Maria Larisa Ivănescu, Gabriela Martinescu, Liviu Dan Miron
ABSTRACT. Borreliosis or Lyme disease is a disease transmitted by ixodidae ticks during feeding on blood (Ixodes pacificus and Ixodes scapularis in the USA, Ixodes persulcatus in Asia, Ixodes ricinus in Europe) and is widespread in the entire northern hemisphere. In Romania, the geographic distribution and prevalence of Borrelia burgdorferi sensu lato was 1.4% in 41 counties, with a prevalence between 0.75–18.8%. B. burgdorferi sensu lato. had a prevalence of 3.8%, being found inside ticks in 55 of 183 localities. Successful treatment and full recovery can only be achieved through early diagnosis. The clinical and serologic diagnosis of Lyme disease is particularly difficult because of the phenotypic heterogeneity within and among spirochete species. A case study is presented in this paper: an eight-year-old male Yorkshire terrier dog, which was diagnosed positive for Lyme disease, based on a test which uses a peptide called C6 and which comes from the VlsE protein of B. burgdorferi, used to detect antibodies in dogs. The results demonstrate the reliability of the commercial SNAP 4Dx Plus Test for B. burgdorferi, which uses C6 to differentiate antibodies produced by natural infection from antibodies formed after vaccination. In addition, using real-time PCR, the diagnosis was negative, confirming the results from the literature, according to which the PCR technique is only recommended for research, the positivity percentage being low, especially when the sample is blood (0.1%). We conclude that the tests for the detection of antibodies specific to Lyme disease are recommended and useful.
Keywords: Lyme disease; antibodies; real-time PCR.
Cite
ALSE and ACS Style
Ivănescu, M.L.; Martinescu, G.; Miron, L.D. Effective diagnostic techniques in Borrelia burgdorferi infestation in dogs. Journal of Applied Life Sciences and Environment 2022, 55(2), 219-232.
https://doi.org/10.46909/alse-552059
AMA Style
Ivănescu ML, Martinescu G, Miron LD. Effective diagnostic techniques in Borrelia burgdorferi infestation in dogs. Journal of Applied Life Sciences and Environment. 2022; 55 (2): 219-232.
https://doi.org/10.46909/alse-552059
Chicago/Turabian Style
Ivănescu, Maria Larisa, Gabriela Martinescu, and Liviu Dan Miron. 2022. “Effective diagnostic techniques in Borrelia burgdorferi infestation in dogs” Journal of Applied Life Sciences and Environment 55, no. 2: 219-232.
https://doi.org/10.46909/alse-552059
View full article (HTML)
Effective Diagnostic Techniques in Borrelia Burgdorferi Infestation in Dogs
Maria Larisa IVĂNESCU, Gabriela MARTINESCU* and Liviu Dan MIRON
Iasi University of Life Sciences, Faculty of Veterinary Medicine, Department of Clinics, 8, Mihail Sadoveanu Alley, 700489, Iasi, Romania; e-mail: larisssa81@yahoo.com, livmiron@yahoo.com
*Correspondence: martinescugabi11@yahoo.co.uk
Received: Nov. 03, 2022. Revised: Dec. 06, 2022. Accepted: Dec. 21, 2022. Published online: Feb. 09, 2023
ABSTRACT. Borreliosis or Lyme disease is a disease transmitted by ixodidae ticks during feeding on blood (Ixodes pacificus and Ixodes scapularis in the USA, Ixodes persulcatus in Asia, Ixodes ricinus in Europe) and is widespread in the entire northern hemisphere. In Romania, the geographic distribution and prevalence of Borrelia burgdorferi sensu lato was 1.4% in 41 counties, with a prevalence between 0.75–18.8%. B. burgdorferi sensu lato. had a prevalence of 3.8%, being found inside ticks in 55 of 183 localities. Successful treatment and full recovery can only be achieved through early diagnosis. The clinical and serologic diagnosis of Lyme disease is particularly difficult because of the phenotypic heterogeneity within and among spirochete species. A case study is presented in this paper: an eight-year-old male Yorkshire terrier dog, which was diagnosed positive for Lyme disease, based on a test which uses a peptide called C6 and which comes from the VlsE protein of B. burgdorferi, used to detect antibodies in dogs. The results demonstrate the reliability of the commercial SNAP 4Dx Plus Test for B. burgdorferi, which uses C6 to differentiate antibodies produced by natural infection from antibodies formed after vaccination. In addition, using real-time PCR, the diagnosis was negative, confirming the results from the literature, according to which the PCR technique is only recommended for research, the positivity percentage being low, especially when the sample is blood (0.1%). We conclude that the tests for the detection of antibodies specific to Lyme disease are recommended and useful.
Keywords: Lyme disease; antibodies; real-time PCR.
INTRODUCTION
In the context of global changes, primarily climate warming, land use, and increased mobility of people and animals, the risk of vector-borne diseases increases with alterations of vector distribution, host distribution and ecosystems (Aenishaenslin et al., 2017).
Thus, the increase in temperatures has led to a change in the distribution of ticks and the temporal pattern of their activity, now being found at higher altitudes and further north as a result of milder winters (Akl et al., 2019), which leads to an increase in the risk of pathogen transmission.
Borreliosis or Lyme disease is a disease transmitted by ixodidae ticks during feeding on blood (Ixodes pacificus and Ixodes scapularis in the USA, Ixodes persulcatus in Asia, Ixodes ricinus in Europe) (Snydman and Hu, 2021) and is widespread in the entire northern hemisphere. The pathogen involved in the occurrence of Lyme disease is a spirochete which is part of the Spirochaetaceae class of the Borreliaceae family, being isolated for the first time in 1982 by the American entomologist Willy Burgdorfer, from the intestine of a tick (Ixodes scapularis), later being connected with erythema migrans in humans. Later, other species associated with Lyme disease (B. afzelii, B. garinii, B. spielmanii) were isolated in Asia and Europe, which differ from those previously described in the United States, remaining under the name of Borrelia burgdorferi sensu stricto, and the group of bacteria involved in Lyme disease was named B. burgdorferi sensu lato. The genome of B. burgdorferi sensu stricto was sequenced in 1997 (Fraser et al., 1997), with a large number of Borrelia reported, resulting in the Borreliaceae family comprising of two groups: the Borreliella group, which includes species capable of inducing erythema migrans (more than 20 species being included), and the Borrelia group responsible for relapsing fever. The first report of Lyme disease was in Connecticut, USA, based on a group of patients with symptoms similar to juvenile rheumatoid arthritis, later diagnosed as Lyme arthritis. It is now called Lyme disease based on clinical features which include, besides arthritis, cardiac, dermatological and neurological symptoms. Usually, the first symptom is the appearance of a skin erythema, called ‘erythema migrans’. Approximately 4-8% of patients develop cardiac problems, 11% develop neurological symptoms and 45–60% of patients develop arthritis (Borchers et al., 2015).
B. burgdorferi sensu stricto (B. burgdorferi) is responsible for the production of the disease in the USA and in a much lower percentage, it is produced by B. mayonnaise. The species B. afzelii and B. garinii predominate in Europe being implicated in the production of Lyme disease and in a lower percentage, B. burgdorferi and two rare species, B. speilmanii and B. bavariensis (Snydman and Hu, 2021) have also been implicated.
A study conducted in the north-eastern USA showed that the regional average for the percentage of seropositive dogs is 11.6% (Rhodes et al., 2013). Lyme disease can affect many wild and domestic animals, but clinical signs appear in few. The dog is considered a sentinel species, spreading ticks and being the closest to humans. Thus, a study by Kalmár et al. in 2013, based on immunological tests, showed a prevalence of 20.4% for B. burgdorferi s.l. using the expression of p41, OmpA, OmpC and p100 proteins. Using the immunoblot method, the presence of antibodies against B. burgdorferi sensu stricto was highlighted in one case (2%) and B. afzeli in five cases (10.2%), and the rest were probably infections with other Borrelia species. The study of dog contamination with B. burgdorferi is valuable in providing information on the risk of human contamination (Iordache et al., 2015). A wide range of wild and domestic animals are affected, but only a small proportion show clinical symptoms. The importance of dogs as a sentinel species has been highlighted, as dogs are hosts for sharing vector ticks, and are in close physical contact with humans
A study conducted on the prevalence and geographical distribution of B. burgdorferi sensu lato (s.l.) by PCR in 41 counties in Romania showed an infection rate of 1.4%, with an average local prevalence between 0.75–18.8%. B. burgdorferi s.l. was found inside ticks in 55 of 183 localities, the global prevalence being 3.8%. Three genospecies of Borrelia were detected, with B. afzelii being the most widespread, followed by B. garinii and B. burgdorferi sensu stricto (s.s.) (Kalmár et al., 2013).
An incidence of Lyme disease of approximately 50/100,000 people per year was reported between 2009–2017 in France, Germany, Belgium and Switzerland. The peak incidence of 104/100,000 was observed in 2018, but the reality is considered to be different and the seroprevalence is much higher among the population, increasing with age and varying from one region to another (up to 20% in Alsace). The most common manifestation observed was erythema migrans (95%), which usually appears within 1–2 weeks of the tick bite (range of 3–30 days). The bull’s-eye appearance of the erythema was observed in 30–40% of those with erythema migrans, but in most cases, it had an erythematous, homogeneous appearance. Iridocyclitis, optic neuritis, vasculitis, keratitis, conjunctivitis and uveitis were also reported but in a much lower percentage. Myopericarditis can occur in the acute form of the disease, in approximately 12% of patients, with varying degrees of complication, even with sudden cardiac death. If the disease is not diagnosed in the early stages, it presents itself as arthritis, which develops several months after the initial infection in about 30% of untreated cases. Arthritis cases have been less frequently reported in Europe, possibly due to differences among the predominant strains of Borrelia (Schwartz et al., 2021).
Encephalopathy was reported in the more advanced stage of Lyme disease, with mild cognitive impairment, as well as memory problems. Spastic paraparesis and cognitive impairment were reported in B. garinii infection. Early diagnosis is essential for successful treatment and full recovery. In any case, the clinical and serological diagnosis of Lyme disease is particularly difficult due to the phenotypic heterogeneity within and among spirochete species (Smith, 2005). Even in regions where only one species of B. burgdorferi is found, Lyme disease develops very differently from one patient to another (Baranton et al., 2001). Serology can be misleading in early forms, such as radiculitis (Coiffier and Tattevin, 2021).
The diagnosis is usually serological, but it is not recommended for patients with erythema migrans, as the results yield a false negative, these patients being in the early stage of the disease (Snydman and Hu, 2021). In general, an enzyme immunoassay is used, which increases the specificity of the result, supplemented with western blot or two enzyme immunoassays can be combined. The criteria given by the Centres for Disease Control (CDC) should be used in the interpretation of western blot results, since the immunoglobulin (Ig) M western blot can be associated with false positive results. These criteria refer to ignoring IgM testing when symptoms are present for more than 30 days. The C6 enzyme-linked immunoassay captures antibodies specific for both American and European strains. PCR and culture are currently considered tests used only for research purposes (Lantos et al., 2020).
For the diagnosis of Lyme disease in dogs, traditional blood tests are used (joint fluid analysis, PCR, western blot, ELISA), the C6 quantitative test being used more recently (QC6). The C6 test detects antibodies against the C6 protein, which is a very specific protein, being a preliminary blood test. The presence of antibodies to this protein suggests Borrelia infection, being a unique protein for Borrelia bacteria. Thus, four weeks after the bite of an infected tick, C6 antibodies can be detected, even before symptoms appear. The SNAP 4Dx Plud IDEXX test is the most commonly used in veterinary clinics (O’Connor et al., 2013). The newest version of the SNAP test is a multianalyte ELISA used in clinics to detect antibodies against B. burgdorferi, Anaplasma phagocytophilum, A. platys, Ehrlichia ewingii, E. canis and Dirofilaria immitis antigens (Stillman et al., 2014). The test uses the C6 peptide derived from the VlsE protein (Vmp-like sequence, expressed) of B. burgdorferi for the detection of antibodies in dogs.
Vaccinated dogs have been tested with the SNAP 4Dx Plus Test, finding a lack of reactivity to the serum antibodies formed in post-vaccine dogs, showing that these dogs were not infected with B. burgdorferi. This is explained by the fact that the vaccines fail to produce anti-C6 antibodies due to the loss of the lp 28-1 plasmid in the spirochete isolates used to generate whole bacteria vaccines. After administration of the Vanguard crLyme7 Vaccine, sera from dogs receiving it were shown to be nonreactive in the SNAP 4Dx Plus Test, as this vaccine contains OspA and OspC (Stillman et al., 2019). When evaluating patients with suspected Lyme neuroborreliosis involving the peripheral nervous system or the central nervous system, serum antibody testing is recommended rather than PCR or cerebrospinal fluid culture.
The ability of Borrelia burgdorferi to encyst has been proven in animal models, remains dormant and persisting in the body (Feng et al., 2019). The Infectious Diseases Society of America, the American College of Rheumatology and the American Academy of Neurology published, on 30 November 2020, a series of recommendations regarding the diagnosis and treatment of Lyme disease, with doxycycline administration for 10 days for erythema migrans being preferred. The UK National Institute recommended, in 2018, using doxycycline for 21 days. These recommendations are the result of studies that showed that 20 days of doxycycline compared to 10 days of doxycycline did not improve the effectiveness of Lyme disease treatment in the early stages. Another study conducted in Europe demonstrated that administration of doxycycline for 10 days was not inferior to administration for 15 days in patients with erythema migrans (Mushtaq and Kazi, 2021). Treatment with azithromycin is recommended for 5–10 days, preferably seven days (Luft et al., 1996).
A study carried out in 2018 involving several countries, calculated the incidence of borreliosis in humans depending on the incidence of the disease in dogs using mathematical models. In humans, an incidence of 473,000/year was found in the USA, 471,000/year in Germany, 434,000/year in France and 132,000/year in Great Britain; the prevalence being 2.4 million in the USA, 2.4 million in Germany, 2.2 million in France and 667,000 in the UK; with a total of infections in the USA of 10.1 million, Germany 10.0 million, France 9.3 million and Great Britain 2.8 million. Thus, the global estimates for 2018 were an incidence of 12.3 million/year, a prevalence of 62.1 million and a total burden of infections of 262.0 million.
The prevalence of Lyme disease in Europe was 12.7 million cases and for North America it was4.0 million and worldwide was estimated at nearly 250 million. The seropositivity of Lyme disease in dogs compared to humans in England was 6.5% and 2.1%, Finland 6.3% and 4.1%, France 12.2% and 13.9%, Germany 11.1% and 11.8%, Italy 1.0% and 5.3%, Holland 17.0% and 9.0% and Sweden 7.5% and 12.3%, respectively, which highlights that the most important animal reservoir in nature remains the dog, being the closest to humans (Cook and Puri, 2020). Unlike other species of wild animals which create phylogenetic host species for B. burgdorferi s.s., and can even act as an incompetent reservoir host, the dog, like man, can be a carrier of several genospecies of Borrelia burgdorferi s.l., being able to host other agents carried by ticks and double or triple infections can occur. For this reason, the dog is considered the main reservoir in nature for humans. Studies on the prevalence of borreliosis in humans should be done in association with the prevalence in dogs in order to prevent outbreaks (Skotarczak, 2014).
MATERIALS AND METHODS
A case was presented at the Faculty of Veterinary Medicine of an eight-year-old unneutered male Yorkshire terrier dog. The owners came on the occasion of a campaign initiated by the Faculty of Veterinary Medicine for a set of complete tests and sterilisation. Biochemical and haematological blood tests were recommended before surgery.
The biochemistry tests showed normal values of liver and kidney parameters (Table 1). The blood count showed the presence of an infection in the body, with an increase in the number of leukocytes and neutrophils, the symptomatology not being conclusive (Table 2).
Given that the red blood cell count was at the low end, a Diff Quick-stained peripheral blood smear was performed to rule out a vector-borne disease which produces haemolytic anaemia (Table 2).
The result was negative for babesiosis and anaplasmosis, specifying that the smear is recommended only in the acute phase of the disease; in the chronic phase, serological tests are recommended to detect antibodies. Clinically, the patient did not show any symptoms, being brought only for a routine check-up during the campaign and for sterilisation.
Table 1
Results of biochemical tests
|
Parameter |
Values |
Reference range |
|
Creatinine (CRE) |
0.56 mg/dL |
0.5–1.7 |
|
Urea (URE) |
32.2 mg/dL |
16–56 |
|
Alkaline phosphatase (ALP) |
31 U/L |
1–114 |
|
Alanine aminotransferase (ALT) |
17.4 U/L |
10–109 |
|
Aspartate aminotransferase (AST) |
14 U/L |
13–15 |
|
Gamma-glutamyltransferase (GGT) |
5.8 U/L |
1–9.7 |
|
Cholesterol (CHOL) |
127 mg/dL |
135–278 |
|
Total protein (TP) |
7.49 g/dL |
5.4–7.5 |
|
Triglycerides (TRIG) |
35.3 mg/dL |
<150 |
|
Serum glucose (GLU) |
93 mg/dL |
76–119 |
Table 2
Results of haematological tests
|
Parameter |
Values |
Reference range |
|
White blood cells (WBC) |
20.32 x 10^9/l |
6–17 |
|
Lymphocyte (LYM) |
3.39 x 10^9/l |
1–4.8 |
|
Monocyte (MON) |
1.2 x 10^9/l |
0.2–1.5 |
|
Neutrophil (NEU) |
15.48 x 10^9/l |
3–12 |
|
Eosinophil (EOS) |
0.18 x 10^9/l |
0–0.8 |
|
Basophil (BAS) |
0.07 x 10^9/l |
0–0.4 |
|
Lymphocyte% (LY%) |
16.7% |
0–100 |
|
Monocyte% (MO%) |
5.9% |
0–100 |
|
Neutrophil% (NE%) |
76.2% |
0–100 |
|
Eosinophil% (EO%) |
0.9% |
0–100 |
|
Basophil% (BA%) |
0.3% |
0–100 |
|
Red blood cells (RBC) |
5.68 x 10^12/l |
5.5–8.5 |
|
Haemoglobin (HGB) |
12.7 g/dl |
12–18 |
|
Haematocrit (HCT) |
40.88% |
37–55 |
|
Mean corpuscular volume (MCV) |
72 fl |
60–77 |
|
Mean corpuscular haemoglobin (MCH) |
22.4 pg |
19.5–24.5 |
|
Mean corpuscular haemoglobin concentration (MCHC) |
31.1 g/dl |
31–39 |
|
Red cell distribution width concentration (RDWC) |
16.1% |
14–20 |
|
Platelet (PLT) |
350 x 10^9/l |
165–500 |
|
Procalcitonin (PCT) |
0.41% |
|
|
Mean platelet volume (MPV) |
11.7 fl |
3.9–11.1 |
|
Platelet distribution width (PDW) |
41.6% |
|
For this purpose, we used the SNAP 4Dx Plus rapid test, which is recommended in the diagnosis of chronic infections, instead of the May Grunwald Giemsa stained blood smear.
The SNAP 4Dx Plus test is an in vitro diagnostic test for the detection of the D. immitis antigen, A. phagocytophilum antibodies, A. platys antibodies, B. burgdorferi antibodies, E. canis antibodies and E. ewingi antibodies in canine serum, plasma or whole blood. The VlsE-derived C6 peptide of B. burgdorferi is used by this test to detect antibodies. The study results demonstrate the sensitivity and specificity of the commercial C6-based SNAP 4Dx Plus Test for B. burgdorferi, distinguishing between antibodies formed after natural infection and those acquired after vaccination. The lack of antibody response produced by the vaccine is important for clinicians who consistently vaccinate dogs against Lyme disease, as it is an endemic disease in some areas. Thus, the SNAP 4DX Plus rapid test is internationally accepted for the diagnosis of Lyme disease (Stillman et al., 2019).
The test detects antibodies to natural infection with Borrelia burgdorferi, and not after the immunisation with one of the following vaccines: Nobivac Lyme, Galaxy Lyme, Recombitek Lyme and LymeVax.
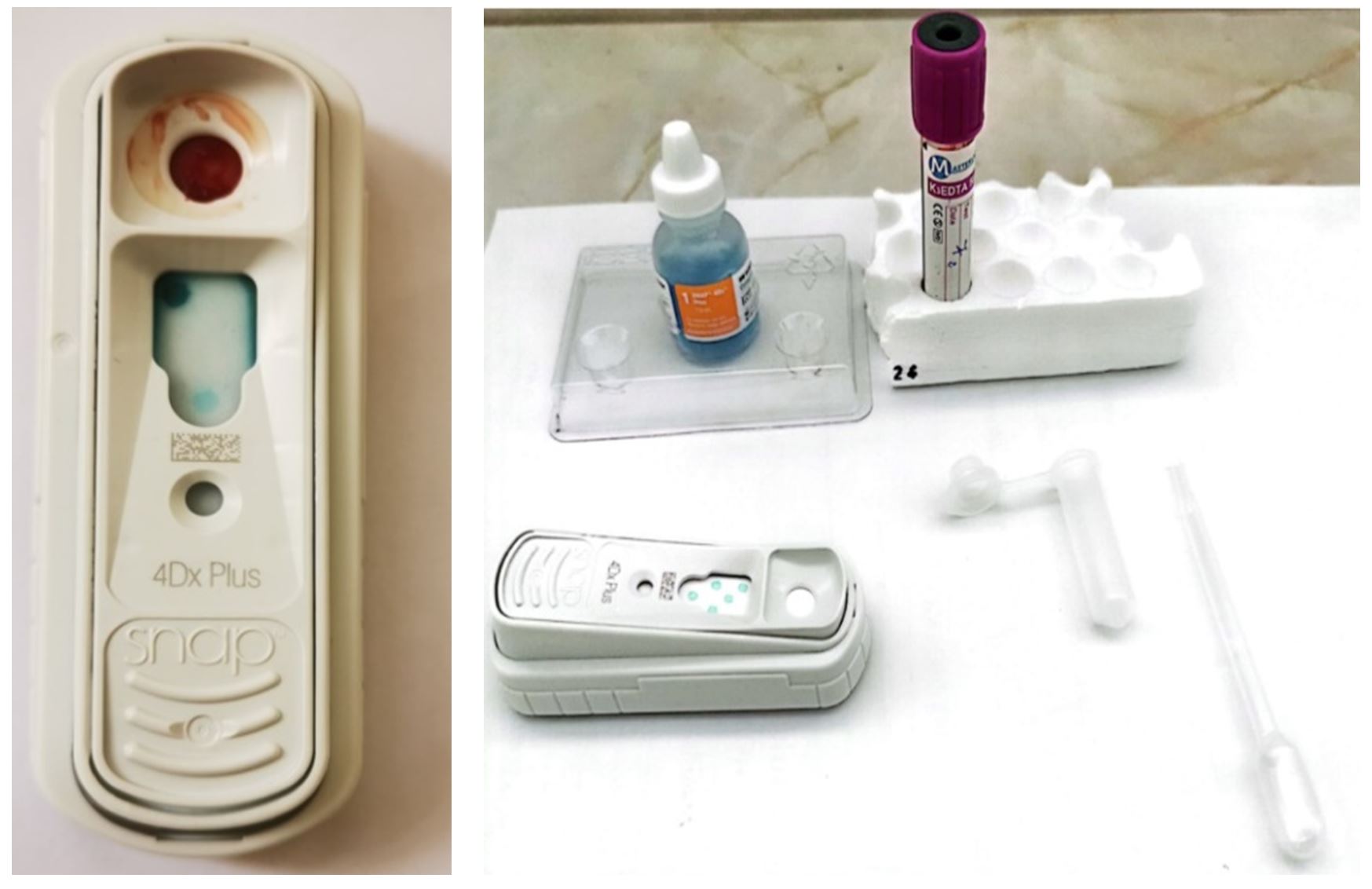
Three drops of the serum obtained after centrifugation of freshly collected blood were placed in the sample tube, over which four drops of conjugate were added and mixed 3–5 times. The contents of the tube were introduced into the device placed on a horizontal surface, and the test was read after 8 minutes.Fresh blood was also used for DNA extraction for the diagnosis of Lyme disease using the real-time PCR technique. The BioMag Pure-Blood DNA Extraction Kit and the BioMag Pure 12 plus extraction equipment were used for DNA extraction (Figure 1). The diagnosis by real-time PCR was made using the device from BIO RAD CFX96 (Figure 2), using the protocol given by Shen et al. (2018) and presented in Table 3.
Table 3
Protocol used for real-time PCR diagnosis

Figure 1 – Automatic extractor BioMag Pure
Figure 2 – Real-time PCR CFX 96 device
RESULTS
The result of the SNAP 4Dx Plus test was positive, showing the presence of antibodies to B. burgdorferi (Figure 3). The test has the ability to detect antibodies against the C6 protein. The C6 protein is very specific and unique to Borrelia, the presence of antibodies suggesting natural infection. C6 antibodies can be detected even before symptoms appear, approximately one month after the tick inoculated the pathogen during the blood meal.
Considering the late onset of symptoms, after inoculation of Borrelia by the tick during the blood feed, we cannot know the precise moment of infestation, but considering the changed blood count, with increased values of leukocytes and neutrophils, we can deduce that the disease was in the acute phase. The diagnosis by real-time PCR was negative, which showed a low concentration of DNA in the sample used. The positivity of the internal control and the expression of the melting curve show that the protocol was correct, and the tested sample was negative (Figure 4).
DISCUSSION
As Borrelia reproduces slowly (every 12 hours), it is likely that after infection, antibodies will not be detected promptly; as it takes time for the immune system to produce detectable amounts of antibodies following contact with Borrelia-specific antigens, even if highly sensitive methods are used to detect them (Baker et al., 2017). The sensitivity of the diagnostic tests that follow the detection of specific antibodies increases in patients with acute or chronic arthritis, in those with neurological abnormalities and in those with specific erythema migrans. Both specificity and sensitivity vary between 85–99% as the infection spreads. Sensitivity is low in early infection (< 50%) (Baker, 2019).
Even years after the infection has been cured, low antibody levels may persist (Baker, 2017), making it difficult to detect active infection. Studies clearly show that a diagnosis cannot be made with certainty only symptomatically, which shows that the administration of antibiotics until the symptoms improve or disappear isnot advisable. Some antibiotics used in medicine, such as tetracycline, have anti-inflammatory properties, which can lead to temporary relief of chronic pain, which has led to the misdiagnosis of Lyme disease (Weinberg, 2005). Other antibiotics have neuroprotective properties, such as doxycycline and ceftriaxone, currently used to treat borreliosis (Rothstein et al., 2006). The administration of antibiotics for a long period, when there is a so-called relapse, is inappropriate and most of the time the diagnosis is incorect (Baker, 2019). A definite diagnosis is essential in the proper management of Lyme disease. Thus, a study conducted on 23,777 specimens tested by real-time PCR in order to establish the percentage of positivity according to the sample used showed that synovial fluid (6.4%) and tissues (6.5%) had the highest percentage of positive rates, and blood (0.1%) and cerebrospinal fluid (0.09%) had the lowest rates, underlining the fact that the PCR technique is not the most relevant in the diagnosis of Lyme disease (Babady et al., 2008).
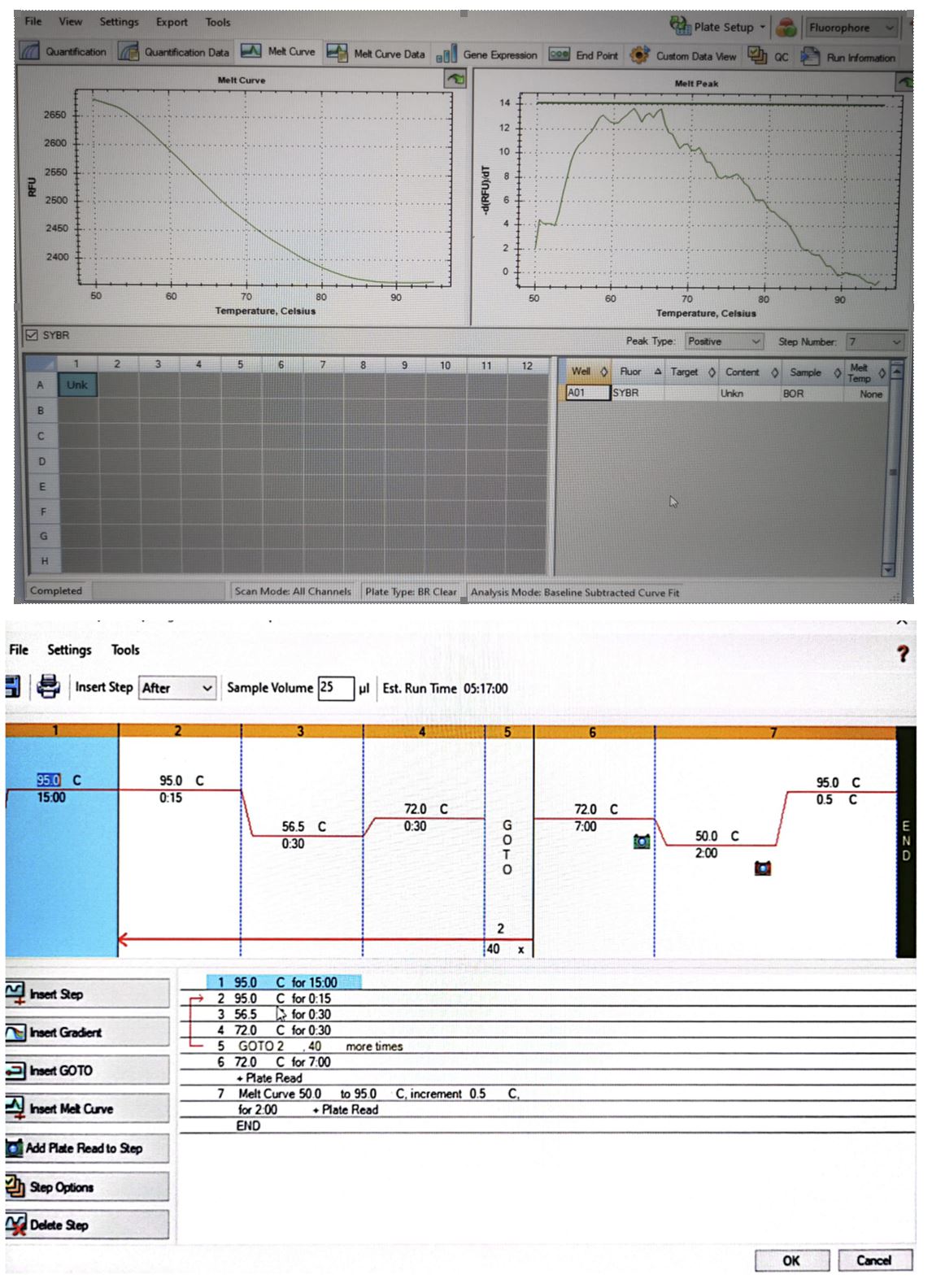
Figure 4 – The result of the diagnosis of Lyme disease using the real-time PCR technique, and the graph of the complete cycles
In this paper we demonstrated the necessity of the mandatory use of serological tests for the detection of antibodies in Lyme disease and the use of molecular biology only if the aim is to identify the species of Borrelia involved. The precise time of infestation should be known in order to obtain a blood sample with a higher DNA concentration.
This paper is of great value to clinicians who are faced with an ever-increasing number of cases of vector-borne diseases, especially with the increase in Lyme disease cases.
Lyme disease is a huge problem in both human and veterinary medicine, because of the difficulty of diagnosis. We conclude that molecular biology techniques do not always represent a diagnosis of certainty, having the highest sensitivity and specificity, and the diagnostic technique must be chosen with great care based on the anamnesis and clinical picture, which are often irrelevant.
CONCLUSION
We conclude that serological tests to detect antibodies are recommended for the diagnosis of Lyme disease. The SNAP 4Dx Plus test is internationally accepted for the diagnosis of Lyme disease, being an in vitro diagnostic test. Additionally, following this study, we can say that this test has increased sensitivity and specificity, and can be used by clinicians. The diagnosis of Lyme disease using the real-time PCR technique is recommended only if we know the moment of infection with Borrelia sp., the technique having a percentage of 0.1% of detecting positive cases. A study by Milich et al. in 2022 showed that the SNAP 4Dx Plus Test had a sensitivity superior to other tests, of 95.5%. In a separate validation study, the sensitivity and specificity were 96.7% and 98.8%, respectively, proving to be valuable for identifying dogs exposed to B. burgdorferi. Additionally, this test does not detect antibodies resulting from the Lyme disease vaccine. Another study carried out in the south of Italy showed the need to use several techniques in the diagnosis of diseases transmitted by ticks, the SNAP 4Dx Plus Test having a sensitivity of 95.7% (Sgroi et al., 2022). Numerous studies suggest that the SNAP® 4Dx® Plus test lends itself to the diagnosis of tick-borne diseases, unlike other techniques with increased sensitivity and specificity (Stillman et al., 2019; Kotwa et al., 2020), for the diagnosis borreliosis, the sensitivity being 97.1% (Liu et al., 2018).
Author Contributions: conceptualization, M.L.I., C.M., L.D.M.; methodology, M.L.I., C.M.; investigation, M.L.I., C.M.; writing-original draft preparation, C.M.; supervision, L.D.M. All authors have read and agreed to the published version of the manuscript.
Funding: There was no external funding for this study.
Conflicts of Interest: The author confirms that there is no conflict of interest regarding the work presented in this article.
REFERENCES
Aenishaenslin, C.; Bouchard, C.; Koffi, J.K.; Ogden N.H. Exposure and preventive behaviours toward ticks and Lyme disease in Canada: results from a first national survey. Ticks and Tick-borne Diseases. 2017, 8, 112-118. https://doi.org/10.1016/j.ttbdis.2016.10.006.
Akl, T.; Bourgoin, G.; Souq, M.L.; Appolinaire, J.; Poirel, M.T.; Gibert, P.; Abi Rizk, G.; Garel, M.; Zenner, L. Detection of tick-borne pathogens in questing Ixodes ricinus in the French Pyrenees and first identification of Rickettsia monacensis in France. Parasite. 2019, 26, 1-9. https://doi.org/10.1051/parasite/2019019.
Babady, N.E.; Sloan, L.M.; Vetter, E.A.; Patel, R.; Binnicker, M.J. Percent positive rate of Lyme real-time polymerase chain reaction in blood, cerebrospinal fluid, synovial fluid, and tissue. Diagnostic microbiology and infectious disease. 2008, 62, 464-466. https://doi.org/10.1016/j.diagmicrobio.2008.08.016.
Baker, P.J. Antibody-based diagnostic tests for Lyme disease. 2017. Available at: https://aldf.com/wp-content/uploads/2017/09/Antibody-Based-Diagnostic-Tests-for-Lyme-Disease-9.1.17.pdf.
Baker, P.J. Is It Possible to Make a Correct Diagnosis of Lyme Disease on Symptoms Alone? Review of Key Issues and Public Health Implications. The American Journal of Medicine. 2019, 132, 1148-1152. https://doi.org/10.1016/j.amjmed.2019.04.001.
Baranton, G.; Seinost, G.; Theodore, G.; Postic, D.; Dykhuizen, D. Distinct levels of genetic diversity of Borrelia burgdorferi are associated with different aspects of pathogenicity. Research in Microbiology. 2001, 152, 149-156. https://doi.org/10.1016/S0923-2508(01)01186-X.
Borchers, A.T.; Keen, C.L.; Huntley, A.C.; Gershwin, M.E. Lyme disease: a rigorous review of diagnostic criteria and treatment. Journal of autoimmunity. 2015, 57, 82-115. https://doi.org/10.1016/j.jaut.2014.09.004.
Coiffier, G.; Tattevin, P. Lyme disease: “End of the debate?”. Joint Bone Spine. 2021, 88, 105181. https://doi.org/10.1016/j.jbspin.2021.105181.
Cook, M.J.; Puri, B.K. Estimates for Lyme borreliosis infections based on models using sentinel canine and human seroprevalence data. Infectious Disease Modelling. 2020, 5, 871-888. https://doi.org/10.1016/j.idm.2020.10.004.
Feng, J; Li, T; Yee, R; Yuan, Y.; Bai, C.; Cai, M.; Shi, W.; Embers, M.; Brayton, C.; Saeki, H.; Gabrielson, K.; Zhang, Y. Stationary phase persister/biofilm microcolony of Borrelia burgdorferi causes more severe disease in a mouse model of Lyme arthritis: implications for understanding persistence. Post-treatment Lyme Disease Syndrome (PTLDS), and treatment failure. Discovery Medicine. 2019, 27, 125-138.
Fraser, C.M.; Casjens, S.; Huang, W.M. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997, 390, 580-586. https://doi.org/10.1038/37551.
Iordache, F.; Nasoiu, L.I.; Istrate, C.; Mihailescu, P.; Mitrea, I.L.; Ionita, M. Screening of Lyme borreliosis in dogs from Romania using immunoblot assay. Journal of Biotechnology. 2015, 208, Suplement, S98. https://doi.org/10.1016/j.jbiotec.2015.06.308.
Kalmár, Z.; Mihalca, A.D.; Dumitrache, M.O.; et al. Geographical distribution and prevalence of Borrelia burgdorferi genospecies in questing Ixodes ricinus from Romania: a countrywide study. Ticks and Tick-Borne Disease. 2013, 4, 403-408. https://doi.org/10.1016/j.ttbdis.2013.04.007.
Kotwa, J.D.; Jardine, C.M.; Pearl, D.L.; Berke, O.; Mercer, N.J.; Peregrine, A.S. Evaluation of the SNAP® 4Dx® plus test for the detection of Dirofilaria immitis antigen and characterization of exposure to tick-borne pathogens in wild canids in southern Ontario. Veterinary Parasitology. 2020, 283, 109176. https://doi.org/10.1016/j.vetpar.2020.109176.
Lantos, P.M.; Rumbaugh, J.; Bockenstedt, L.K.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis, and Treatment of Lyme Disease. Arthritis & Rheumatology. 2021, 73, 12-20. http://doi.org/10.1002/art.41562.
Liu, J.; Drexel, J.; Andrews, B.; Eberts, M.; Breitschwerdt, E.; Chandrashekar, R. Comparative evaluation of 2 in-clinic assays for vector-borne disease testing in dogs. Topics in companion animal medicine. 2018, 33, 114-118. https://doi.org/10.1053/j.tcam.2018.09.003.
Luft B.J.; Dattwyler, R.J.; Johnson, R.C.; Luger, S.W.; Bosler, E.M.; Rahn, D.W.; Masters, E.J.; Grunwaldt, E.; Gadgil, S.D. Azithromycin compared with amoxicillin in the treatment of erythema migrans: a double-blind, randomized, controlled trial. Annals of Internal Medicine. 1996, 124, 785-791. https://doi.org/10.7326/0003-4819-124-9-199605010-00002.
Milich, K.A.; Donga, C.; Rosenkrantzb, W.S.; Herrinc, B.H. Seroprevalence of Borrelia burgdorferi in Shelter Dogs in Los Angeles County. Topics in Companion Animal Medicine. 2022, 50, 100676. https://doi.org/10.1016/j.tcam.2022.100676.
Mushtaq, A.; Kazi, F. New guidelines for Lyme disease diagnosis. The Lancet Infectious Diseases. 2021, 21, 173. https://doi.org/10.1016/S1473-3099(21)00009-8.
O’Connor Jr, T.P.; Lawrence, J.; Andersen, P.; Leathers, V.; Workman, E. Chapter 8.1 – Immunoassay Applications in Veterinary Diagnostics. The immunoassay handbook (Fourth Edition). 2013, 623-645. https://doi.org/10.1016/B978-0-08-097037-0.00053-1.
Rhodes, D.V.L.; Earnhart, C.G.; Mather, T.N.; Meeus, P.F.M.; Marconi, R.T. Identification of Borrelia burgdorferi ospC genotypes in canine tissue following tick infestation: implications for Lyme disease vaccine and diagnostic assay design. The Veterinary Journal. 2013, 198, 412-418. https://doi.org/10.1016/j.tvjl.2013.07.019.
Rothstein, J.D.; Patel, S.; Regan, M.R.; Haenggeli, C.; Huang, Y.H.; Bergles, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005, 433, 73-77. https://doi.org/10.1038/Nature03180.
Schwartz, A.M.; Kugeler, K.J.; Nelson, C.A.; Marx, G.E.; Hinckley, A.F. Use of commercial claims data for evaluating trends in Lyme disease diagnoses, United States, 2010–2018. Emerging infectious diseases. 2021, 27, 499-507. https://doi.org/10.3201/eid2702.202728
Sgroi, G.; Buono, F.; Iatta, R.; Beall, M.; Chandrashekar, R.; Buch, J.; Piantedosi, D.; Veneziano, V.; Otranto, D. Vector-borne pathogens of zoonotic concern in hunting dogs of southern Italy. Acta Tropica. 2022, 232, 106502. https://doi.org/10.1016/j.actatropica.2022.106502.
Shen, Z.; Zhang, M.Z.; Stich, R.W.; Mitchell, W.J.; Zhang, S. Development of a tick-borne pathogen QPCR panel for detection of Anaplasma, Ehrlichia, Rickettsia, and Lyme disease Borrelia in animals. Journal of microbiological methods. 2018, 151, 83-89. https://doi.org/10.1016/j.mimet.2018.05.019.
Skotarczak, B. Why are there several species of Borrelia burgdorferi sensu lato detected in dogs and humans? Infection, Genetics and Evolution. 2014, 23, 182-188. https://doi.org/10.1016/j.meegid.2014.02.014.
Smith, R.P. Current diagnosis and treatment of Lyme disease. Comprehensive therapy. 2005, 31, 284-290. https://doi.org/10.1385/COMP:31:4:284
Snydman, D.R.; Hu, L. Lyme disease. Medicine. 2021, 49, 747-750. https://doi.org/10.1016/j.mpmed.2021.09.004.
Stillman, B.A.; Monn, M.; Liu, J.; et al. Performance of a commercially available in-clinic ELISA for detection of antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii and Dirofilaria immitis antigen in dogs. Journal of the American Veterinary Medical Association. 2014, 245, 80-86. https://doi.org/10.2460/javma.245.1.80.
Stillman, B.A.; Thatcher, B.; Beall, M.J.; et al. Borrelia burgdorferi antibody test results in dogs administered 4 different vaccines. Topics in companion animal medicine. 2019, 37, 100358. https://doi.org/10.1016/j.tcam.2019.100358.
Weinberg, J.M. The anti-inflammatory effects of tetracyclines. Cutis. 2005, 75, 6-11.
Academic Editor: Prof. Dr. Daniel Simeanu
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Ivănescu Larisa Maria, Martinescu Gabriela-Victoria, Miron Liviu Dan