) was taken with a wide- mouthed pipette and placed into the counting chamber of the Sedgwick Rafter. After allowing it to settle for some time, they were counted. Each group was countered at least 5 times, and the average values were noted. The total number of plankton species present in 1 liter of water sample was calculated by using method of Santhanam et al., 1989.
Identification of phytoplankton and zooplankton species
The phytoplankton and zooplankton species were identified by referring to the standard manuals, textbooks, and monographs (Venkataraman, 1939; Iyengar and Venkataraman, 1951; Adoni et al., 1985; Agarker et al., 1994; Battish, 1992; Reddy, 1994; Anand, 1989; Sheil, 1995; Murugan et al., 1998; Altaff, 2004; Manickam et al., 2019a, 2019b). The taxonomic identification was completed under the compound light microscope at a magnification of 40 X to 100 X and they were photomicrographed by using, Inverted Biological Microscope (Model Number INVERSO 3000 (TC-100) CETI) attached to the camera (Model IS 300).
Statistical analyses of phytoplankton and zooplankton species
Species diversity index (H’) was calculated using Shannon and Weaner’s formula (1949); H1 =pi ∑ log2 pi, I = 1s, Where, H1 à species diversity in bits of information per individual, pi – ni / N (proportion of the sample belonging to the species), ni à Number of individual in all the sample; Species Richness (SR) was calculated as described by Gleason (1922); D = 1 – C, Where, C = ∑ pi2 , pi – ni/N, ni – N/S, N à Total number of individuals, S à Number of species in the collection; Evenness index (J1 ) was calculated by using the formula of Pielous (1966); J1 = H1 /log2 s, Where, H1 = species diversity in bits of information per individual, S = Number of species. Shannon and Weaner’s species diversity index (H1), Species Richness (SR), and Evenness index (J) were analysed using the PAST (Palaeontological Statistics), software (ver. 2.02).
RESULTS
Physico-chemical characteristics of lake water
The average values of hydrographical characteristics of lake water depicted in Table 1. During the study period, all the parameters were found to be higher in the summer season, whereas lower in the monsoon season. Moreover, the Dissolved oxygen, Total dissolved solids and ammonia were found to be higher in the monsoon season, whereas lower in the summer season.
Morphologically identifiedphytoplankton species
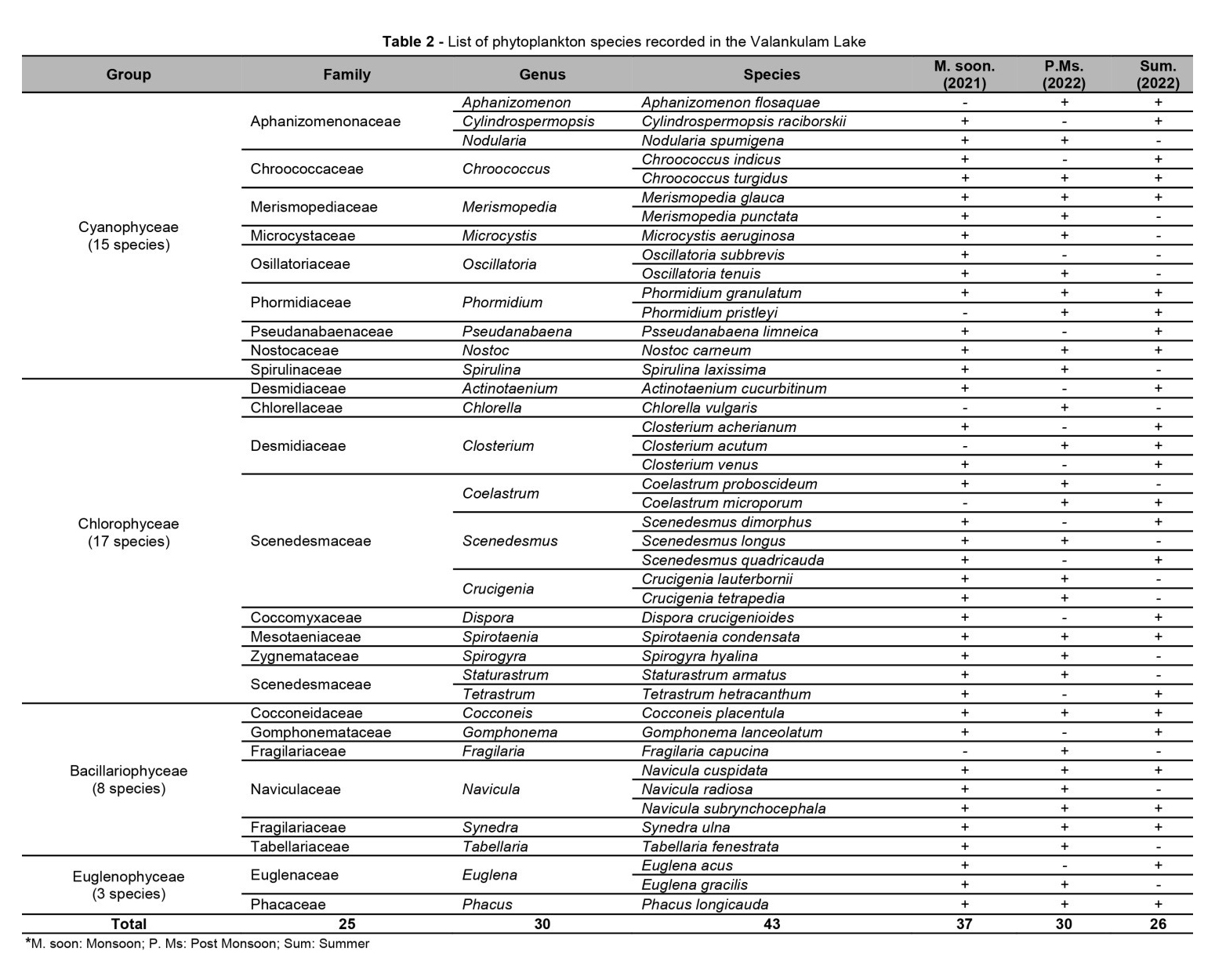
Totally 43 species of phytoplankton were recorded under 25 families and 30 genera, which include 15 species of Cyanophyceae, 17 species of Chlorophyceae, 8 species of Bacillariophyceae: and 3 species of Euglenophyceae as seen in Table 2.
Table 1
Physico-chemical characteristics of the Valankulam Lake, Coimbatore city, Tamil Nadu
|
Parameters |
Monsoon (2021) |
P. Monsoon (2022) |
Summer (2022) |
|
Water temperature (°C) |
24.26±0.96a |
26.99±0.42b |
29.88±0.49c |
|
pH |
7.06±0.25a |
7.20±0.35b |
8.93±0.40c |
|
Salinity (ppt) |
107.92±0.06a |
104.15±0.22b |
132.48±0.20c |
|
DO (mg/l-1) |
8.31±0.50c |
7.79±0.14b |
6.21±0.29a |
|
TDS (mg/l-1) |
109.21±24.06b |
108.28±15.20ab |
104.34±24.06a |
|
EC (µS cm-1) |
193.42±0.16a |
200.03±0.24b |
209.35±0.29c |
|
Phosphate (mg/l-1) |
33.14±0.28a |
34.24±0.09b |
35.75±1.27bc |
|
Chlorides (mg/l-1) |
2.65±0.45a |
2.98±0.70b |
3.69±0.51c |
|
Total alkalinity (mg/l-1) |
120.80±7.04a |
128.23±9.10b |
134.36±8.86c |
|
Total hardness (mg/l-1) |
6.97±0.57a |
7.40±0.73b |
8.30±0.80c |
|
Calcium hardness (mg/l-1) |
68.05±1.05a |
71.43±2.44b |
77.37±3.59c |
|
Nitrate (mg/l-1) |
22.42±1.27a |
23.50±1.80ab |
24.83±1.69bc |
|
Ammonia (mg/l-1) |
2.53±0.80c |
2.45±0.68b |
2.13±0.01a |
DO, dissolved oxygen; TDS, total dissolved solids; EC, electrical conductivity. Each season value is overall average of mean ± SD (n=15; 5 sites × 3 seasons). Mean values within the same row but having different superscript are significantly different (P<0.05).
Population density with percentage composition of phytoplankton species
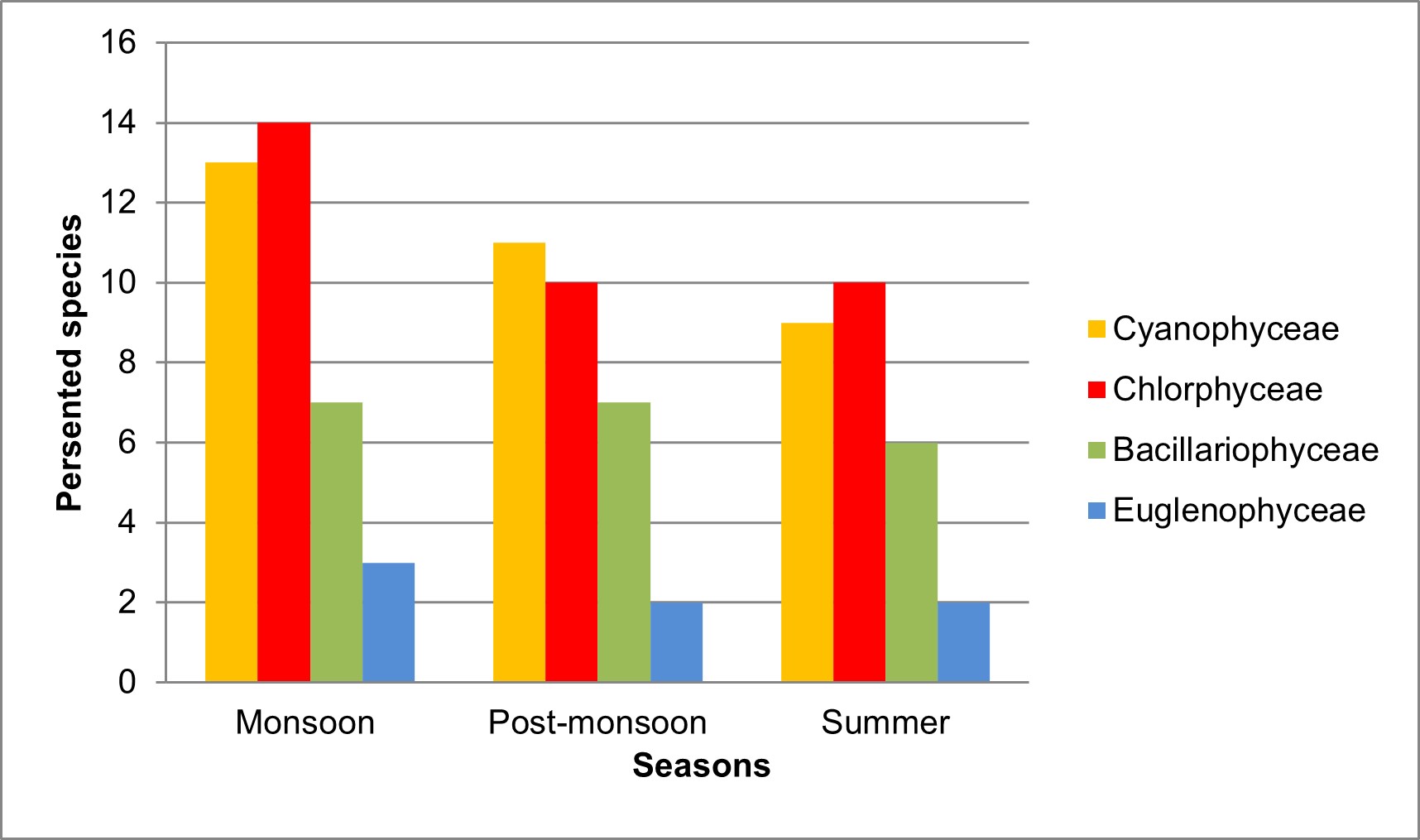
The population density was recorded in the range of 2130 – 3188 Ind./L at Valankulam Lake during September-2021 to May-2022. The total density of population during monsoon time is 3188 Ind/L, during post monsoon 2530 Ind/L and in summer 2130 Ind/L it means that the maximum population density was noticed during the monsoon followed by post-monsoon and summer (Table 3). In the present observation, phytoplankton percentage composition shows that the holds the Cyanophyceae was most abundant in Valankulam Lake. The seasonal wise variations of phytoplankton species values were depicted in Figure 2.
The groups Cyanophyceae were found in predominant in monsoon, followed by post monsoon and summer season with (35.07%) followed by species of Chlorophyceae (29.47%), Bacillariophyceae (25.46%) and Euglenophyceae with (10%) in Figure 3.
Table 3
Phytoplankton density with percentage composition in the Valankulam Lake, Coimbatore city, Tamil Nadu
|
Phytoplankton groups |
Monsoon (2021) |
P. Monsoon (2022) |
Summer (2022) |
Total (Ind./L) & % |
|
Cyanophyceae |
1087±42a |
867±29b |
798±32c |
2752 (35.07%) |
|
Chlorophyceae |
916±30a |
746±34b |
651±27bc |
2313 (29.47%) |
|
Bacillariophyceae |
798±31a |
698±26ab |
502±25c |
1998 (25.46%) |
|
Euglenophyceae |
387±32a |
219±28ab |
179±16bc |
785 (10%) |
|
Total |
3188 |
2530 |
2130 |
7848 |
Each season value is overall average of mean ± SD (n=15; 5 sites × 3 seasons). Mean values within the same row but having different superscript are significantly different (P<0.05).
Diversity indices of phytoplankton species
The calculated seasonal diversity indices, such as Simpson’s species dominance (D), Shannon-Wiener’s diversity (H), Buzas and Gibson’s evenness (e^H/S) and Margalef’s (R1) species richness for each group of phytoplankton species recorded at Valankulam Lake is presented in (Table 4). In overall diversity index for D was recorded in the order of, Cyanophyceae > Chlorophyceae > Bacillariophyceae > Euglenophyceae (0.162, 0.154, 0.144 and 0.137, respectively).
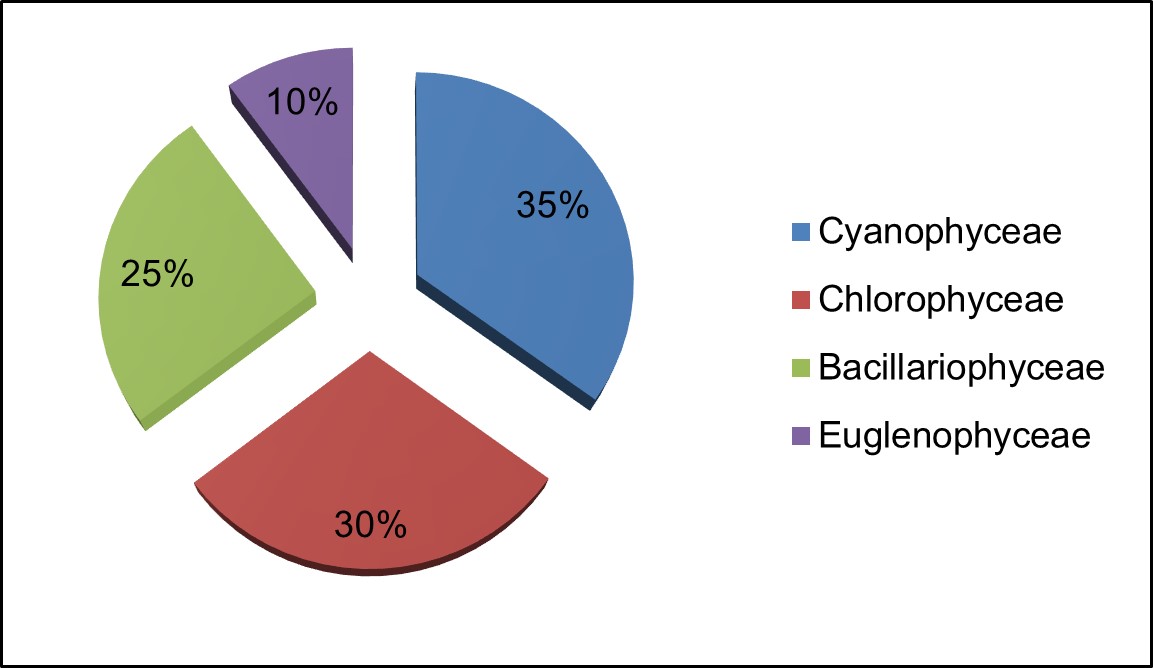
Figure 3 – Percentage composition of different groups of phytoplankton recorded in the Valankulam Lake
Table 4
Species diversity indices of phytoplankton in the Valankulam Lake, Coimbatore city, Tamil Nadu
|
Phytoplankton groups |
Diversity indices |
Monsoon (2021) |
P. Monsoon (2022) |
Summer (2022) |
|
Cyanophyceae (15 Species) |
Dominance (D) |
0.162±0.007a |
0.154±0.005b |
0.146±0.009c |
|
Shannon (H) |
1.983±0.040a |
1.967±0.041b |
1.991±0.038c |
|
|
Evenness_e^H/S |
0.981±0.024a |
0.978±0.024b |
0.971±0.028bc |
|
|
Margalef (R1) |
0.821±0.047a |
0.829±0.042ab |
0.831±0.045bc |
|
|
Chlorophyceae (17 Species) |
Dominance (D) |
0.154±0.006a |
0.144±0.008b |
0.130±0.006c |
|
Shannon (H) |
1.963±0.042a |
1.956±0.043b |
1.973±0.042c |
|
|
Evenness_e^H/S |
0.974±0.023a |
0.970±0.021ab |
0.967±0.027bc |
|
|
Margalef (R1) |
0.812±0.045bc |
0.817±0.042a |
0.820±0.046a |
|
|
Bacillariophyceae (8 Species) |
Dominance (D) |
0.144±0.004a |
0.136±0.006b |
0.127±0.008c |
|
Shannon (H) |
1.954±0.040a |
1.948±0.038ab |
1.969±0.032c |
|
|
Evenness_e^H/S |
0.968±0.020a |
0.961±0.017ab |
0.957±0.022b |
|
|
Margalef (R1) |
0.815±0.040a |
0.811±0.037ab |
0.807±0.040b |
|
|
Euglenophyceae (3 Species) |
Dominance (D) |
0.137±0.004a |
0.126±0.006b |
0.112±0.008c |
|
Shannon (H) |
1.926±0.040a |
1.914±0.038b |
1.929±0.032c |
|
|
Evenness_e^H/S |
0.952±0.020a |
0.912±0.017b |
0.895±0.022c |
|
|
Margalef (R1) |
0.727±0.040c |
0.734±0.037b |
0.805±0.040a |
Each season value is overall average of mean ± SD (n=15; 5 sites × 3 seasons). Mean values within the same row but having different superscript are significantly different (P<0.05).
The overall diversity index for H was recorded in the order of, Cyanophyceae > Chlorophyceae > Bacillariophyceae > Euglenophyceae (1.991, 1.973, 1.969 and 1.929, respectively). The diversity value for evenness was observed in the order of, Cyanophyceae > Chlorophyceae > Bacillariophyceae > Euglenophyceae (0.981, 1.974, 1.968 and 0.952, respectively). The R1 was observed in the order of, Cyanophyceae > Chlorophyceae > Bacillariophyceae > Euglenophyceae (0.831, 0.820, 0.815 and 0.805, respectively). Simpson’s species dominance (D), and Buzas and Gibson’s evenness (e^H/S) was higher in monsoon and lower in summer season. Shannon-Wiener’s diversity (H), and Margalef’s (R1) was higher in summer and lower in monsoon season.
Morphologically identified zooplankton species
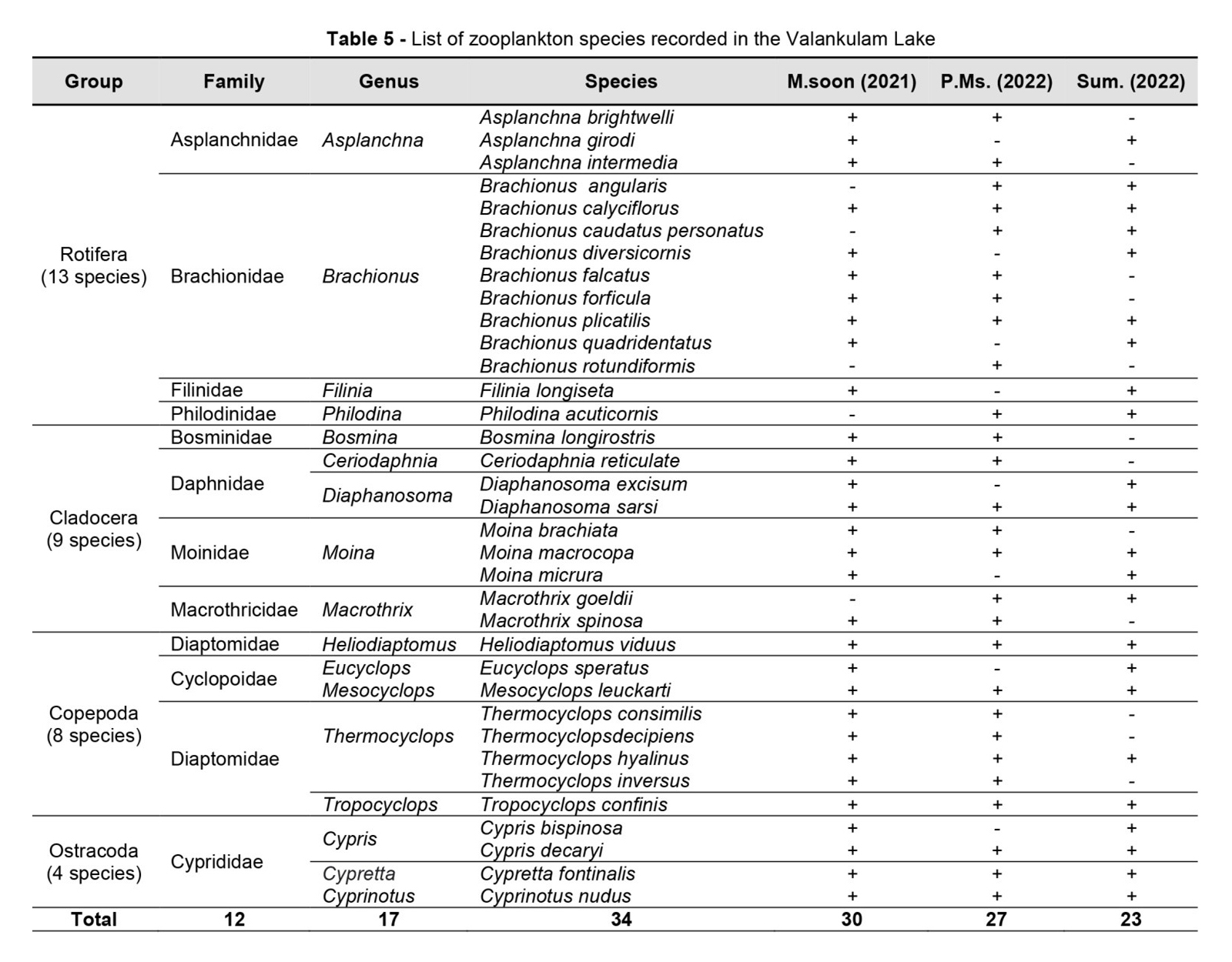
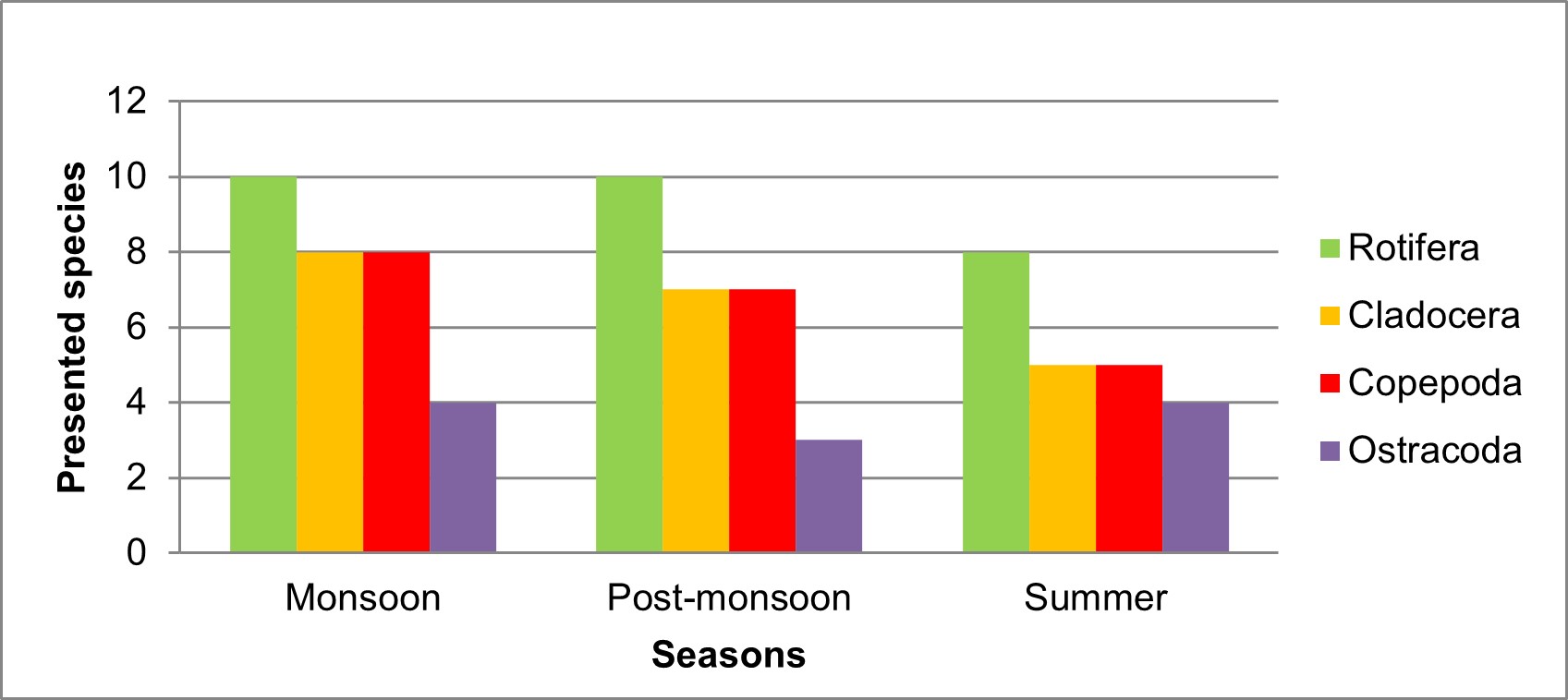
Totally 34 species of zooplankton was recorded under 12 families and 17 genera, which include 13 species of Rotifer, 9 species of Cladocera, 8 species of Copepoda, and 4 species of Ostrocoda as shown in Table 5.
Population density with percentage composition of zooplankton species
The population density was recorded in the range of 3,828 – 4,815 Ind./L at Valankulam Lake during September-2021 to May-2022. The total density of population during monsoon time is 4815 Ind./L, during post monsoon 4274 Ind./L and in summer 3828 Ind./L it means that the maximum population density was noticed during the monsoon followed by post-monsoon and summer (Table 6). The seasonal wise variations of zooplankton species depicted in Figure 4.
In the present observation, zooplankton percentage composition shows that the rotifer were most abundant in Valankulam Lake. The groups Rotifera were (33.36%) followed by species of Cladocera (24.80%), Copepoda (22.44%) and Ostrocoda with (19.40%) in Figure 5.
Diversity indices of zooplankton species
The calculated seasonal diversity indices, such as Simpson’s species dominance (D), Shannon-Wiener’s diversity (H), Buzas and Gibson’s evenness (e^H/S) and Margalef’s (R1) species richness for each group of zooplankton species recorded at Valankulam Lake is presented in (Table 7). In overall diversity index for D was recorded in the order of, Rotifera > Cladocera > Copepoda > Ostrocoda (0.165, 0.160, 0.153 and 0.142, respectively). The overall diversity index for H was recorded in the order of, Rotifera > Cladocera > Copepoda > Ostrocoda (1.977, 1.967, 1.965 and 1.861, respectively). The diversity value for evenness was observed in the order of, Rotifer > Cladocerans > Copepoda > Ostrocoda (0.988, 0.980, 0.978 and 0.862, respectively). The R1 was observed in the order of, Rotifera > Cladocera > Copepoda > Ostrocoda (0.837, 0.831, 0.828 and 0.717, respectively). Simpson’s species dominance (D), and Buzas and Gibson’s evenness (e^H/S) was higher in monsoon and lower in summer season. Shannon-Wiener’s diversity (H), and Margalef’s (R1) was higher in summer and lower in monsoon season.
Table 6
Zooplankton density with percentage composition in the Valankulam Lake, Coimbatore city, Tamil Nadu
|
Zooplankton groups |
Monsoon (2021) |
P. Monsoon (2022) |
Summer (2022) |
Total (Ind./L) & % |
|
Rotifera |
1,590±43a |
1,432±39b |
1,287±41c |
4,309 (33.36%) |
|
Cladocera |
1,158±36a |
1,067±42ab |
978±38c |
3,203 (24.80%) |
|
Copepoda |
1,089±37a |
956±32ab |
853±31b |
2,898 (22.44%) |
|
Ostrocoda |
978±27a |
819±32ab |
710±33bc |
2,507 (19.40%) |
|
Total |
4,815 |
4,274 |
3,828 |
12,917 |
Each season value is overall average of mean ± SD (n=15; 5 sites × 3 seasons). Mean values within the same row but having different superscript are significantly different (P<0.05)
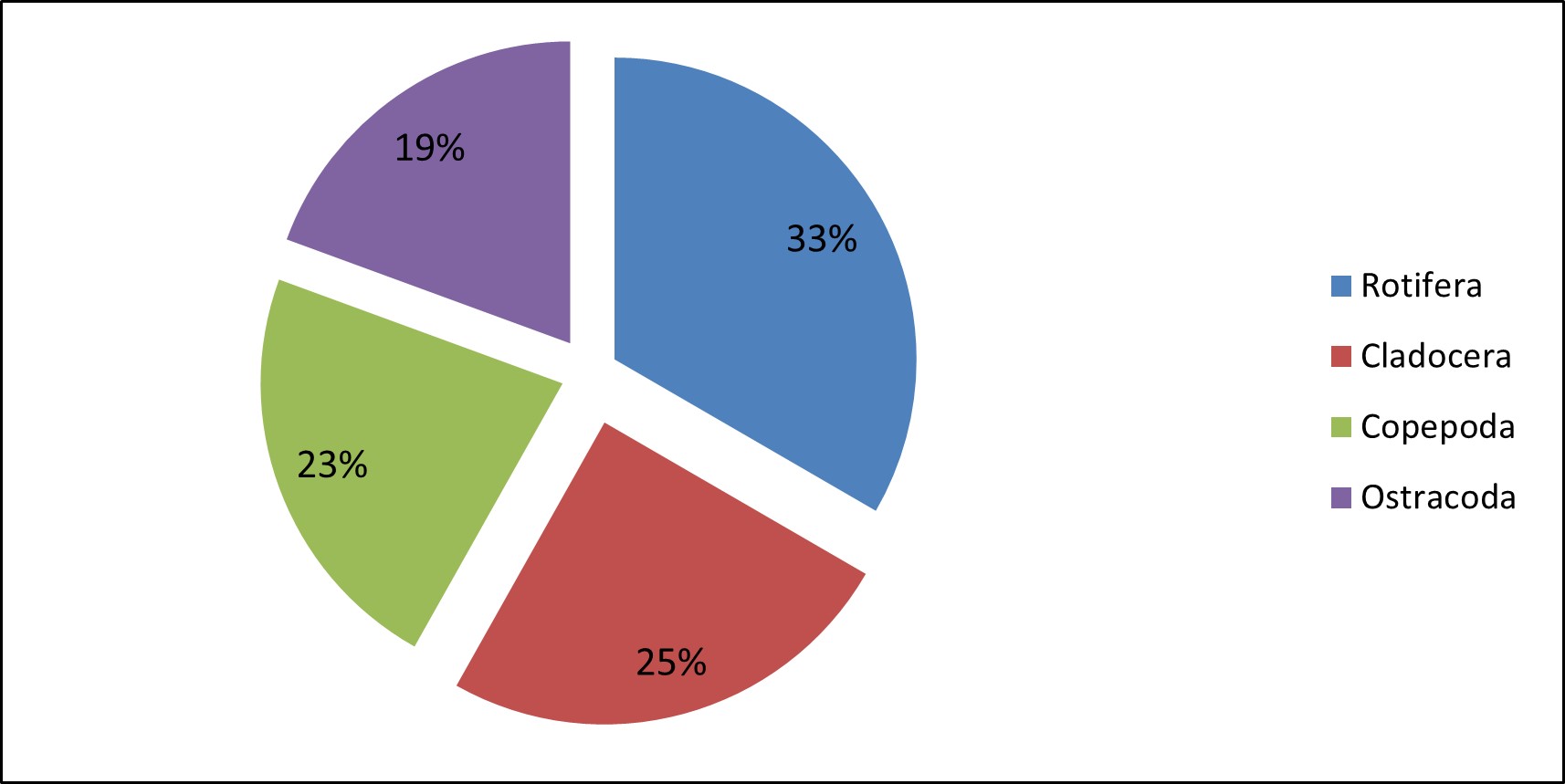
Figure 5 – Percentage composition of different groups of zooplankton recorded in the Valankulam Lake
Table 7
Species diversity indices of phytoplankton in the Valankulam Lake
|
Zooplankton Groups |
Diversity Indices |
Monsoon (2021) |
P. Monsoon (2022) |
Summer (2022) |
|
Rotifera (13 Species) |
Dominance (D) |
0.165±0.009a |
0.157±0.004b |
0.149±0.008c |
|
Shannon (H) |
1.969±0.042a |
1.960±0.038ab |
1.977±0.038c |
|
|
Evenness_e^H/S |
0.988±0.026a |
0.979±0.023ab |
0.973±0.025bc |
|
|
Margalef (R1) |
0.835±0.048a |
0.832±0.045ab |
0.837±0.041bc |
|
|
Cladocera (9 Species) |
Dominance (D) |
0.160±0.004a |
0.153±0.007b |
0.142±0.009c |
|
Shannon (H) |
1.963±0.040a |
1.957±0.032ab |
1.967±0.035c |
|
|
Evenness_e^H/S |
0.980±0.025a |
0.971±0.024ab |
0.964±0.021bc |
|
|
Margalef (R1) |
0.818±0.037bc |
0.824±0.041ab |
0.831±0.042a |
|
|
Copepoda (8 Species) |
Dominance (D) |
0.153±0.006a |
0.147±0.008b |
0.140±0.008c |
|
Shannon (H) |
1.960±0.038a |
1.954±0.030ab |
1.965±0.034c |
|
|
Evenness_e^H/S |
0.978±0.022a |
0.968±0.025ab |
0.961±0.023bc |
|
|
Margalef (R1) |
0.815±0.032bc |
0.821±0.038ab |
0.828±0.040a |
|
|
Ostrocoda (4 Species) |
Dominance (D) |
0.142±0.005a |
0.135±0.009b |
0.131±0.008c |
|
Shannon (H) |
1.854±0.031a |
1.847±0.035b |
1.861±0.036c |
|
|
Evenness_e^H/S |
0.862±0.021a |
0.857±0.029ab |
0.852±0.026bc |
|
|
Margalef (R1) |
0.704±0.031bc |
0.711±0.034ab |
0.717±0.045a |
Each season value is overall average of mean ± SD (n=15; 5 sites × 3 seasons). Mean values within the same row but having different superscript are significantly different (P<0.05).
DISCUSSION
A notable aspect of the present study was focused on the impact of seasonal variation in freshwater phytoplankton and zooplankton species at Valankulam Lake based on the prevailing hydrographical characteristics in the aquatic environment. If any moderate fluctuation in the biological density of water bodies, the hydrographical characteristics, and biological circumstances must be taken into consideration immediately. The hydrographical characteristics play a crucial role in species diversity and their community structure in the aquatic environment (Sharma et al., 2016). The water temperature plays important role in physiological activities and their life process such as feeding, reproduction, movements, density, and diversity of the organisms are greatly influenced by the high temperature in water bodies. Moreover, the maintenance of water temperature is essential for aquatic biota, there is a significant similarity between water temperature and atmospheric temperature in the environment. The temperature mainly depends upon the intensity of solar radiation, evaporation, and freshwater influx (Ma et al., 2019). The results from the study revealed, the water temperature increased during the summer season and decreased during the monsoon season respectively. PH is the most important index of alkalinity and acidity. The rise of PH levels in aquatic ecosystems indicates that increased level of pollution in water bodies (Jeyaraj et al., 2016; Zhou et al., 2008), due to the erosion of carbonated rocks, and higher evaporation, will definitely affects the aquatic organisms. In the present study, the PH level was increased during in summer season due to the higher level of pollution, and the occurrence of blooms in the aquatic environment, and the PH level was decreased during the monsoon season due to the heavy dilution of water. Salinity is the most important parameter for living aquatic organisms and helps to determine the biological process in the aquatic environment. In the present investigation, high-level salinity was found in summer and lows in the monsoon season. The salinity level increases due to high evaporation of water and low precipitation in aquatic ecosystems. When the salinity level increase, at the same time PH level will also increase, because salinity induce the hydrogen ions in water surfaces. In aquatic ecosystems DO level varies according to their trophic levels. A DO fluctuate seasonally or daily with variations due to the consumption of DO owing by the respiration of aquatic organisms and mainly influenced by atmospheric temperature, decomposition of organic matter, and occurrence of various chemical reactions in the aquatic environment (Bhateria and Jain, 2016; Omer, 2019). Sewage from the surrounding areas that flows down, anthropogenic activities, and agricultural runoff will reduce the DO level in aquatic ecosystems (Sasakova et al., 2018). The maximum level of DO was noticed during the monsoon season and the minimum level was noticed during the summer season respectively. The total dissolved solids will represent a total concentration of soluble salts in given water samples that may be organic or inorganic but precisely total dissolved solids are mainly composed of carbonates, sulfate, calcium, phosphate, nitrate, and potassium (Chebet et al., 2020). The TDS content was increased during the monsoon and lower in the summer respectively. A high level of TDS content in Valankulam Lake might be attributed due to the higher amount of industrial effluents, municipal waste, and sewage sludge adjoining the Valankulam Lake. Electrical conductivity will decide the pollution status of the water bodies. In the present study, a maximum level of EC was noticed during the summer and a minimum in monsoon respectively. Total alkalinity represents a chemical property that accelerates and neutralizes the acidic nature of the water bodies and hardness shows the presence of total polyvalent captions in the water and they will mostly be divalent cations, known to be calcium and magnesium. A high level of alkalinity in water bodies has a bitter taste and is harmful to the irrigation process, can damage soil properties, and affects the life of aquatic communities (Ma et al., 2020; Patil et al., 2012). In the present study, the total alkalinity and hardness level was higher in summer and lower in the monsoon season. The calcium is essential for all aquatic organisms, and they are present due to the dissolution of gypsum and calcareous rock within aquatic ecosystems (Ayoob and Gupta, 2006; Anim-Gyampo et al., 2018). In the present study, the maximum level of calcium was found in summer and the minimum in monsoon season respectively. Nitrate, phosphate, and chlorides are the most important nutrient, which is generally found in a higher level of organic polluted water bodies. Nitrate and phosphate are good indicators of eutrophication in aquatic ecosystems, and chloride is one of the important water quality parameters, to assess water pollution in water bodies. The Nitrate, phosphate, and chlorides enter the lake via different sources like municipal waste, agricultural runoff, and other anthropogenic activities (Isiuku and Enyoh, 2020; Mushatq et al., 2013). The nitrate, phosphate, and chloride values indicate that Valankulam Lake is loaded with a higher amount of organic matter with various substances (Halder and Islam, 2015) and ammonia plays important role in the nitrogen cycle of aquatic ecosystems. Moreover, higher amounts of ammonia were found in the monsoon season and lower in the summer season. In the present study, all the parameters are found to be higher in summer and lower in monsoon and except for DO, TDS, and ammonia. The Valankulam Lake has been experiencing great pressure from human activities that alter the hydrographical profile and biological process. Therefore, conservation and management of the lake environment are necessary to classify according to the paved certain standard platform for other biologists to work on these primary producers and consumers of aquatic ecosystems (Pan et al., 2014). Freshwater lakes are facing tremendous ecological stress due to various anthropogenic activities in the environment and lead to in the extinction of some species in the aquatic environment.
Plankton (phytoplankton and zooplankton) are unique organisms which present in the aquatic environment. The distribution patterns of planktonic communities are strongly correlated with environmental factors, and can be used to assess the pollution status of water bodies (Jiang et al., 2014; Nandan and Sajeevan, 2018). The freshwater phytoplankton diversity and their population density are highly influenced by the interaction of some hydrographical and biological factors acting simultaneously to maintain health and wealth of aquatic ecosystems (Mohan and Priyadarshinee, 2022). In the present findings, a total of 43 species of phytoplankton was recorded under 25 families and 30 genera, which include 15 species of Cyanophyceae, 17 species of Chlorophyceae, 08 species of Bacillariophyceae, and 03 species of Euglenophyceae in Valankulam Lake. In the percentage compositions of phytoplankton species, Cyanophyceae were found to be the predominant group at 35.07% followed by Chlorophyceae at 29.47%, Bacillariophyceae at 25.46%, and Euglenophyceae at 10%. Cyanophyceae are the major group of freshwater algae and can produce cyano-toxins. The Cyanobacterial toxins are considered to be important because of their spreading tropical to the temperate environment and they are highly correlated with the global warming phenomena. The present study shows that there are certain members of species in the Cyanophyceae and Chlorophyceae which are tolerant of organic pollution and resist the stress caused by pollutants (Rajagopal et al., 2010; Annalakshmi and Amsath, 2012). However, Phormidium, Oscillatoria, Spirillum, Cylindrospermopsis, and Merisomopedia in Cyanophyceae and Chlorella, Closterium, Tetrastrum and Scenedesmus in chlorophyceae were found to be dominant genera in Valankulam Lake. These species are mainly used as bio-indicator (bio-monitoring agents) of water quality in aquatic ecosystems (Xue et al., 2018; Sarwade and Kamble, 2014). Gomphonema, Fragilaria, and Navicula in Bacillariophyceae and Euglena in Euglenophyceae are dominant genera in Valankulam Lake. Oscillatoria, Scendesmus, and Navicula sp are mainly found in organic-rich water bodies. Moreover, Phacus, Fragilaria capucina, Navicula radiosa, and Navicula subryn chocephala were found in highly polluted water bodies and they correlated with the intensity of pollution (Bhat et al., 2015). Freshwater phytoplankton communities have numerous environmental functions related to the recycling of nutrients and the enormous increase in the numbers of some species (Cylindrospermopsis raciborskii; Oscillatoria tenuis) has made the water unfit for drinking and any other purpose (Khan et al., 2011). Blooms producing Cyanobacterial species like Cylindrospermopsis raciborskii, Aphanizomenon flosaquae, Nostoc corneum, and Microcystis aeruginosa are affects the water ecosystems due to the releasing of Cyanotoxins substances. The cyanobacterial blooms will impair the physico-chemical characteristics (Merel, et al., 2013). Therefore, chlorination is best method to treat drinking water, for removing the cyanobacterial blooms. In the past few years, Indian freshwater lakes are facing various anthropogenic activities due to rising pollution from rapid industrialization, urbanization, high human population, and human exploitation. Therefore, conservation freshwater bodies are very important for the purpose of future. Coimbatore city may future aggravate the level of pollution due to Urbanization and industrial revolution.
Crustacean zooplankton is an essential aquatic organism, occurring abundantly in all types of aquatic habits, and has an important role in energy transfer at the base of aquatic ecosystems. They hold the center position in the aquatic food chain. Zooplankton not only forms an integral part of the lentic community but also contributes a significant role in biological productivity in the aquatic ecosystem (Le Loc’h et al., 2008; Xu and Zhang, 2012; Mohan and Priyadarshinee, 2022). The zooplankton community often exhibits rapid changes to hydrographical characteristics in the aquatic environment (Hsu et al., 2008). In the present findings, a total of 34 species of zooplankton was recorded under 12 families and 17 genera, which include 13 species of rotifer, 09 species of Cladocerans, 08 species of Copepods, and 04 species of Ostracoda in Valankulam Lake. In the percentage composition of zooplankton species, rotifers were found to be the predominant group at 33.36% followed by Cladocerans at 24.80%, Copepods at 22.44%, and Ostracoda at 19.40%. The rotifer species were found to be predominant in municipal and industrial discharges water, and they play important role in energy flow and nutrient cycling, according to more than 50% of the zooplankton production in some freshwater ecosystem (Kaur et al., 2018; Singh et al., 2021). In the present study, among the four major groups of zooplankton species; Rotifera was found to be predominant, which are the good bio-indicators of eutrophication in water bodies. Continuous measures must be taken to reduce water pollution by regulating human activities and other anthropogenic activities in the watershed area (Verma and Prakash, 2020; Shen et al., 2021). Among the zooplankton species, Asplanchna brightwelli, Asplanchna girodi, Brachionus calyciflorus, B. diversicornis, B. rotundiformis, and B. falcatus, in rotifer, Diaphanosoma, Moina macrocopa, and Moina brachiata in cladoceran Thermocyclops consimilis, T. decipiens, T. hyalinus, Tropocyclops confinis, and Heliodiaptomus viduus in Copepoda and Cypretta fontinalis, and Cypris decaryi in Ostracoda were dominantly recorded zooplankton species in Valankulam Lake and the presence has been recorded in both eutrophic and oligotrophic lakes reported by Vadadi Fülöp et al., (2008); Makino et al., (2020) and Kalpana et al. (2017). The copepods species contain an important link in the aquatic food web, and they are in the intermediate trophic level among bacteria, algae, and protozoa. The presents of cyclopoids suggest that preponderance in higher trophic level of water, and good bio-indicators of high turbidity due to the suspended solids (Balakrishna et al., 2013; da Conceicao et al., 2021). The quantitative and qualitative assessment of the planktonic community in aquatic ecosystems is essential to explain the status of water quality and biological productivity. Hence, the present study gives the seasonal-wise distribution of diversity among the phytoplankton and zooplankton from the given area which can be useful as data to identify one of the biological aspects. A relationship between plankton communities and hydrographical profile (phytoplankton, zooplankton, diversity, temperature, pH, alkalinity, hardness, nitrates, and phosphates) by calculating Dominance (D), Shannon (H’), Evenness e^H/S, Margalef (R1) to analyses their interrelationships (Imran et al., 2014; Perumal et al., 2009). In the present study, Simpson’s species dominance (D), and Buzas and Gibson’s evenness (e^H/S) was higher in monsoon and lower in summer season. Shannon-Wiener’s diversity (H), and Margalef’s (R1) was higher in summer and lower in monsoon season. The higher value of Shannon (H’) indices and the population of plankton communities are found higher in the summer season and lower in the monsoon season (Panwar and Malik, 2016; Malik et al., 2020; Nandy and Mandal, 2020). The Evenness indices are relatively higher in the monsoon season (Offem et al., 2011) and lower in summer season, R1 indices are higher in summer season and lower in monsoon season, and this species abundance indicated the stable environmental conditions in Valankulam Lake. The analyses of species composition and community structure of phytoplankton and zooplankton species are very important because there are ecologically important organisms in aquatic ecosystems. The plankton population is affected by the impact of seasonal variations and they show a different pattern in different months.
CONCLUSIONS
The increase in industrialization, urbanization, human population, and global warming-induce latitudinal shifts in climate zones resulting in a hydrological regime that has serious implications for aquatic organisms including phytoplankton zooplankton species. Results from this study revealed hydrographical characteristics, plankton diversity, and population density are more useful tools to assess the lake ecosystem. The hydrographical characteristics of Valankulam Lake are almost suitable for all aquatic forms. The analysis of diversity indices from September-2021 to May-2022 indicated that Valankulam Lake had a richness of phytoplankton and zooplankton species. The maximum plankton diversity was observed during the monsoon and the minimum diversity was observed during in summer season. Totally 78 species of plankton were recorded, under 39 families and 47 genera, which includes 44 species of phytoplankton and 34 species of zooplankton. However, hydrographical characteristics of Valankulam Lake, are categorized as moderately polluted. It is understood that affected by various anthropogenic activities such as entry of agricultural runoff (insecticides and pesticides) from surrounding agricultural fields, dumping of municipal waste, and mixing of sewage sludge into the lake, and seems that the major cause is eutrophication. Among the phytoplankton and zooplankton species, Cyanophyceae and Rotifera were found to be predominant groups, and they may serve as good bio-indicators of eutrophication. Continuous measures must be taken to minimize pollution in water bodies by regulating human activities in the watershed area. Moreover, Valankulam Lake has more potential for fish resources. Further, water quality and quantity in this lake should be maintained by the desilting process. The present study concluded that strict vigilance and general awareness are required so that proper conservation of water can be done, which supports a rich biodiversity of flora and fauna in their surrounding environment.
Author Contributions: Conceptualization BM, DP; methodology BM, PSB, PS; analysis SP, PSB, NM; writing BM, NM, review BM, SP, DP; supervision PS, DP. All authors declare that they have read and approved the publication of the manuscript in this present form.
Funding: There was no external funding for this study.
Acknowledgments: Authors are thankful to Environmental Monitoring & Bioremediation Laboratory, Department of Environmental Sciences, and Crustacean Biology Laboratory, Department of Zoology in Bharathiar University, Coimbatore – 641 046, Tamil Nadu, India. PG & Research Department of Zoology, Government Arts College, Coimbatore – 641 018, Tamil Nadu, India and Marine Planktonology & Aquaculture Laboratory, Department of Marine Science, Bharathidasan University, Tiruchirappalli – 620 024, Tamil Nadu, India.
Conflicts of Interest: There are no conflicts of interest associated with this study or work.
REFERENCES
Adoni, A.; Joshi, D.G.; Gosh, K.; Chourasia, S.K.; Vaishya, A.K.; Yadav, M.M.; Verma, H.G. Work Book on Limnology, Pratibha Publisher Sagar, 1985, 1-166.
Agarker, M.S.; Goswami, H.K.; Kaushik, S.; Mishra, S.M.; Bajpai, A.K.; Sharma U.S. Biology, conservation and management of Bhojwtland, Upper lake ecosystem in Bhopal. Bionature. 1994, 14, 73-119.
Alan Yeakley, J.; Ervin, D.; Chang, H.; Granek, E.F.; Dujon, V.; Shandas, V.; Brown, D. Ecosystem services of streams and rivers. In River Science (eds D.J. Gilvear, M.T. Greenwood, M.C. Thoms and P.J. Wood). 2016, https://doi.org/10.1002/9781118643525.ch17.
Altaff, K. A Manual of Zooplankton. Department of Zoology, The New College, Chennai. Tamil Nadu, India, 2004, 19-145.
Anand, N. Handbook of Blue-Green Algae, Bishen Singh Mahendra Pal Singh, Pub-lishers, Dehra Dun, India, 1989, 1-79.
Anim-Gyampo, M.; Anornu, G.K.; Appiah-Adjei, E.K.; Agodzo, S.K. Hydrogeochemical evolution and quality assessment of groundwater within the Atankwidi basin: the case of northeastern Ghana. Arabian Journal of Geosciences. 2018, 11, 1-14. https://doi.org/10.1007/s12517-018-3753-6.
Anju, A.; Ravi, S.P.; Bechan, S. Water pollution with special reference to pesticide contamination in India. Journal of Water Resource and Protection. 2010, 2. http://dx.doi.org/10.4236/jwarp.2010.25050.
Annalakshmi, G.; Amsath, A. Studies on the hydrobiology of river Cauvery and its tributaries arasalar from Kumbakonam region (Tamilnadu, India) with reference to phytoplankton. International Journal of Plant, Animal and Environmental Sciences. 2012, 2, 37-46.
Ayoob, S.; Gupta, A.K. Fluoride in drinking water: a review on the status and stress effects. Critical reviews in environmental science and technology. 2006, 36, 433-487. https://doi.org/10.1080/10643380600678112.
Battish, S.K. Freshwater Zooplankton of India. Oxford and IBH Publication Co. Pvt. Ltd, New Delhi, India, 1992, 1-233.
Balakrishna, D.; Reddy, T.R.; Reddy, K.V.; Samatha, D. Physico-chemical parameters and plankton diversity of Ghanpur Lake, Warangal, AP, India. International Journal of Zoology Research. 2013, 3, 44.
Beckers, L.M.; Busch, W.; Krauss, M.; Schulze, T.; Brack, W. Characterization and risk assessment of seasonal and weather dynamics in organic pollutant mixtures from discharge of a separate sewer system. Water research. 2018, 135, 122-133. https://doi.org/10.1016/ j.watres.2018.02.002.
Bhat, N.A.; Wanganeo, A.; Raina, R. Variability in water quality and phytoplankton community during dry and wet periods in the Tropical Wetland, Bhopal, India. Journal of Ecosystem & Ecography. 2015, 5, 1.
Bhateria, R.; Jain, D. Water quality assessment of lake water: a review. Sustainable Water Resources Management. 2016, 2, 161-173. https://doi.org/10.1016/j.aca.2007.11.018.
Chebet, E.B.; Kibet, J.K.; Mbui, D. The assessment of water quality in river Molo water basin, Kenya. Applied Water Science. 2020, 10, 1-10. https://doi.org/10.1007/s13201-020-1173-8.
da Conceicao, L.R.; Demoner, L.E.; Pereira, J.B.; Perassoli, F.; Ghisolfi, R.D.; Bastos, A.C.; Fernandes, L.F.L. Copepod community structure after a mining dam disaster in the Southwestern Atlantic Ocean. Estuarine, Coastal and Shelf Science. 2021, 254, 107325. https://doi.org/10.1016/j.ecss.2021.107325.
Dey, S.; Botta, S.; Kallam, R.; Angadala, R.; Andugala, J. Seasonal variation in water quality parameters of Gudlavalleru Engineering College pond. Current Research in Green and Sustainable Chemistry. 2021, 4, 100058. https://doi.org/10.1016/j.crgsc.2021.100058.
Frederiksen, M.; Edwards, M.; Richardson, A.J.; Halliday, N.C.; Wanless, S. From plankton to top predators: bottom‐up control of a marine food web across four trophic levels. Journal of Animal Ecology. 2006, 75, 1259-1268. https://doi.org/10.1111/j.1365-2656.2006. 01148.x.
Ghosh, D.; Biswas, J.K. Zooplankton diversity indices: assessment of an ox-bow lake ecosystem for sustainable management in West Bengal. International Journal of Advanced Biotechnology and Research. 2015, 6, 37-43.
Gleason, H.A. On the relation between species and area. Ecology. 1922, 3 158-162.
Gołdyn, R.; Kowalczewska-Madura, K. Interactions between phytoplankton and zooplankton in the hypertrophic Swarzędzkie Lake in western Poland. Journal of Plankton Research. 2008, 30, 33-42. https://doi.org/10.1093/plankt/fbm086.
Gökçe, D. Algae as an indicator of water quality. Algae-Organisms for Imminent Biotechnology, 2016, 81-101.
Halder, J.N.; Islam, M.N. Water pollution and its impact on the human health. Journal of environment and human. 2015, 2, 36-46.
Hsu, P.K.; Lo, W.T.; Shih, C.T. The coupling of copepod assemblages and hydrography in a eutrophic lagoon in Taiwan: seasonal and spatial variations. Zoological Studies-Taipei. 2008, 47, 172.
Imran, S.; Fatima, M.; Khan, D.; Qari, R. Seasonal variation in abundance and species diversity in a crustacean assemblage from sandy beach of Clifton (Karachi), Pakistan. International Journal of Biology and Biotechnology. 2014, 11, 207-220.
Isiuku, B.O.; Enyoh, C.E. Pollution and health risks assessment of nitrate and phosphate concentrations in water bodies in South Eastern, Nigeria. Environmental Advances. 2020, 2, 100018. https://doi.org/10.1016/j.envadv.2020.100018.
Iyengar, M.O.P.; Venkataraman, G. The ecology and seasonal succession of the algal flora of the River Cooum at Madras with special reference to Diatomaceae. J. Madras Univ. 1951, 21, 463-485.
Jiang, Y.J.; He, W.; Liu, W.X.; Qin, N.; Ouyang, H.L.; Wang, Q.M.; Xu, F.L. The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a large eutrophic Chinese lake (Lake Chaohu). Ecological Indicators. 2014, 40, 58-67. https://doi.org/10.1016/j.ecolind.2014.01.006.
Jeyaraj, M.; Ramakrishnan, K.; Jai, A.; Arunachalam, S.; Magudeswaran, P.N. Investigation of physico-chemical and biological characteristics of various lake water in coimbatore district, Tamilnadu, India. Oriental Journal of Chemistry. 2016, 32, 2087-2094. https://dx.doi.org/10.13005/ojc/320436.
Kaur, A.; Hundal, S.S.; Aulakh, R.K. Seasonal study of zooplankton diversity in the polluted water stretch of Buddha Nullah, Ludhiana. Journal of Entomology and Zoology Studies. 2018, 6, 2241-2245.
Khan, M.; Hussain, F.; Musharaf, S. A fraction of fresh water algae of Kalpani stream and adjoining area of district Mardan, Pakistan. International Journal of Biosciences. 2011, 1, 45-50.
Li, Z.; Yang, Q.; Yang, Y.; Ma, H.; Wang, H.; Luo, J.; Martin, J.D. Isotopic and geochemical interpretation of groundwater under the influences of anthropogenic activities. Journal of Hydrology. 2019, 576, 685-697. https://doi.org/10.1016/j.jhydrol.2019.06.037.
Le Loc’h, F.; Hily, C.; Grall, J. Benthic community and food web structure on the continental shelf of the Bay of Biscay (North Eastern Atlantic) revealed by stable isotopes analysis. Journal of Marine Systems. 2008, 72, 17-34. https://doi.org/10.1016/j.jmarsys.2007.05.011
Ma, C.; Mwagona, P.C.; Yu, H.; Sun, X.; Liang, L.; Mahboob, S.; Al-Ghanim, K.A. Seasonal dynamics of zooplankton functional group and its relationship with physico-chemical variables in high turbid nutrient-rich Small Xingkai Wetland Lake, Northeast China. Journal of Freshwater Ecology. 2019, 34, 65-79. https://doi.org/10.1080/02705060.2018.1443847.
Ma, J.; Wu, S.; Shekhar, N.V.; Biswas, S.; Sahu, A.K. Determination of physicochemical parameters and levels of heavy metals in food waste water with environmental effects. Bioinorganic Chemistry and Applications. 2020. https://doi.org/10.1155/2020/8886093.
Makino, W.; Machida, R.J.; Okitsu, J.; Usio, N. Underestimated species diversity and hidden habitat preference in Moina (Crustacea, Cladocera) revealed by integrative taxonomy. Hydrobiologia. 2020, 84, 857-878. https://doi.org/10.1007/s10750-019-04147-3.
Maltby, E.; Ormerod, S.; Acreman, M.; Dunbar, M.; Jenkins, A.; Maberly, S.; Ward, R. Freshwaters: open waters, wetlands and floodplains, (chapter 9), In UK National Ecosystem Assessment: Understanding Nature’s Value to Society, 2011, 295-360.
Malik, D.S.; Sharma, A.K.; Sharma, A.K. Status of zooplankton diversity, abundance in relation to hydrography of Ganga River and its Tributaries at Rishikesh. Indian Journal of Ecology. 2020, 47, 739-745.
Manickam, N.; Bhavan, P.S.; Santhanam, P.; Muralishankar, T.; Dhinesh kumar, S.; Balakrishnan, S.; Anath, S.; Shenba Devi. Phytoplankton biodiversity in the two perennial lakes of Coimbatore, Tamil Nadu, India. Acta Ecologica Sinica. 2019a, CHNAES658. https://doi.org/10.1016/j.chnaes.2019.05.014.
Manickam, N.; Santhanam, P.; Bhavan, P.S. Techniques in collection, preservation and Morphological Identification of Freshwater Zooplankton In. Basic and Applied Zooplankton Biology Springe. Editors Santhanam P, Begum A, Perumal P, Springer Singapore, 2019b, 139-195.
Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environment international. 2013, 59, 303-327. https://doi.org/10.1016/j.envint.2013.06.013.
Mohan, B.; Priyadarshinee, S. A study on the diversity and community structure of freshwater zooplankton and Ichthyofunal in kumarasawamy lake, Coimbatore district, Tamil Nadu, India. Journal of Materials and Environmental Science. 2022, 13, 1327-1338.
Mohan, B.; Piryadarshinee, S. Phytoplankton as bio indicators of water quality in two perennial lakes of Coimbatore district, Tamil Nadu, India. International Journal of Entomology Research. 2023, 8, 10-17.
Murugan, N.; Murugavel, P.; Kodarkar, M.S. Cladocera. Indian Association of Aquatic Biologist, Hyderabad, 1998, 5, 1-55.
Mushatq, B.; Raina, R.; Yaseen, T.; Wanganeo, A.; Yousuf, A.R. Variations in the physico-chemical properties of Dal Lake, Srinagar, Kashmir. African Journal of Environmental Science and Technology. 2013, 7, 624-633.
Nandan, S.B.; Sajeevan, K. Distribution and abundance of phytoplankton in Vembanad estuary, a Ramsar site on south west coast India. International journal of engineering technologies and management research. 2018, 5, 75-87.
Nandy, T.; Mandal, S. Unravelling the spatio-temporal variation of zooplankton community from the river Matla in the Sundarbans Estuarine System, India. Oceanologia. 2020, 62, 326-346. https://doi.org/10.1016/j.oceano.2020.03.005.
Nilsson, C; Renöfält, B.M. Linking flow regime and water quality in rivers: a challenge to adaptive catchment management. Ecology and Society. 2008, 13.
Offem, B.O.; Ayotunde, E.O.; Ikpi, G.U.; Ada, F.B.; Ochang, S.N. Plankton-based assessment of the trophic state of three tropical lakes. Journal of Environmental Protection. 2011, 2, 304. http://dx.doi.org/10.4236/jep.2011.23034.
Omer, N.H. Water quality parameters. Water Quality – Science, Assessments and Policy. 2019, 18, 1-34. https://doi.org/10.5772/INTECHOPEN.89657.
Onyemesili, O.O.; Egbueri, J.C.; Ezugwu, C.K. Assessing the pollution status, ecological and health risks of surface waters in Nnewi urban, Nigeria: Implications of poor waste disposal. Environmental forensics. 2022, 23, 346-360. https://doi.org/101080/15275922.2020. 1850564.
Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environmental Technology & Innovation. 2020, 17, 100526. https://doi.org/10.1016/j.eti.2019.100526.
Pan, B.; Wang, H.; Wang, H. A floodplain-scale lake classification based on characteristics of macroinvertebrate assemblages and corresponding environmental properties. Limnologica. 2014, 49, 10-17. https://doi.org/10.1016/j.limno.2014.07.003.
Panikkar, P.; Saha, A.; Prusty, A.K.; Sarkar, U.K.; Das, B.K. Assessing hydrogeochemistry, water quality index (WQI), and seasonal pattern of plankton community in different small and medium reservoirs of Karnataka, India. Arabian Journal of Geosciences. 2022, 15, 1-17. https://doi.org/10.1007/s12517-021-09291-6.
Panwar, S.; Malik, D.S. Zooplankton diversity, species richness and their distribution pattern in Bhimtal Lake of Kumaun region, (Uttarakhand). Hydrology Current Research. 2016, 7, 219. http://dx.doi.org/10.4172/2157-7587.1000219.
Patil, P.N.; Sawant, D.V.; Deshmukh, R.N. Physico-chemical parameters for testing of water-a review. International journal of environmental sciences. 2012, 3, 1194.
Perumal, N.V.; Rajkumar, M.; Perumal, P.; Rajasekar, K.T. Seasonal variations of plankton diversity in the Kaduviyar estuary, Nagapattinam, southeast coast of India. Journal of Environmental Biology. 2009, 30, 1035-1046.
Pielou, E.C. The measurement of diversity of different types of biological collections. Journal of Theoretical Biology. 1966, 13 131-144. https://doi.org/10.1016/0022-5193(66)90013-0.
Pinheiro, J.P.S; Windsor, F.M.; Wilson, R.W.; Tyler, C.R. Global variation in freshwater physico‐chemistry and its influence on chemical toxicity in aquatic wildlife. Biological Reviews. 2021, 96, 1528-1546. https://doi.org/10.1111/brv.12711.
Rajagopal, T.; Thangamani, A.; Archunan, G. Comparison of physico-chemical parameters and phytoplankton species diversity of two perennial ponds in Sattur area, Tamil Nadu. Journal of Environmental Biology. 2010, 31, 787.
Reddy, Y.R. Guides to the identification of the micro invertebrates of the continental waters of the world. Editor Dumont HJF. Copepoda: Calanoida: Diaptomidae. SPB Aca Pub, The Netherlands, 1994, 221.
Qureshi, A.; Dube, P. Studies on phytoplankton diversity of Chandrasarovar Pond of Jhalawar (Rajasthan). EPRA International Journal of Multidisciplinary Research (IJMR). 2022, 8, 1-1. https://doi.org/10.36713/epra9394.
Santhanam, R.; Velayutham, P.; Jegatheesan, G. A Manual of Freshwater Ecology, Daya Publishing House, Delhi, India, 1989, 1-109.
Sarwade, A.B.; Kamble, N.A. Plankton diversity in Krishna River, Sangli, Maharashtra. Journal of Ecology and the Natural Environment. 2014, 6, 174-181.
Sasakova, N.; Gregova, G.; Takacova, D.; Mojzisova, J.; Papajova, I.; Venglovsky, J.; Kovacova, S. Pollution of surface and ground water by sources related to agricultural activities. Frontiers in Sustainable Food Systems. 2018, 2, 42. https://doi.org/10.3389/fsufs.2018.00042.
Shannon, C.E.; Weaner’s, W. The Mathematical Theory of Communications, University of Lllinois Press, Urbana, 1949, 117.
Sharma, R.C.; Singh, N.; Chauhan, A. The influence of physico-chemical parameters on phytoplankton distribution in a head water stream of Garhwal Himalayas: a case study. The Egyptian Journal of Aquatic Research. 2016, 42, 11-21. https://doi.org/10.1016/j.ejar.2015.11.004.
Shen, J.; Qin, G.; Yu, R.; Zhao, Y.; Yang, J.; An, S.; Wan, Y. Urbanization has changed the distribution pattern of zooplankton species diversity and the structure of functional groups. Ecological Indicators. 2021, 120, 106944. https://doi.org/10.1016/j.ecolind.2020.106944.
Shiel, R.J. A guide to identification of rotifers, Cladocerans and copepods from Australian inland waters. Presented at Taxonomy Workshop, Murray-Darling Freshwater Research Centre, 1995, 8-10.
Singh, S.; Kumari, V.; Usmani, E.; Dutta, R.; Kumari, R.; Kumari, J.; Arif, M. Study on Zooplankton Diversity in A Fresh Water Pond (Raja Bandh) of Jamtara, Jharkhand, India. International Journal of Advancement in Life Sciences Research. 2021, 4, 5-13. https://doi.org/10.31632/ijalsr.2021.v04i02.002.
Vadadi Fülöp, C.; Mészáros, G.; Jablonszky, G.; Hufnagel, L. The zooplankton of the Ráckeve-Soroksár Danube: spatio-temporal changes and similarity patterns. Applied Ecology and Environmental Research. 2008, 6, 121-148.
Verma, A.K.; Prakash, S.A. Zooplankton diversity in Guthia Taal, Wetland of Bahraich (UP), India. International journal of Zoology and Research, 2020, 10, 09-18.
Venkataraman, G. A systematic account of some south Indian Diatoms, Proceedings / Indian Academy of Sciences. 1939, 6, 293-368. https://doi.org/10.1007/BF03039988.
Xiong, W.; Huang, X.; Chen, Y.; Fu, R.; Du, X.; Chen, X.; Zhan, A. Zooplankton biodiversity monitoring in polluted freshwater ecosystems: A technical review. Environmental Science and Ecotechnology. 2020, 1, 100008. https://doi.org/10.1016/j.ese.2019.100008.
Xu, J.; Zhang, M. Primary consumers as bioindicator of nitrogen pollution in lake planktonic and benthic food webs. Ecological Indicators. 2012, 14, 189-196. https://doi.org/10.1016/j.ecolind.2011.02.012.
Xue, Y.; Chen, H.; Yang, J.R.; Liu, M.; Huang, B.; Yang, J. Distinct patterns and processes of abundant and rare eukaryotic plankton communities following a reservoir cyanobacterial bloom. The ISME Journal. 2018, 12, 2263-2277. https://doi.org/10.1038/s4139 6-018-0159-0.
Xue, H.; Sayama, T.; Takara, K.; He, B.; Huang, G.; Duan, W. Non-point source pollution estimation in the Pingqiao River Basin, China, using a spatial hydrograph-separation approach. Hydrological Sciences Journal. 2019, 64, 962-973. https://doi.org/10.1080/0262 6667.2019.1617867.
Yusuf, Z.H. Phytoplankton as bioindicators of water quality in Nasarawa reservoir, Katsina State Nigeria. Acta Limnologica Brasiliensia. 2020, 32. https://doi.org/10.1590/S2179-975X3319.
Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Analytica chimica acta. 2008, 606, 135-150. https://doi.org/10.1016/j.aca.2007.11.018.
Academic Editor: Dr. Isabela Maria Simion
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Bhavan Periyakali Saravana, Kalpana Ramaswamy, Manickam Narasimman, Mohan Bala, Prabha Duraiswamy, Priyadarshinee Sheela, Santhanam Perumal