Andrei Daniel Tudor, Ciprian Bolohan, Valerica Tudor, Răzvan Ionuț Teodorescu
ABSTRACT. The consumption of fruits, leaves, and roots of Lycium barbarum L. and Lycium chinense (Mill.) species has a long tradition, especially on the Asian continent, due to their health benefits. In recent decades, social and economic factors, along with scientific progress, have stimulated the expansion of the consumption and cultivation of goji plants on a global scale, but mostly in Western countries. The traditional therapeutic properties attributed to goji plants, scientifically demonstrated through clinical and pharmacological studies in vitro and in vivo, are due to a diversified content in antioxidants (polysaccharides, flavonoids, carotenoids, and antioxidant capacity). With the development of technological capabilities for the detection and extraction of biocompounds from plant resources (including from secondary metabolisms), the completeness of research on the beneficial and secondary effects of the use of these species in human nutrition has increased. In most of the published studies, the chemical profile of L. barbarum or L. chinense species was analysed in terms of the therapeutic benefits of the variety, the different plant components subjected to extraction, the prior processing of these components, the method of extraction of active biocompounds, and to some extent, the correlation of this profile with geographical origin. The objective of this study is to provide a comprehensive and updated summary on some chemical compounds with therapeutic effects from Lycium spp. plants, addressing the correlation of the phytochemical composition in relation to their cultivation area, in the perspective of identifying and creating new goji varieties with high adaptability to local pedoclimatic conditions.
Keywords: L. barbarum; L. chinense; chemical profile; cultivation areas.
Cite
ALSE and ACS Style
Tudor, A.D.; Bolohan, C.; Tudor, V.; Teodorescu, R.I. Main active components of goji berry and their nutritional importance – a review. Journal of Applied Life Sciences and Environment 2022, 55 (2), 111-132.
https://doi.org/10.46909/alse-552053
AMA Style
Tudor AD, Bolohan C, Tudor V, Teodorescu R.I. Main active components of goji berry and their nutritional importance – a review. Journal of Applied Life Sciences and Environment. 2022; 55 (2): 111-132.
https://doi.org/10.46909/alse-552053
Chicago/Turabian Style
Tudor, Andrei Daniel, Ciprian Bolohan, Valerica Tudor, and Răzvan Ionuț Teodorescu. 2022. “Main active components of goji berry and their nutritional importance – a review” Journal of Applied Life Sciences and Environment 55, no. 2: 111-132.
https://doi.org/10.46909/alse-552053
View full article (HTML)
Main Active Components of Goji Berry and Their Nutritional Importance – A Review
Andrei Daniel TUDOR, Ciprian BOLOHAN, Valerica TUDOR* and Răzvan Ionuț TEODORESCU
University of Agronomic Sciences and Veterinary Medicine of Bucharest 59, Marasti Blvd, District 1 Bucharest 011464, Romania; e-mail: tudorandreidaniel10@gmail.com; cippy_bollo@yahoo.com; razvan.iteodorescu@gmail.com
*Correspondence: valericatudor@gmail.com
Received: Oct. 27, 2022. Revised: Nov. 23, 2022. Accepted: Dec. 12, 2022. Published online: Jan. 26, 2023
ABSTRACT. The consumption of fruits, leaves, and roots of Lycium barbarum L. and Lycium chinense (Mill.) species has a long tradition, especially on the Asian continent, due to their health benefits. In recent decades, social and economic factors, along with scientific progress, have stimulated the expansion of the consumption and cultivation of goji plants on a global scale, but mostly in Western countries. The traditional therapeutic properties attributed to goji plants, scientifically demonstrated through clinical and pharmacological studies in vitro and in vivo, are due to a diversified content in antioxidants (polysaccharides, flavonoids, carotenoids, and antioxidant capacity). With the development of technological capabilities for the detection and extraction of biocompounds from plant resources (including from secondary metabolisms), the completeness of research on the beneficial and secondary effects of the use of these species in human nutrition has increased. In most of the published studies, the chemical profile of L. barbarum or L. chinense species was analysed in terms of the therapeutic benefits of the variety, the different plant components subjected to extraction, the prior processing of these components, the method of extraction of active biocompounds, and to some extent, the correlation of this profile with geographical origin. The objective of this study is to provide a comprehensive and updated summary on some chemical compounds with therapeutic effects from Lycium spp. plants, addressing the correlation of the phytochemical composition in relation to their cultivation area, in the perspective of identifying and creating new goji varieties with high adaptability to local pedoclimatic conditions.
Keywords: L. barbarum; L. chinense; chemical profile; cultivation areas.
INTRODUCTION
Due to the nutritional properties and the high content of different phytochemical compounds with multiple effects on health, goji fruits have received the generic name of ‘superfruit’ or ‘superfood’ (Van Straten and Griggs, 2006; Llorent-Martínez et al., 2013; Kulczyński and Gramza-Michałowska, 2016; Chang et al., 2018; Ma et al., 2019; Jiao and Liu, 2020; Chang et al., 2020; Wang et al., 2022). Lately, the special attention generally enjoyed by the species of the Lycium genus emerges from the research carried out on them by numerous authors, including through reviews that cover an increasing amount of data. Qian et al. (2017a) showed that in a period of 41 years (1975-2016), more than 350 chemical compounds have been isolated from different parts of plants of the genus Lycium and described. Lu et al. (2017) mentioned the consumption of goji fruits due to the special taste as a ‘unique aroma’ and talked about the detection of 193 volatile substances in the fruits of Lycium barbarium L. grown in the Ningxia Region of China, and Kim et al. (2009) detected 130 volatile substances in the fruits of Lycium chinensis Miller (Chungchungnam-do, South Korea).
Geographical distribution and taxonomy
The generic name ‘goji’ is attributed to the fruit and, by extension, to the perennial shrubs of the family Solanaceae, genus Lycium. The genus includes between 75 (Miller, 2002) and 80 species (Levin and Miller, 2005; Zhang et al., 2018; Liu and Sun, 2020), with a fragmented distribution in temperate, arid, or semi-arid climate areas and temperatures between 15 °C and 40 °C (Jatoi et al., 2017; Yao et al., 2018a) (Figure 1). Carl Linneaus made the first description of the genus, with three of its species (Lycium europaeum, Lycium barbarum, and Lycium afrum), in his 1753 work, ‘Species Plantarum’ (Yao et al., 2018a), where he gives the species name L. barbarum. Miller named the species Lycium chinense in 1768 (Kulczyński and Gramza-Michałowska, 2016). The first taxonomy, including 43 species of the genus Lycium from the Northern Hemisphere, was made by Hitchcock in 1932 (Yao et al., 2018a).
The generic name ‘goji’ is a derivative of the term ‘gouqi’ (Potterat and Hamburger, 2008) or ‘gou qi’ (Chen et al., 2018; Yao et al., 2018a), resulting from the extrapolation of several native Chinese words and stated in this form for the first time in 1973 by Tanaduk Botanical Research Institute researchers (Amagase and Farnsworth, 2011 cited by Shahrajabian et al., 2018). The first mention of the term ‘gou qi’ is found in “Shen Nong Ben Cao Jing”, a book of Chinese origin written between 200 and 250 AD that describes agricultural practices and presents information about medicinal plants (Chen et al., 2018).
The endemic species of the genus Lycium are mainly found in North America, South America, South of Africa, Asia, Europe, and Australia (Table 1). Three taxonomic varieties and seven species of goji are found in China (Zhang et al., 2018). Analyses carried out by genetic sequencing (Miller et al., 2011; Cao et al., 2021) support the hypothesis that species of the genus Lycium migrated from South America to North America and Africa, then to Eurasia, East Asia, and Australia.
L. barbarum and L. chinense were the species intensively promoted under the name of goji, and they spread worldwide from the point of view of cultivation for commercial purposes, on the background of the superior quality of the fruits, exploited as food with therapeutic effects.
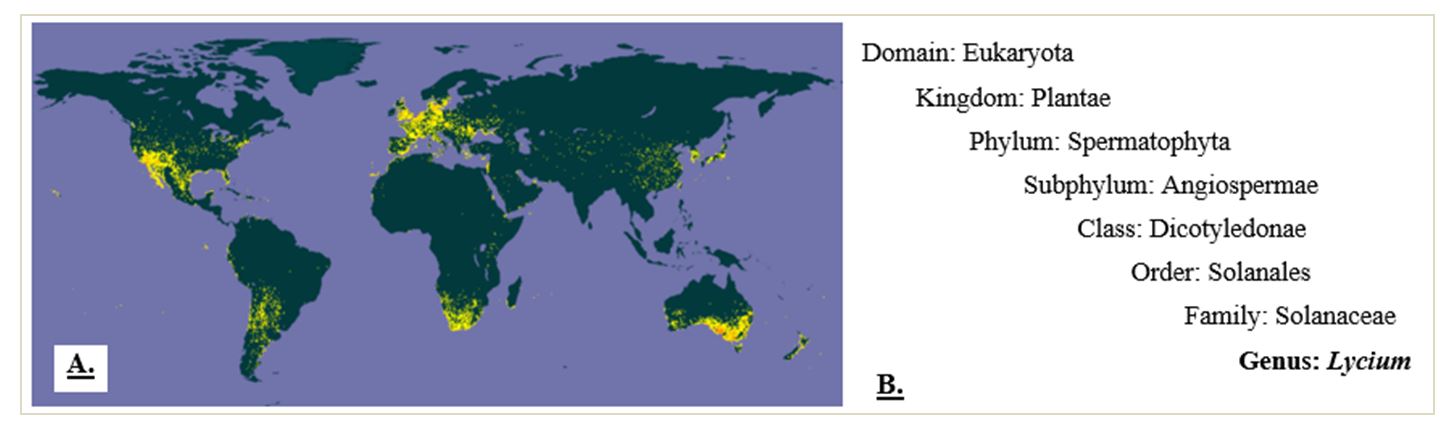
Figure 1 – Genus Lycium distribution and taxonomy. A) Global distribution of genus Lycium based on the records associated with the number of occurrences (yellow dots) of its species from 1600 to 2022 (cumulated values) in the Global Biodiversity Information Facility (GBIF, 2022). B) Genus Lycium taxonomic tree (CABI, 2022)
Table 1
Species of the genus Lycium
|
Continent |
Lycium spp. |
Authors |
|
Asia |
L. chinense, Lycium ruthenicum, L. barbarum, Lycium truncatum, Lycium dasystemum, Lycium cylindricum, L. chinense, Lycium yunnanense, Lycium changjicum, Lycium depressum |
Levin and Miller, 2005; Cao et al., 2021 |
|
Europe |
Lycium ruthenicum |
Levin and Miller, 2005 |
|
Lycium europeaum |
Yao et al., 2018 |
|
|
South of Africa |
Lycium arenicol, Lycium bosciifolium, Lycium cinereum, Lycium ferocissimum, Lycium hirsutum, Lycium. horridum, Lycium oxycarpum, Lycium pilifolium C, Lycium schizocalyx, Lycium shawii, Lycium sp. N-309, Lycium tenue, Lycium villosum, Lycium villosum, |
Levin and Miller, 2005 |
|
Australia |
Lycium australe |
Levin and Miller, 2005 |
|
North America |
L. barbarum, Lycium berlandieri, Lycium californicum, Lycium carolinianum, Lycium cooperi, Lycium exsertum, Lycium exsertum, Lycium fremontii, Lycium macrodon, Lycium pallidum, Lycium parishi, Lycium puberulum, Lycium Shockley, Lycium sp. 202, Lycium texanum, Lycium torreyi, |
Levin and Miller, 2005; Cao et al., 2021; Shahrajabian et al., 2018 |
|
South America |
Lycium ameghinoi, Lycium. americanum, Lycium andersonii, Lycium brevipes, Lycium cestroides, Lycium chilense, Lycium ciliatum, Lycium cuneatum, Lycium elongatum, Lycium fremontii, Lycium gilliesianum, Lycium infaustum, Lycium moronga, Lycium nodosum, Lycium vimineum |
Levin and Miller, 2005; Shahrajabian et al., 2018 |
Although the quality and productivity of other species or subspecies of the Lycium genus may be lower, they are valued locally under the same common name (goji) (Wetters et al., 2018) or similar names (e.g., wolfberry or boxthorn). Based on the distribution of the two species, Yao et al. (2021) postulated the impossibility of differentiating the two based on their geographical origin, given the overlap of their distribution areas (Figure 2).
According to some authors (Wenli et al., 2021), goji is native from China, a perception justified by the fact that this country is the main grower of goji for commercial purposes (Sun et al., 2017; Chen et al., 2018). The most recent data (Yao et al., 2018b) indicated an area of about 150 thousand hectares (at the level of 2015) that was cultivated mainly in the regions of north and northwest China in the provinces of Inner Mongolia (Inner Mongolia), Xinjiang, Gansu, Qinghai, Ningxia, and Hebei (Potterat and Hamburger, 2008; Sun et al., 2017; Yao et al., 2018b; Zhang et al., 2018). Other authors (Kulczyński and Gramza-Michałowska, 2016) advance values of about 82,000 hectares in terms of cultivated area, with an annual production of about 95,000 tons. Moreover, the beginning of the domestication and cultivation of the species L. barbarum and L. chinese about 600 years ago in northwest China, the endemic character of the species L. ruthenicum for this area (Shahrajabian et al., 2018; Zhang et al., 2018), and the use of different component parts of goji plants (fruits, roots, leaves, calyx, bark, or even the whole plant) in traditional Chinese medicine or as food for about 4,000 years (Wang et al., 2015; Yao et al., 2018a) have facilitated the perception of goji as a native plant in China. This perception is to some extent justified for the commercial goji varieties that were obtained over 600 years of natural and artificial selection (Zhang et al., 2018). In addition, in the last decades, significant government resources have been allocated to support the cultivation and promotion of the consumption of goji berries, especially L. barbarum and L. chinense, a fact that led to the expansion and international recognition of the two species, assimilated most frequently under the name of goji (Yao, 2019).
Goji was first introduced to Europe in the 18th century (Sopher, 2013 cited by Kulczyński and Gramza-Michałowska, 2016); however, since the 2000s, due to effective marketing strategies promoting goji berries as a quasi-miracle remedy for health and anti-aging (Potterat and Hamburger, 2008; Potterat, 2010; Jiao and Liu, 2020), the two species (L. barbarum and L. chinense) were introduced for cultivation in North America, southeastern Europe (Romania, Bulgaria), and in Mediterranean agro-climatic conditions (Italy, Portugal) (Amagase and Farnsworth, 2011) where new varieties were developed (Dzhugalov et al., 2015; Protti et al., 2017; Mocan et al., 2018, 2019). In the southern region of Italy, L. barbarum is cultivated on about 38 ha, the largest plantation in Europe (Juan-Garcia et al., 2019), and the fruits are sold as a fresh, dried, or processed products. The geographical origin of the fruit is essential from the point of view of quality because the chemical composition changes depending on the climate, water, soil, and cultivation conditions (Li et al., 2017). In Romania, Stavrescu-Bedivan et al. (2022) showed that the species L. Barbarum was reported in 37 counties out of a total of 41, including the Bucharest area.
Phytochemical constituents
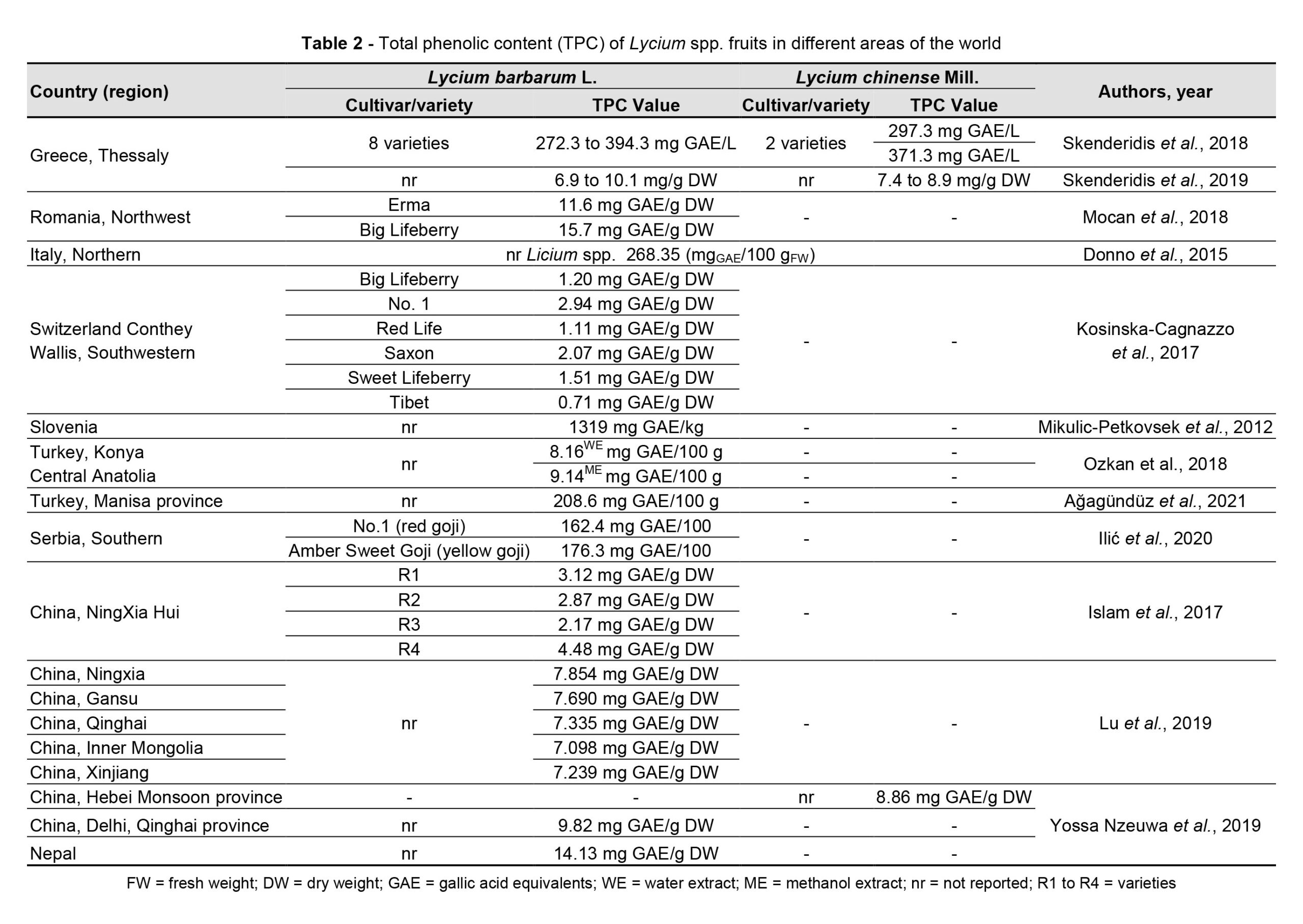
The interest in researching goji species derives from the complexity of the plant’s chemical composition. This is especially attributed to the content of different classes of biocompounds with antioxidant, anti-inflammatory, and antineoplastic effects (Chen et al., 2018), with the predominant ones being polysaccharides, flavonoids, carotenoids, lipids, steroids, alkaloids, terpenoids, and phenolic compounds (Protti et al., 2017; Chen et al., 2018; Jiang et al., 2021). In the different parts of the plant (reproductive: fruits, flowers, and seeds, but also vegetative: roots, leaves, and bark), Lycium spp. contain 355 active compounds that can be grouped into glycerogalactolipids (6%), phenylpropanoids (9%), coumarins (3%), lignans (4%), flavonoids (9%), amides (10%), alkaloids (20%), anthraquinones (1%), organic acids (9%), terpenoids (11%), peptides (1%), and sterols, steroids, and other constituents (16%) (Qian et al., 2017a; Jiao and Liu, 2020). Total phenolic content differed according to cultivation conditions and variety (Table 2). Lycium spp. fruit contain a total of 186 phenolic compounds, with flavonoids and phenolic acids being the predominant classes (Jiang et al., 2021) in red goji berries (L. barbarum, L. chinense) and anthocyanins being the main polyphenols in black goji berries (varieties of L. ruthenicum) (Sun et al., 2017).
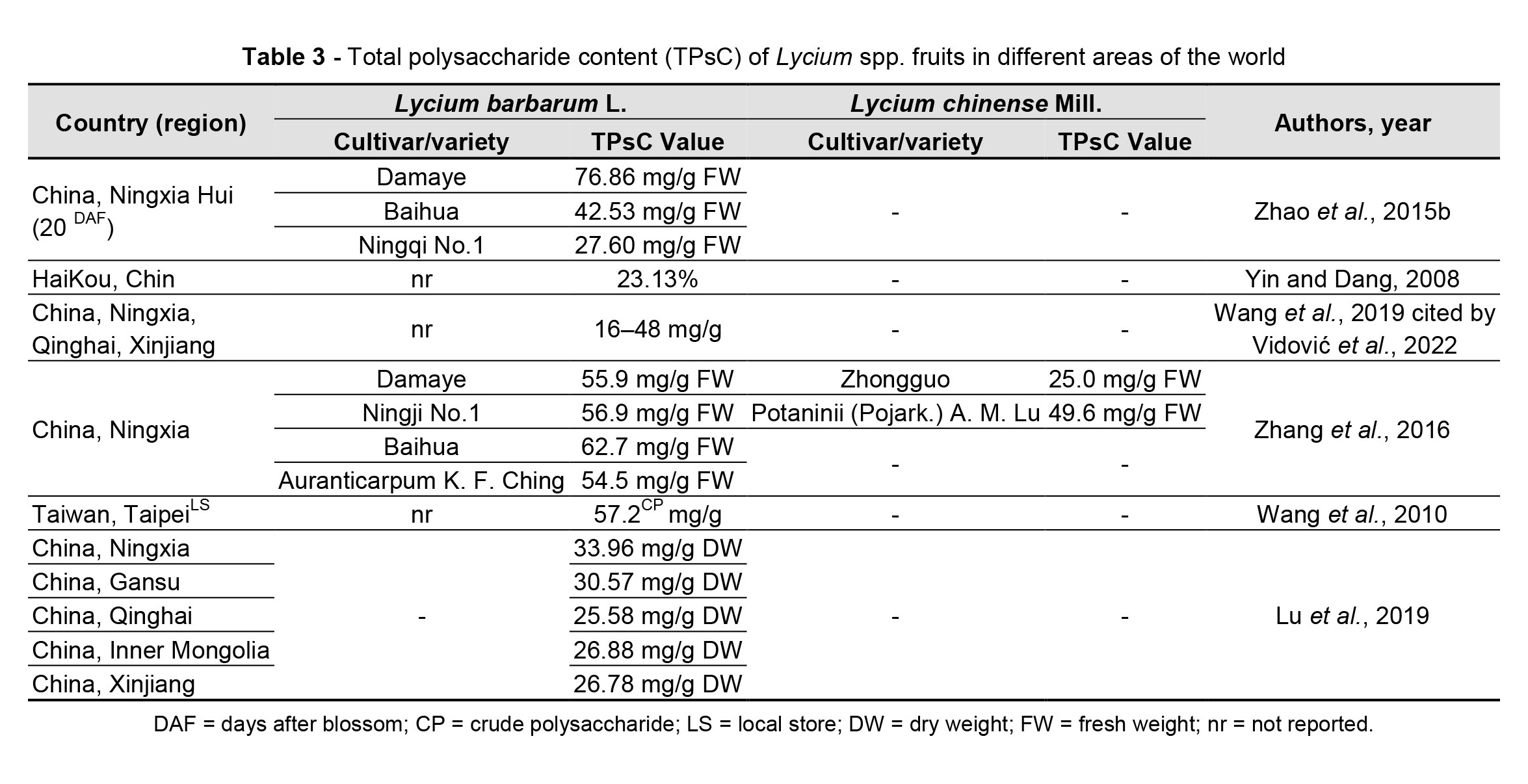
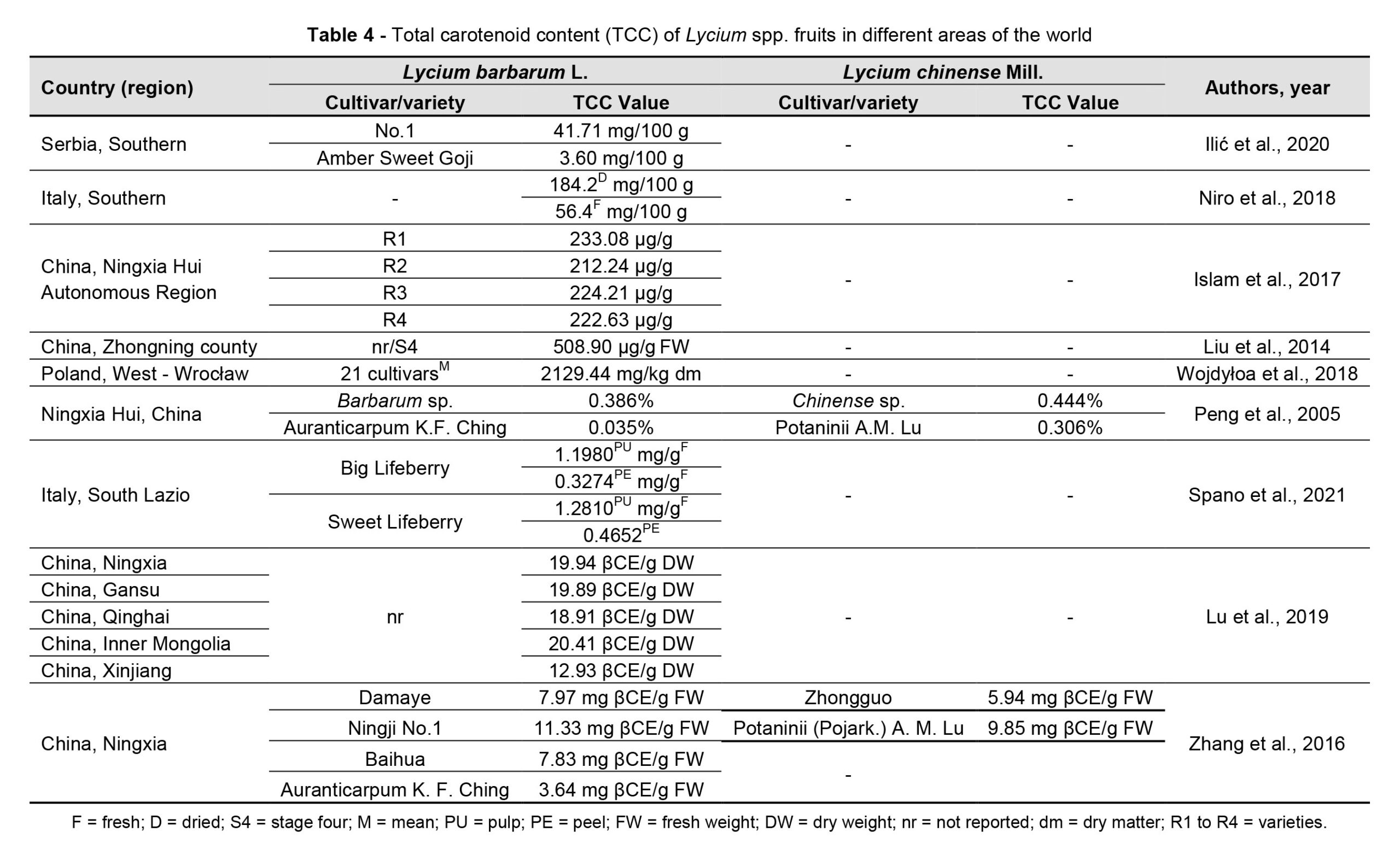
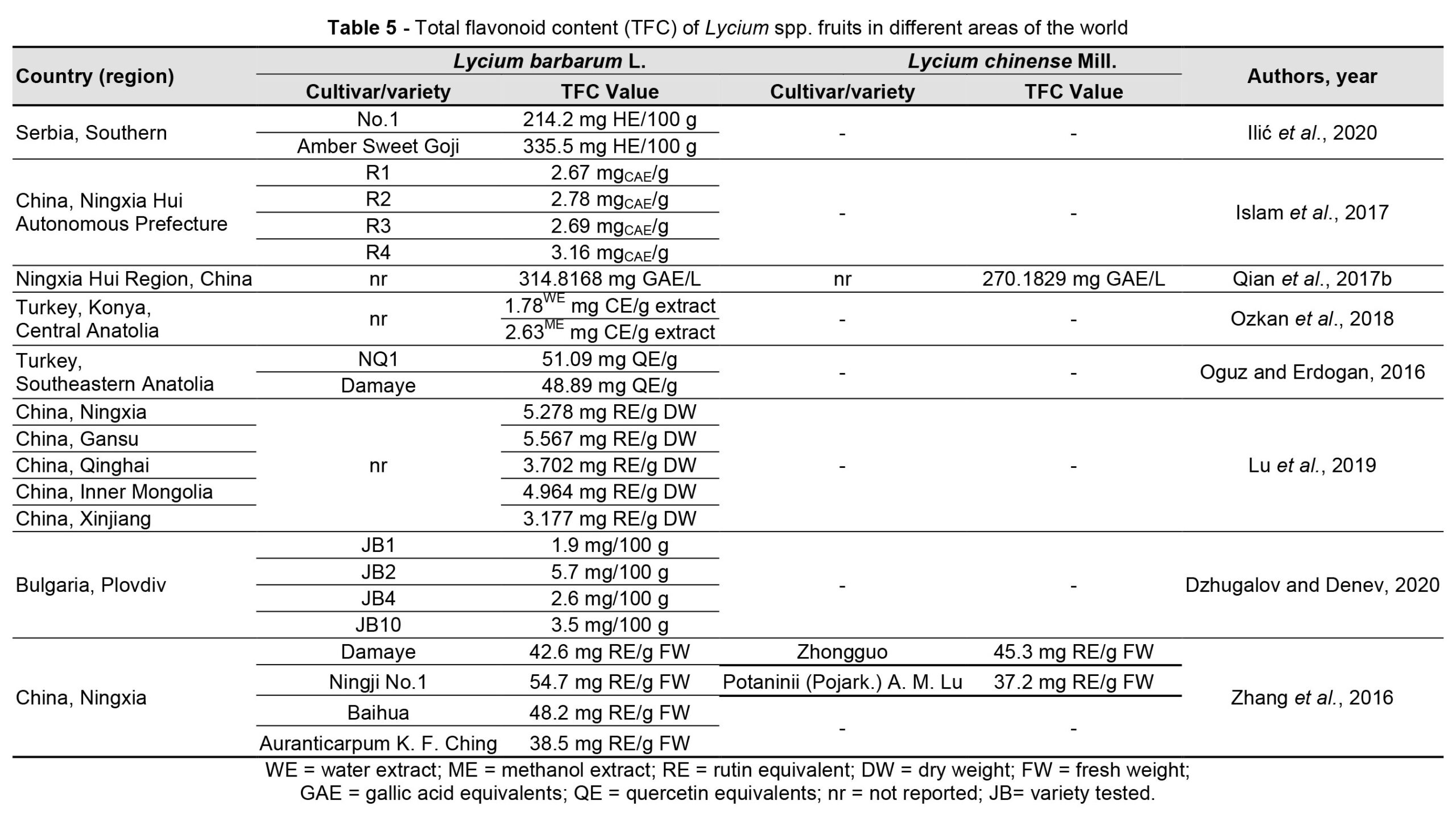
Goji berries are a source of potentially bioactive substances, but the chemical profile could differ depending on their origin, cultivation, and/or processing method (Mocan et al., 2019). Environmental conditions influence both the fruit appearance and the metabolic profile (Zhang et al., 2012; Shen et al., 2016) of goji plants, in terms of the amount of polysaccharides (Table 3), carotenoids (Table 4), flavonoids (Table 5) and betaine.
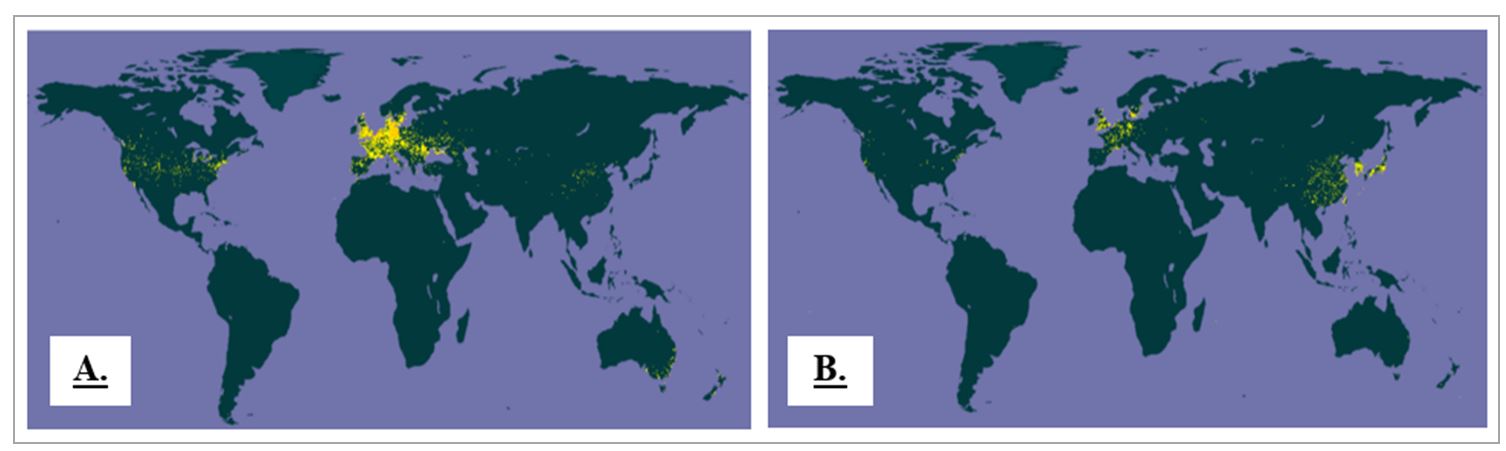
Figure 2 – Distribution maps of L. barbarum (A) and L. chinense (B); data reflect the number of occurences of the species (yellow areas) based on the number of occurrences from 1600 to 2022 (cumulated values) and are based on the Global Biodiversity Information Facility (GBIF, 2022)
Yang et al. (2021) identified strong correlations between chemical profile constituents and geographic distribution of L. chinense with higher concentrations of flavonoids and polysaccharides in cultivars from central and southern China. Zheng et al. (2010) observed such variations in a study on the fructose, glucose, sucrose, polysaccharide, and sugar content of L. barbarum and L. chinense fruits from different cultivars and regions. The research revealed that the pedological specificity of the cultivation area, from the point of view of pH, organic matter, and nitrogen content but also the salt content (HCO3–, Na+, Ca2+, Mg2+, and Cl–), influences the accumulation of sugar in the fruits of goji. Bondia-Pons et al. (2014) highlighted the importance of the geographical origin in analysing the chemical profile of the plants, as it is influenced by climate and soil conditions and cultivation methods, which can change the chemical composition and impact the quality of the fruits or other parts of the plant.
Bertoldi et al. (2019) identified a higher total carotenoid content (355 mg/ 100 g DWcompared to 198 mg/100 g DW), a higher magnesium content (from 72 to 267 mg/100 g compared to values between 78 and 161 mg/100 g), and higher values of the micronutrient content (K, B, Cu, Mo, Se, Zn) in Italian goji berries compared to those of Asian origin.
The sterol content of goji berries differs according to the geographic area of cultivation (Cossignani et al., 2018). The results of the research carried out by Cossignani et al. (2018) showed that goji berry samples of Italian origin had a higher content of β-sitosterol, whereas the content of Δ5-avenasterol and Δ-5,23-stigmastadienol was representative of fruits of Asian origin. Moreover, the content of essential fatty acids was higher for samples from China and Mongolia (61.0% and 61.6%) and lower (47.8%) for Italian fruits, and the content of phytosterols varied between 42.8 mg/100 g for Italian fruits and 130.1 mg/100 g for Mongolian ones. Wojdyło et al. (2018) analysed 21 new cultivars of L. barbarum obtained in a breeding program developed in Poland and identified differences in the biochemical profile and functional properties of the new varieties, postulating the opportunity to use only some of them as a source of bioactive compounds with high biological activity.
Yossa Nzeuwa et al. (2019) identified slight differences in the chemical profile of dried fruits from different varieties of L. barbarum and L. chinense cultivated in China (in the regions devoted to these species, like Ningxia, Xinjiang, Qinghai, and Gansu) and Nepal, without obtaining specific results to support the prevalence of a geographical area in terms of chemical composition (Table 2).
Research on the chemical composition and antioxidant activity of the dried fruits of L. barbarum (cultivated in Greece, China, and Mongolia) and L. chinense (cultivated in Greece) highlights differences both in the total carbohydrate content and the total phenolic content (Skenderidis et al., 2019). The highest total carbohydrate content was recorded in L. barbarum fruits grown in Greece and harvested in August (490 mg/g dw), and the highest total phenolic content (10.9 mg/g dw) is identified in the Mongolian variety. Moreover, L. chinense shows a lower concentration of carbohydrates and phenols compared to L. barbarum varieties (Skenderidis et al., 2019). The antioxidant activity of Lycium spp. fruits has been validated by numerous authors (Table 6), and the values of this parameter vary depending on the variety/cultivar and the cultivation area.
Nutritional uses
Between 31 (Yao et al., 2018) and 36 species (Yao, 2019) of the genus Lycium are exploited for food or medicinal purposes globally. L. barbarum, L. chinense, and L. ruthenicum are the species most often reported in Asia, especially in China (Yao et al., 2018) where the fruits have been used in traditional medicine for thousands of years (Cossignani et al., 2018). Goji berries are also used in traditional Korean, Vietnamese, Japanese, and Tibetan medicine (Potterat and Hamburger, 2008; Shahrajabian et al., 2018). As food, the fruits are usually consumed fresh or dried, or they are processed as juices, tinctures, or jellies (Kulczyński and Gramza-Michałowska, 2016; Jiao and Liu, 2020). Along with the fruits, the bark and leaves of Lycium spp. are boiled for infusions, individually or in combination with Chrysanthemum morifolium, Chrysanthemum indicum, Zizisiphus jujube, or Camellia sinensis (Sun et al., 2017). In Chinese cuisine, goji berries are often cooked before consumption and used in vegetable soups in combination with rice porridge, chicken, or pork (Kulczyński and Gramza-Michałowska, 2016; Sun et al., 2017). Goji wines are another form of processing the fruits of these species (Xia et al., 2016; Kulczyński and Gramza-Michałowska, 2016; Jiao and Liu, 2020). Traditionally, goji wine is obtained by macerating the fruit in an alcohol (Sun et al., 2017), and fermented goji wines have gained special attention in recent years due to their distinctive aroma and taste (Xia et al., 2016). In the last decades, L. barbarum and L. chinense have been promoted on a global scale as ‘superfood’ (Yao et al., 2018) or ‘superfruit’ (Jiao and Liu, 2020), terms used to define a food rich in elements, nutrients, and antioxidants (Chang et al., 2018) with properties to prevent or treat certain conditions (Jiao and Liu, 2020). Although L. europeaum, L. intricatum, and L. shawii are endemic species reported in the Mediterranean area and the Middle East, L. pallidum in North America, and L. afrum in Africa (Yao et al., 2018), L. barbarum and L. chinense have been promoted for cultivation on a global scale and are culturally accepted under the name goji. The two species were initially promoted for consumption in Europe, America, and Australia in juice form (Potterat and Hamburger, 2008). Later, the fruits were integrated into cakes, protein bars, chocolate, muesli, sausages, and even soups (Potterat and Hamburger, 2008), but most frequently they are consumed as a food supplement or used in the form of tea (Sun et al., 2017).
The popularization of goji consumption in Western countries is in close interdependence with the expansion of research on deepening the nutritional profile of the species but also investigating the potential for new uses of them for food purposes.
Dried and fresh goji fruits are a rich source of proteins and minerals (Niro et al., 2017; Pires et al., 2018). One hundred dry grams of goji berries can contain: 1) 10.2% (Niro et al., 2017) –14.26% (Potterat, 2010) protein; 2) 101.3–190 mg of calcium; 3) 861.9–2,233 mg of potassium; 4) 3.4–9 mg of iron; 5) 0.17–0.5 mg of selenium; 6) 0.33–1.3 mg of vitamin B2 (riboflavin); 7) 29–148 mg of vitamin C; 8) 45.9–140 mg of magnesium; 9) 209.8–448 mg of natrium; and 10) 140–174.3 mg of phosphorus (Potterat, 2010; Niro et al., 2017; Pedro et al., 2018; Shahrajabian et al., 2018).
Rybicka et al. (2021) compared dried goji berries with a selection of other dried fruits (raisins, prunes, sea buckthorn, rose hips, etc.; all commercially available in Poland), and the results obtained show the importance of dried goji berries as an alternative to snack foods, due to their high protein content (13.3%).
Other authors (Pop et al., 2013; Bora et al., 2019) successfully test the use of goji fruit and powder to improve the nutritional and sensory properties of muffins and cookies. Ducruet et al. (2017) successfully developed an antioxidant-rich beer based on goji berries.
Through a study on the development of yogurt microflora, Rotar et al. (2015) validates the fact that goji berries can be successfully used as a potentiator of the level of probiotics in yogurt. The high antioxidant content makes Lycium spp. plants a candidate for obtaining extracts with potential use in the food industry. Using non-toxic solvents, Pedro et al. (2018) obtained a goji fruit extract with applications as a natural antioxidant in the food industry, especially for edible oils. Other research (Juan-Garcia et al., 2019) suggests the consumption of goji berries have a preventive or curative role in the case of phytotoxic effects generated by the mycotoxin beauvericin, attributed to Fusarium infestation of cereals and cereal-based products. Fadiloglu and Çoban (2019) proved the effectiveness of goji extracts as antimicrobial and antioxidant agents to extend the shelf life of smoked fish sausages by up to seven days.
Potential health and therapeutic uses
Through their chemical composition, goji fruits have a high potential in reducing oxidative stress, including preventing the effect of free radicals on the damage of proteins, lipids, and DNA (Ma et al., 2019). They are also used to treat diabetes and a variety of problems related to blood circulation (Chen et al., 2018). The Asian pharmacopeia postulates the therapeutic use of the fruits and peel of L. barbarum / L. chinense (Chinese pharmacopoeia) and sometimes of the aerial parts of L. barbarum and L. europeaum (Indian pharmacopoeia), while the European one includes only the dried fruits of L. barbarum (Yao et al., 2018).
Various modern in vitro or in vivo clinical research validated the therapeutic effects of the species (Jiang et al., 2021). Research by He et al. (2012) demonstrated that goji plants, through their content in polysaccharides, have the potential to be used in protection against cancer. This action is attributed to the ability of Lycium Barbarum Polysaccharide to stop the cell cycle and inhibit some signalling flows, eliminating excess abnormal cells (Jin et al., 2013). Li et al. (2007) proved the positive effects of L. barbarum polysaccharides in reducing the risk of lipid peroxidation and the decline of the total antioxidant capacity in the body of mice. Hsu et al. (2017) identified the ability of a carotenoid nanoemulsion from L. barbarum to inhibit the growth of HT-29 cancer cells, associated with colon cancer.
In vitro research (Huang et al., 2019) reveals the positive effect of the pectic polysaccharide XLBP-I-I, extracted from the fruits of L. barbarum, in reducing endoplasmic reticulum stress and protecting cells against apoptosis induced by this type of stress. Gan et al. (2004) analysed the regulatory capacity of a polysaccharide-protein complex extracted from L. barbarum on the immune system and the size of S180 sarcoma tumours in mice, their results indicated a very significant effect on tumour weight in the case of treatment with 10 mg/kg polysaccharide-protein extract from L. barbarum, associated with immunostimulatory activity.
The phenolic compounds extracted from L. barbarum are correlated with the inhibition of lipid peroxidation and the increase of the antioxidant and hepatoprotective effects, applicable in the case of high-fat diets, according to the in vivo research (on laboratory mice) carried out by Cui et al. (2011). Ming et al. (2009) also observed the positive effect obtained as a result of the administration of polysaccharides extracted from L. barbarum in the sense of decreasing, on the background of antioxidant activity, both total cholesterol and LDL, HLD fractions, triglycerides, and glucose content in the blood and glycogen from the liver. Kulczyński and Gramza-Michałowska (2016) identified the reduction of cholesterol concentration (LDL and HDL) in the case of laboratory mice subjected to a high-fat diet, which were administered polysaccharide fractions extracted from L. barbarum.
Cheng and Kong (2011) demonstrate that the polysaccharides specific to the L. barbarum species have a significant impact in improving liver damage, preventing the progression of fatty liver associated with alcohol consumption, and improving the antioxidant activity of the body of laboratory mice. Other in vivo research postulates the hypoglycaemic and antidiabetic effect of the polysaccharide extract from L. barbarum in particular (Zhou et al., 2009; Jing and Yin, 2010; Zhao et al., 2015a), and the results are supported by in vitro studies (Wojdyło et al., 2018).
CONCLUSIONS
The rediscovery of the food value and the commercial importance of goji fruit is reflected in the effort of the scientific literature of the last decades to validate and deepen the state of knowledge of the species of the genus Lycium.
Numerous studies have followed the chemical composition of Lycium spp. plants, mainly analysing the chemical profile of L. barbarum fruits, since this is generally accepted as the superior quality species from the point of view of human consumption. Therefore, future research can also focus on L. chinense, also accepted as goji, or other species of the genus Lycium.
The scientific literature reports differences in the chemical composition and antioxidant action of goji plants; these changes are associated with the cultivar/variety, geographical origin, and the cultivation technology or the processing method.
Any change in the chemical profile of goji plants influences the nutritional or therapeutic effect on the body, in addition to affective consumption. Therefore, either their commercial destinations (food, medicinal, industrial) are adapted accordingly, or the possibility of acclimatization or creation can be deepened by new cultivars/varieties of goji in different areas to ensure the supply of these ‘superfruits’ locally.
Author Contributions: Conceptualization V.T.; methodology V.T., R.I.T; resources A.D.B.; writing T.V., C.B.; review T.V.; supervision R.I.T. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: There are no conflicts of interest related to this work.
REFERENCES
Ağagündüz, D.; Köseler-Beyaz, E.; Duman, S. Assessment of the Physicochemical and Antioxidant Profile of Dried Goji Berries (Lycium barbarum). Progress in Nutrition. 2021, 23, 1-8. https://doi.org/10.23751/pn.v23i4.11321
Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food research international. 2011, 44, 1702-1717. https://doi.org/10.1016/j.foodres.2011.03.027.
Bertoldi, D.; Cossignani, L.; Blasi, F.; Perini, M.; Barbero, A.; Pianezze, S.; Montesano, D. Characterisation and geographical traceability of Italian goji berries. Food chemistry. 2019, 275, 585-593. https://doi.org/10.1016/j.foodchem.2018.09.098.
Bondia-Pons, I.; Savolainen, O.; Torronen, R.; Martinez, J.A.; Poutanen, K.; Hnhineva, K. Metabolic profiling of goji berry extracts for discrimination of geographical origin by non-targeted liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Research International. 2014, 63, 132-138. https://doi.org/10.1016/j.foodres.2014.01.067.
Bora, P.; Ragaee, S.; Abdel-Aal, S.M. Effect of incorporation of Goji berry by-product on biochemical, physical and sensory properties of selected bakery products. LWT – Food Science and Technology. 2019, 112, 108225. https://doi.org/10.1016/J.LWT.2019.05.123.
CABI (Digital Library). Lycium barbarum (Matrimonyvine). Available online: https://www.cabi.org/isc/datasheet/31905 (accessed on 13 August 2022).
Cao, Y.L.; Li, Y.I.; Fan, Y.F. Wolfberry genomes and the evolution of Lycium (Solanaceae). Communications Biology. 2021, 4, 671. https://doi.org/10.1038/s42003-021-02152-8.
Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects – A comprehensive review. Critical Reviews in Food Science and Nutrition. 2018, 59, 1580-1604. https://doi.org/10.1080/10408398.2017.1422111.
Chen, J.; Chao, C.T.; Wei, X. Gojiberry breeding: current status and future prospects, In Breeding and health benefits of fruit and nut crops. IntechOpen., London, United Kingdom, 2018, pp. 2-15.
Cheng, D.; Kong, H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules. 2011, 16, 2542-2550. https://doi.org/10.3390/molecules16032542.
Çolak, A.M.; Okatan, V.; Güçlü, S.F.; Korkmaz, N.; Polat, M. Chemical characteristics and antioxidant activities of four native goji (Lycium barbarum L.) genotypes. “Scientific Papers” Iași University of Life Sciences (IULS), Series Horticulture. 2016, 59, 29-34. https://repository.uaiasi.ro/xmlui/handle/20.500.12811/2027.
Cossignani, L.; Blasi, F.; Simonetti, M.S.; Montesano, D. Fatty acids and phytosterols to discriminate geographic origin of Lycium barbarum berry. Food analytical methods. 2018, 11, 1180-1188. https://doi.org/10.1007/s12161-017-1098-5.
Cui, B.; Liu, S.; Lin, X.; Wang, J.; Li, S.; Wang, Q.; Li, S. Effects of Lycium barbarum aqueous and ethanol extracts on high-fat-diet induced oxidative stress in rat liver tissue. Molecules. 2011, 16, 9116-9128. https://doi.org/10.3390/molecules16119116.
Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. Journal of functional foods. 2015, 18, 1070-1085.
Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with Goji berries – The effect on bioactive compound content and sensorial properties. Food Chemistry. 2017, 226,109-118. https://doi.org/10.1016/j.foodchem.2017.01.047.
Dzhugalov, H.; Lichev, V.; Yordanov, A.; Kaymakanov, P.; Dimitrova, V.; Kutoranov, G. First results of testing Goji berry (Lycium barbarum L.) in Plovdiv region, Bulgaria. Scientific Papers. Series B, Horticulture. 2015, 59, 47-50.
Dzhugalov, H.; Denev, P. Growth, reproductive characteristics and antioxidant activity of four goji berry (Lycium barbarum L.) Cultivars. AGRICULTURAL SCIENCES – Agricultural University – Plovdiv. 2020, 12, 86-91. https://doi.org/10.22620/agrisci.2020.27.013.
Endes, Z.; Uslu, N.; Özcan, M.M.; Er, F. Physico-chemical properties, fatty acid composition and mineral contents of goji berry (Lycium barbarum L.) fruit. Journal of agroalimentary processes and technologies. 2015, 21, 36-40.
Fadiloglu, E.E.; Çoban, M.Z. The Effects of Goji Berry (Lycium barbarum L.) Extract on Some Chemical, Microbiological and Sensory Characteristics of Liquid Smoked Common Carp (Cyprinus carpio L., 1758) Sausages. Yuzuncu Yıl University Journal of Agricultural Sciences. 2019, 29, 702-710. https://doi.org/10.29133/yyutbd.632966
GBIF. Available online: https://www.gbif.org/species/2928808 (accessed on 17 August 2022)
He, N.; Yang, X.; Jiao, Y.; Tian, L.; Zhao, Y. Characterisation of antioxidant and antiproliferative acidic polysaccharides from Chinese wolfberry fruits. Food Chemistry. 2012, 133, 978-989. https://doi.org/10.1016/j.foodchem.2012.02.018.
Hsu, H.J.; Huang, R.F.; Kao, T.H.; Inbaraj, B.S.; Chen, B.H. Preparation of carotenoid extracts and nanoemulsions from Lycium barbarum L. and their effects on growth of HT-29 colon cancer cells. Nanotechnology. 2017, 28, 135103. https://doi.org/10.1088/1361-6528/aa5e86.
Huang, C.; Yao, R.; Zhu, Z.; Pang, D.; Cao, X.; Feng, B.; Paulsen, B.S.; Li, L.; Yin, Z.; Chen, X.; Jia, R.; Song, X.; Ye, G.; Luo, Q.; Chen, Z.; Zou, Y. A pectic polysaccharide from water decoction of Xinjiang Lycium barbarum fruit protects against intestinal endoplasmic reticulum stress. International journal of biological macromolecules. 2019, 130, 508-514. https://doi.org/10.1016/j.ijbiomac.2019.02.157.
Ilić, T.; Dodevska, M.; Marčetić, M.; Božić, D.; Kodranov, I.; Vidović, B. Chemical characterization, antioxidant and antimicrobial properties of goji berries cultivated in Serbia. Foods. 2020, 9, 1614. https://doi.org/10.3390/foods9111614.
Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chemistry Central Journal. 2017, 11, 1-8. https://doi.org/10.1186/s13065-017-0287-z.
Jatoi, M.A.; Jurić, S.; Vidrih, R.; Vinceković, M.; Vuković, M.; Jemrić, T. The effects of postharvest application of lecithin to improve storage potential and quality of fresh goji (Lycium barbarum L.) berries. Food chemistry. 2017, 230, 241-249. https://doi.org/10.1016/j.foodchem.2017.03.039.
Jiang, Y.; Fang, Z.; Leonard, W.; Zhang, P. Phenolic compounds in Lycium berry: Composition, health benefits and industrial applications. Journal of Functional Foods. 2021, 77, 104340. https://doi.org/10.1016/j.jff.2020.104340
Jiao, Y.; Liu, G. Goji Berry: a Novel Nutraceutical “Superfruit” for Florida Master Gardeners: HS1391, 10/2020. EDIS. 2020, 5, 1-5. http://dx.doi.org/10.32473/edis-hs1391-2020.
Jing, L.; Yin, L. Antihyperglycemic activity of polysaccharide from Lycium barbarum. Journal of Medicinal Plants Research. 2010, 4, 023-026. https://doi.org/10.5897/JMPR09.301.
Jin, M.; Huang, Q.; Zhao, K.; Shang, P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. International journal of biological macromolecules. 2013, 54, 16-23. https://doi.org/10.1016/j.ijbiomac.2012.11.023.
Juan-García, A.; Montesano, D.; Mañes, J.; Juan, C. Cytoprotective effects of carotenoids-rich extract from Lycium barbarum L. on the beauvericin-induced cytotoxicity on Caco-2 cells. Food and Chemical Toxicology. 2019, 133, 110798. https://doi.org/10.1016/j.fct.2019.110798.
Kim, J.S.; Chung, H.Y. GC-MS Analysis of the Volatile Components in Dried Boxthorn (Lycium Chinensis) Fruit. Journal of the Korean Society for Applied Biological Chemistry. 2009, 52, 516-524. https://doi.org/10.3839/jksabc.2009.088
Kosińska-Cagnazzo, A.; Weber, B.; Chablais, R.; Vouillamoz, J.F.; Molnar, B.; Crovadore, J.; François, F.; Andlauer, W. Bioactive compound profile and antioxidant activity of fruits from six goji cultivars cultivated in Switzerland. Journal of Berry Research. 2017, 7, 43-59. https://doi.org/10.3233/JBR-160144.
Kulczyński, B.; Gramza-Michałowska, A. Goji berry (Lycium barbarum): composition and health effects–a review. Polish Journal of Food and Nutrition Sciences. 2016, 66, 67-76. https://doi.org/10.1515/pjfns-2015-0040.
Levin, R.A.; Miller, J.S. Relationships within tribe Lycieae (Solanaceae): paraphyly of Lycium and multiple origins of gender dimorphism. American Journal of Botany. 2005, 92, 2044-2053. https://doi.org/10.3732/ajb.92.12.2044.
Li, Q.; Yu, X.; Xu, L.; Gao, J.M. Novel method for the producing area identification of Zhongning Goji berries by electronic nose. Food Chemistry. 2017, 221, 1113-1119. https://doi.org/10.1016/j.foodchem.2016.11.049.
Liu, Y.; Zeng, S.; Sun, W.; Wu, M.; Hu, W.; Shen, X.; Wang, Y. Comparative analysis of carotenoid accumulation in two goji (Lycium barbarum L. and L. ruthenicum Murr.) fruits. BMC plant biology. 2014, 14, 1-14.
Liu, B.; Xu, Q.; Sun, Y. Black goji berry (Lycium ruthenicum) tea has higher phytochemical contents and in vitro antioxidant properties than red goji berry (Lycium barbarum) tea. Food quality and safety. 2020, 4, 193-201. https://doi.org/10.1093/fqsafe/fyaa022.
Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Ortega-Barrales, P.; Ruiz-Medina, A. Characterization and comparison of the chemical composition of exotic superfoods. Microchemical Journal. 2013, 110, 444-451. https://doi.org/10.1016/j.microc.2013.05.016.
Lu, J.; Li, H.; Quan, J.; An, W.; Zhao, J.; Xi, W. Identification of characteristic aroma volatiles of Ningxia goji berries (Lycium barbarum L.) and their developmental changes. International journal of food properties. 2017, 20, S214-S227. https://doi.org/10.1080/10942912.2017.1295254.
Lu, Y.; Guo, S.; Zhang, F.; Yan, H.; Qian, D.W.; Wang, H.Q.; Jin, L.; Duan, J.A. Comparison of functional components and antioxidant activity of Lycium barbarum L. fruits from different regions in China. Molecules. 2019, 24, 2228. https://doi.org/10.3390/molecules24122228.
Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Yang, L.; Ma, T.; Dong, Z.; Zhang, Y.; Zhu, Y. Goji berries as a potential natural antioxidant medicine: An insight into their molecular mechanisms of action. Oxidative Medicine and Cellular Longevity. 2019, 9, 2437397. https://doi.org/10.1155/2019/2437397.
Mikulic‐Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. Journal of food science. 2012, 77, C1064-C1070. https://doi.org/10.1111/j.1750-3841.2012.02896.x.
Miller, J.S. Phylogenetic relationships and the evolution of gender dimorphism in Lycium (Solanaceae). Systematic Botany. 2002, 27, 416-428. https://doi.org/10.1043/0363-6445-27.2.416.
Miller, J.S.; Kamath, A.; Damashek, J.; Levin, R.A. Out of America to Africa or Asia: inference of dispersal histories using nuclear and plastid DNA and the S-RNase self-incompatibility locus. Molecular Biology and Evolution. 2011, 28, 793-801. https://doi.org/10.1093/molbev/msq253.
Ming, M.; Guanhua, L.; Zhanhai, Y.; Guang, C.; Xuan, Z. Effect of the Lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chemistry. 2009, 113, 872-877. https://doi.org/10.1016/j.foodchem.2008.03.064.
Mocan, A.; Moldovan, C.; Zengin, G.; Bender, O.; Locatelli, M.; Simirgiotis, M.; Atalay, A.; Vodnar D.C.; Rohn, S.; Crișan, G. UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory, and real-time cytotoxicological evaluation. Food and chemical toxicology. 2018, 115, 414-424. https://doi.org/10.1016/j.fct.2018.01.054
Mocan, A.; Cairone, F.; Locatelli, M.; Cacciagrano, F.; Carradori, S.; Vodnar, D.C.; Crișan, G.; Simonetti, G.; Cesa, S. Polyphenols from Lycium barbarum (Goji) fruit European cultivars at different maturation steps: Extraction, HPLC-DAD analyses, and biological evaluation. Antioxidants. 2019, 8, 562. https://doi.org/10.3390/antiox8110562.
Niro, S.; Fratianni, A.; Panfili, G.; Falasca, L.; Cinquanta, L.; Alam, M.R. Nutritional evaluation of fresh and dried goji berries cultivated in Italy. Italian Journal of Food Science. 2017, 29, 398-408. https://doi.org/10.14674/1120-1770/ijfs.v649.
Oğuz, H.I.; Erdoğan, O. A study on the development performances of goji berry (Lycium barbarum L.) Varieties. Fresenius Environmental Bulletin. 2016, 25, 5581-5586.
Ozkan, E.E.; Ozden, T.Y.; Toplan, G.G.; Mat, A. Phenolic content and biological activities of Lycium barbarum L (Solanaceae) fruits (Goji berries) cultivated in Konya, Turkey. Tropical Journal of Pharmaceutical Research. 2018, 17, 2047-2053. http://dx.doi.org/10.4314/tjpr.v17i10.22.
Pedro, A.C.; Maurer, J.B.B.; Zawadzki-Baggio, S.F.; Ávila, S.; Maciel, G.M.; Haminiuk, C.W.I. Bioactive compounds of organic goji berry (Lycium barbarum L.) prevents oxidative deterioration of soybean oil. Industrial crops and products. 2018, 112, 90-97. https://doi.org/10.1016/j.indcrop.2017.10.052.
Peng, Y.; Ma, C.; Li, Y.; Leung, K.S.Y.; Jiang, Z.H.; Zhao, Z. Quantification of zeaxanthin dipalmitate and total carotenoids in Lycium fruits (Fructus Lycii). Plant Foods for Human Nutrition. 2005, 60, 161-164. https://doi.org/10.1007/s11130-005-9550-5.
Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic compounds profile, nutritional compounds and bioactive properties of Lycium barbarum L: A comparative study with stems and fruits. Industrial Crops & Products. 2018, 122, 574-581. https://doi.org/10.1016/j.indcrop.2018.06.046.
Potterat, O.G. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Medicinal. 2010, 76, 7-19. https://doi.org/10.1055/s-0029-1186218.
Potterat, O.; Hamburger, M. Goji juice: a novel miraculous cure for longevity and well-being? A review on composition, pharmacology, health-related claims and benefits. Schweizerische Zeitschrift für Ganzheitsmedizin. 2008, 20, 399-405.
Protti, M.; Gualandi, I.; Mandrioli, R.; Zappoli, S.; Tonelli, D.; Mercolini, L. Analytical profiling of selected antioxidants and total antioxidant capacity of goji (Lycium spp.) berries. Journal of pharmaceutical and biomedical analysis. 2017, 143, 252-260. https://doi.org/10.1016/j.jpba.2017.05.048.
Qian, D.; Zhao, Y.; Yang, G.; Huang, L. Systematic review of chemical constituents in the genus Lycium (Solanaceae). Molecules. 2017a, 22, 1-33. https://doi.org/10.3390/molecules22060911.
Qian, D.; Yang, J.; Kang, L.P.; Ji, R.F.; Huang, L.Q. Variation of sweet chemicals in different ripening stages of wolfberry fruits. Chinese Herbal Medicines. 2017b, 9, 329-334. https://doi.org/10.1016/S1674-6384(17)60112-6.
Rotar, A.M.; Vodnar, D.C.; Bunghez, F.; Cătunescu, G.M.; Jimborean, M.; Semeniuc, C.A. Effect of Goji Berries and Honey on Lactic Acid Bacteria Viability and Shelf Life Stability of Yoghurt. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2015, 43, 196-203. https://doi.org/10.15835/nbha4319814.
Rybicka, I.; Kiewlicz, J.; Kowalczewski, P.Ł.; Gliszczyńska-Świgło, A. Selected dried fruits as a source of nutrients. European Food Research and Technology. 2021, 247, 2409-2419. https://doi.org/10.1007/s00217-021-03802-1.
Shahrajabian, M.H.; Sun, W.; Cheng, Q. A review of goji berry (Lycium barbarum) in traditional Chinese medicine as a promising organic superfood and superfruit in modern industry. Academia Journal of Medicinal Plants. 2018, 6, 437-445. http://dx.doi.org/10.15413/ajmp.2018.0186.
Shen, T.; Zou, X.; Shi, J.; Li, Z; Huang, X.; Xu, Y.; Chen, W. Determination geographical origin and flavonoids content of goji berry using near-infrared spectroscopy and chemometrics. Food Analytical Methods. 2016, 9, 68-79. https://doi.org/10.1007/s12161-015-0175-x.
Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicology reports. 2018, 5, 251-257. https://doi.org/10.1016/j.toxrep.2018.02.001.
Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical properties, fatty-acid composition, and antioxidant activity of goji berry (Lycium barbarum L. and Lycium chinense Mill.) fruits. Antioxidants. 2019, 8, 60. https://doi.org/10.3390/antiox8030060.
Spano, M.; Maccelli, A.; Di Matteo, G.; Ingallina, C.; Biava, M.; Crestoni, M.E.; Bardaud, J-X.; Giusti, A.M.; Mariano, A.; Scotto D’Abusco, A.; Sobolev, A.P.; Lasalvia, A.; Fornarini, S.; Mannina, L. Metabolomic Profiling of Fresh Goji (Lycium barbarum L.) Berries from Two Cultivars Grown in Central Italy: A Multi-Methodological Approach. Molecules. 2021, 26, 5412. https://doi.org/10.3390/molecules26175412.
Stavrescu-Bedivan, M.M.; Pelcaru, C.F.; Croitoru, C.M.; Mihai, C.D.; Ciceoi, R. Preliminary survey for mapping the distribution of spontaneous goji berry shrubs in Romania. Scientific Papers. Series B, Horticulture. 2022, LXV, 907-912.
Sun, Y.; Rukeya, J.; Tao, W.; Sun, P.; Ye, X. Bioactive compounds and antioxidant activity of wolfberry infusion. Scientific reports. 2017, 7, 1-8. https://doi.org/10.1038/srep40605.
Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A Ž.; Pešić, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants. 2022, 11, 248. https://doi.org/10.3390/antiox11020248.
Wang, B.; Han, L.; Liu, J.-M.; Zhang, J.; Wang, W.; Li, B.-G.; Dong, C.-X.; Bai, C.-C. Lycium Genus Polysaccharide: An Overview of its Extraction, Structures, Pharmacological Activities and Biological Applications. Separations. 2022, 9, 1-41. https://doi.org/10.3390/separations9080197.
Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food chemistry. 2010, 120, 184-192. https://doi.org/10.1016/j.foodchem.2009.10.005.
Wang, Y.; Chen, H.; Wu, M.; Zeng, S.; Liu, Y.; Dong, J. Chemical and genetic diversity of wolfberry. In Lycium barbarum and Human Health. Springer, Berlin, Germany, 2015, pp. 1–26.
Wang, Y.; Jin, H.; Dong, X.; Yang , S.; Ma, S.; Ni, J. Quality evaluation of Lycium barbarum (wolfberry) from diferent regions in China based on polysaccharide structure, yield and bioactivities. Chinese Medicine. 2019, 14, 1-10. https://doi.org/10.1186/s13020-019-0273-6.
Wenli, S.; Shahrajabian, M.H.; Qi, C. Health benefits of wolfberry (Gou Qi Zi, Fructus barbarum L.) on the basis of ancient Chinese herbalism and Western modern medicine. Avicenna Journal Phytomedicine. 2021, 11, 109-119. https://doi.org/10.22038/ajp.2020.17147.
Wetters, S.; Horn, T.; Nick, P. Goji who? Morphological and DNA based authentication of a “superfood”. Frontiers in plant science. 2018, 9, 1-14. https://doi.org/10.3389/fpls.2018.01859.
Wojdyło, A.; Nowicka, P.; Bąbelewski, P. Phenolic and carotenoid profile of new goji cultivars and their anti-hyperglycemic, anti-aging and antioxidant properties. Journal of functional foods. 2018, 48, 632-642. https://doi.org/10.1016/j.jff.2018.07.061.
Xia, Q.; Niu, M.; Wu, C.; Zhou, R. Formation of ethyl carbamate in Goji wines: Effect of Goji fruit composition. Food science and biotechnology. 2016, 25, 921-927. https://doi.org/10.1007/s10068-016-0151-2.
Van Straten, M.; Griggs B. Superfoods: Nutrient-dense foods to protect your health. London: DK Publishing, 2006.
Yang, L.; Liang, Q.; Zhang, Y.; Wang, S.; Yuan, F.; Wang, J. Variation of phytochemical composition of Lycium chinense leaves as an endemic high-value healthy resource. Scientia Horticulturae. 2021, 281, 109910. https://doi.org/10.1016/j.scienta.2021.109910.
Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. Journal of ethnopharmacology. 2018a., 212, 50-66. https://doi.org/10.1016/j.jep.2017.10.010.
Yao, R.; Heinrich, M.; Zou, Y.; Reich, E.; Zhang, X.; Chen, Y.; Weckerle, C.S. Quality Variation of Goji (Fruits of Lycium spp.) in China: A Comparative Morphological and Metabolomic Analysis. Frontiers in Pharmacology. 2018b, 9, 151. https://doi.org/10.3389/fphar.2018.00151.
Yao, R. The globalization of goji (fruits of Lycium barbarum L. and L. chinense Mill.): What are the drivers?SC2| Short Communication 2-Regulation/ Globalization. 19th International Congress of the International Society for Ethnopharmacology. 13 June, 2019. Dresden, Germany.
Yao, R.; Heinrich, M.; Zhao, X.; Wang, Q.; Wei, J.; Xiao, P. What’s the choice for goji: Lycium barbarum L. or L. chinense Mill.? Journal of Ethnopharmacology. 2021, 10, 114185. https://doi.org/10.1016/j.jep.2021.114185.
Yin, G.; Dang, Y. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydrate Polymers. 2008, 74, 603-610. https://doi.org/10.1016/j.carbpol.2008.04.025.
Yossa Nzeuwa, I.B.; Guo, B.; Zhang, T.; Wang, L.; Ji, Q.; Xia, H.; Sun, G. Comparative Metabolic Profiling of Lycium Fruits (Lycium barbarum and Lycium chinense) from Different Areas in China and from Nepal. Journal of Food Quality. 2019, 1-6. https://doi.org/10.1155/2019/4396027.
Zhang, L.; Zheng, G.; Teng, Y.; Wang J. Comparison research on fruit quality of Lycium barbarum L. in different regions. Northwest Pharmaceutical Journal. 2012, 27, 195-197.
Zhang, D.; Xia, T.; Dang, S.; Fan, G.; Wang, Z. Investigation of Chinese wolfberry (Lycium spp.) germplasm by restriction site-associated DNA sequencing (RAD-seq). Biochemical genetics. 2018, 5, 575-585. https://doi.org/10.1007/s10528-018-9861-x.
Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chemistry. 2016, 200, 230-236. https://doi.org/10.1016/j.foodchem.2016.01.046.
Zhao, R.; Jin, R.; Chen, Y.; Han, F.M. Hypoglycemic and hypolipidemic effects of Lycium barbarum polysaccharide in diabetic rats. Chinese herbal medicines. 2015a, 7, 310-315. https://doi.org/10.1016/S1674-6384(15)60057-0.
Zhao, J.; Li, H.; Xi, W.; An, W.; Niu, L.; Cao, Y.; Wang, H.; Wang, Y.; Yin, Y. Changes in sugars and organic acids in wolfberry (Lycium barbarum L.) fruit during development and maturation. Food Chemistry. 2015b, 173, 718-724. https://doi.org/10.1016/j.foodchem.2014.10.082.
Zheng, G.Q.; Zheng, Z.Y.; Xu, X.; Hu, Z.H. Variation in fruit sugar composition of Lycium barbarum L. and Lycium chinense Mill. of different regions and varieties. Biochemical Systematics and Ecology. 2010, 38, 275-284. https://doi.org/10.1016/j.bse.2010.01.008.
Zhou, Z.; Jing, L.; Cui, G.; Feng, Q.; Xiao, Y. Effects of polysaccharide from Lycium barbarum in alloxan-induced diabetic mice. African Journal of Biotechnology. 2009, 8, 6634-6637. https://doi.org/10.5897/AJB09.484.
Academic Editor: Dr. Isabela Maria Simion
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Bolohan Ciprian, Teodorescu Răzvan Ionuț, Tudor Andrei Daniel, Tudor Valerica