Liviu Dan Miron, Larisa Maria Ivănescu, Raluca Mîndru, Simona Mătiuț, Gabriela-Victoria Martinescu, Ilie Bodale
ABSTRACT. West Nile virus (WNV) is a re-emerging zoonotic pathogen that represents a threat to both human and animal health. It is difficult to estimate the impact of WNV in the future, although many of the climatic factors influencing its spread have been identified. In this study, we used bioclimatic indices to estimate those periods that favour the growth of vector mosquito populations and the incubation periods for the virus. To this end, we studied the climatic changes in the Romanian regions where cases of WN infection have been reported. Simulations were carried out for 2100 based on long-term scenarios. Identifying the bioclimatic conditions which can cause WNV outbreaks in Romania is necessary to anticipate and thereby prevent future epidemics. However, no extraordinary weather events were registered in the years with WNV outbreaks which could explain such a high number of cases. Thus, in the High Scenario (which will occur if actions to control (GHG) gas emissions are not taken or implemented effectively), the hatching period is extended until November, with the risk that adult mosquitoes are active throughout the year, ensuring a high survival rate of the virus within mosquitoes. In addition, in the High Scenario, the transmission period of the virus is extended from April to October, which underlines the need to establish monitoring and control programmes for both mosquito populations and the spread of the virus among the animal and human populations.
Keywords: climatic change effects; mosquitoes; temperature long-term projection; West Nile outbreak predictions.
Cite
ALSE and ACS Style
Miron, L.D.; Ivănescu, L.M.; Mîndru, R.; Mătiuț, S.; Martinescu, G.-V.; Bodale, I. Establishing the evolution of West Nile virus outbreaks in Romania by using climatic scenarios. Journal of Applied Life Sciences and Environment 2023, 56 (3), 387-412.
https://doi.org/10.46909/alse-563107
AMA Style
Miron LD, Ivănescu LM, Mîndru R, Mătiuț S, Martinescu G-V, Bodale I. Establishing the evolution of West Nile virus outbreaks in Romania by using climatic scenarios. Journal of Applied Life Sciences and Environment. 2023; 56 (3): 387-412.
https://doi.org/10.46909/alse-563107
Chicago/Turabian Style
Miron, Liviu Dan, Ivănescu Larisa Maria, Mîndru Raluca, Mătiuț Simona, Martinescu Gabriela-Victoria, and Bodale Ilie. 2023. “Establishing the evolution of West Nile virus outbreaks in Romania by using climatic scenarios” Journal of Applied Life Sciences and Environment 56, no. 3: 387-412.
https://doi.org/10.46909/alse-563107
View full article (HTML)
Establishing the Distribution of West Nile Virus Outbreaks in Romania by Using Climatic Scenarios
Liviu Dan MIRON1, Larisa Maria IVĂNESCU1, Raluca MÎNDRU1, Simona MĂTIUȚ2, Gabriela-Victoria MARTINESCU1* and Ilie BODALE3
1Clinics Department, Faculty of Veterinary Medicine, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 8, Mihail Sadoveanu Alley, 700489, Iasi, Romania; email: lmiron@uaiasi.ro; l.ivanescu@uaiasi.ro; raluca.mindru@uaiasi.ro
2Praxis Medical Laboratory Iasi, Romania; email: simona.matiut@laboratorpraxis.ro
3Exact Sciences Department, Faculty of Horticulture, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 3, Mihail Sadoveanu Alley, 700490, Iasi, Romania; email: ilie.bodale@uaiasi.ro
*Correspondence: martinescugabi11@yahoo.co.uk
Received: Oct. 10, 2023. Revised: Nov. 16, 2023. Accepted: Nov. 17, 2023. Published online: Dec. 08, 2023
ABSTRACT. West Nile virus (WNV) is a re-emerging zoonotic pathogen that represents a threat to both human and animal health. It is difficult to estimate the impact of WNV in the future, although many of the climatic factors influencing its spread have been identified. In this study, we used bioclimatic indices to estimate those periods that favour the growth of vector mosquito populations and the incubation periods for the virus. To this end, we studied the climatic changes in the Romanian regions where cases of WN infection have been reported. Simulations were carried out for 2100 based on long-term scenarios. Identifying the bioclimatic conditions which can cause WNV outbreaks in Romania is necessary to anticipate and thereby prevent future epidemics. However, no extraordinary weather events were registered in the years with WNV outbreaks which could explain such a high number of cases. Thus, in the High Scenario (which will occur if actions to control (GHG) gas emissions are not taken or implemented effectively), the hatching period is extended until November, with the risk that adult mosquitoes are active throughout the year, ensuring a high survival rate of the virus within mosquitoes. In addition, in the High Scenario, the transmission period of the virus is extended from April to October, which underlines the need to establish monitoring and control programmes for both mosquito populations and the spread of the virus among the animal and human populations.
Keywords: climatic change effects; mosquitoes; temperature long-term projection; West Nile outbreak predictions.
INTRODUCTION
Pathogens transmitted by mosquitoes affect both animals and humans. Vector-borne diseases are difficult to manage once they become established, especially when the natural reservoirs are wild animals (Tolle, 2009).
The incidence of mosquito-borne diseases varies geographically, with the timing of transmission changing in response to the ongoing interaction among hosts, pathogens, vectors and the environment (Gangoso et al., 2020). Usually, the emergence or re-emergence, as well as the spread of mosquito-borne diseases, are closely related to changes in the distribution of the main vectors, either as a result of introduction by human actions or as a consequence of changes in the prevailing environmental conditions (Ebi et al., 2013). In Europe, West Nile virus (WNV) cases in humans were reported more frequently in years with extremely warm temperatures (Patz et al., 1996; Tran et al., 2014; Tabachnick, 2016). Climate combined with other factors, such as human population growth, livestock numbers, global trade, travel, urbanisation and land use change, can increase the risk of new diseases occurring in Europe (Naish et al., 2014; Patz et al., 1998; Watts et al., 2021).
Health risks have arisen in Europe, especially in recent decades, with the emergence of vector-borne diseases such as West Nile, chikungunya, crimean-congo haemorrhagic fever and dengue (Hotez, 2016; Olesen and Ackermann, 2017; Tabachnick, 2010). Of these, WNV represents a threat to both animal and human health as it is a re-emerging zoonotic pathogen (Ometto et al., 2013).
The US Environmental Protection Agency now believes that the WNV may be an indicator of climate change (EPA, 2023). The perpetuation and maintenance of transmission cycles of some arboviruses in nature are dependent on several factors. These factors, which are intrinsic and extrinsic, influence the biology of viruses but also that of the vectors and hosts, as well as their corresponding interactions. For a vector arthropod to be able to transmit a virus to a vertebrate host, it must be a competent vector. In medical entomology, the notion of “vector competence” refers to the inherent ability of a blood-feeding insect to transmit a particular virus. Competent vectors ingest pathogens during blood feeding and permit gut infection and subsequent dissemination to the secondary sites of replication, especially in the salivary glands. From the salivary glands, the virus is inoculated with the saliva into the cutaneous vascular tissue of the vertebrate host during subsequent blood feeding. In 2015, Paz conducted a review of existing studies linking climate change to changes in WNV distribution patterns. Meteorological conditions can both directly and indirectly influence the competence of the vectors (acquisition, maintenance and transmission of the virus), the dynamics of the vector population and the multiplication rate of the virus into the mosquito. The impacts of climatic factors (precipitation, relative humidity, temperature and winds) on the epidemiology of the WNV is favored by climate change (Paz, 2015). In Europe, there has been a marked expansion of WNV outbreaks in recent decades, causing more than 2,000 symptomatic cases in 2018 alone (Farooq et al., 2022). Paz (2015) emphasises that only permanent monitoring and surveillance would keep the WNV and its effect on the population under control in the context of current and future climate impacts. Biotic factors include birds, the hosts of the WNV, which vary by species in their susceptibility to virus infection (Paz and Semenza, 2013).
Taking climatic factors into account, cold-season temperatures between 2°C and 6°C best predicted the number of annual WNV infections; a cause could be the survival of infected mosquitoes over the winter (Culex pipiens) resulting in an increase in outbreaks the following year (Paz and Semenza, 2013). In Europe, Culex pipiens s.s. is found in two behaviourally and ecologically distinct forms (Di Pol et al., 2022) – an aboveground form, Culex pipiens pipiens, which enters diapause in winter and feeds mainly on birds, and a belowground form, molestus, feeds on mammals. The two forms can hybridise, complicating predictions of vectorial capacity and is hypothesised to have contributed to the emergence of the WNV in Europe and the Americas in recent decades (Haba and McBride, 2022a). Studies show that the titre of the WNV is much higher in infected female mosquitoes incubated at 22°C–30°C than in those kept at 14°C–18°C, emphasising the essential role of temperature in the occurrence of WNV outbreaks (Reisen et al., 2006). In another study, female mosquitoes kept at 14°C transmitted the virus for the first time at 36 days post-infection, whilst females kept at 18°C transmitted the virus for the first time at 22 days post-infection. Conversely, females kept at 26°C and 30°C were able to transmit the virus 5 days post-infection (Reisen et al., 2006). Some studies show that lack of rain can accelerate the spread of the virus. Lower rainfall was positively associated with a higher risk of occurrence of WNV cases (Farooq et al., 2022). Changes in the geographic range of mosquitoes may occur as a result of climate warming. These climate changes could lead to the emergence of infectious diseases, defined as infections that increased in incidence or appeared in new regions or new populations in the past 20 years (Hoover and Barker, 2016).
Climate change with the occurrence of extreme weather events, such as increased precipitation leading to floods and heat waves, has contributed to the modification of mosquito activity patterns, creating suitable conditions for the transmission of viruses (Hoover and Barker, 2016).
The species within the Culex pipiens complex can transmit a wide range of pathogens, including Usutu virus, West Nile virus, Sindbis virus, St. Louis encephalitis virus, filaria (Wuchereria, Dirofilaria spp.) and haemosporidia (avian Plasmodium) (Bravo-Barriga et al., 2016; Brugman et al., 2018; Farajollahi et al., 2011). According to the Worldwide Catalog of Mosquitoes maintained by the Walter Reed Biosystematics Unit, the complex includes the following species: Cx. quinquefasciatus, Cx. Australicus, Culex pipiens and Cx. globocoxitus. Also, based on morphological and ecological similarities, some authors have also included the related species Cx. torrentium within the complex. Culex pipiens and Cx. quinquefasciatus are the most widespread species within the complex, with Cx. pipiens being a notable example of vector range shift (Ciota et al., 2013; Martinez-de la Puente et al., 2016; Tesh et al., 2004; Turell et al., 2005). This species feeds on birds, mammals and humans (Brugman et al., 2018; Fritz et al., 2015). A behavioural characteristic relevant to the transmission of arboviruses is the feeding pattern of the vector. Environmental factors include the local and seasonal presence of vertebrate hosts, the host’s defence mechanisms and the presence of pathogens in the arthropod, host or both, which influence the interaction between hosts and vectors. The importance of mosquitoes in the Cx. pipiens complex in WNV transmission resides in the large number of viral isolates from specimens collected in the field (Greece (Tsioka et al., 2022), Italy (Savini et al., 2012), Romania (Crivei et al., 2023), Germany (Kampen et al., 2020), Serbia (Petrović et al., 2021)) from their vector competence for the WNV (Vogels et al., 2015).
The duration of the biological cycle of Culex mosquitoes is dependent on temperatures. For example, eggs hatch after only 1 day at 30°C, after 3 days at 20°C and after 10 days at 10°C; below 7°C, embryonic development cannot be completed (Becker et al., 2010). The WNV survives inside the vector in diapause during the winter, but it cannot be transmitted, even though species such as Cx. pipiens molestus also feed in winter. Starting from 14°C, the virus begins to replicate, but only after two gonotrophic cycles of the females is the salivary gland titre high enough for the virus to infect a host. At a temperature of 30°C, the replication rate begins to decrease since the optimal temperature which allows replication to be completed within a single gonotrophic cycle is between 24°C and 26°C (Anderson et al., 2010; Fay et al., 2022). Models predict that virus transmission will peak in intermediate temperatures and decline in extremes of cold and heat (Paz and Semenza, 2013; Reisen et al., 2006).
The highest abundance of long-lived mosquitoes was recorded at temperatures above 20°C and up to 30°C, with the most noticeable decreases in longevity at extremely hot or cold temperatures. Studies show a decrease in the longevity of female mosquitoes at temperatures above 32°C (Sauer et al., 2021).
Culex mosquitoes are adapted to a synanthropic and an urban/sylvatic cycle, which can overlap, so that areas with humidity, close to human dwellings, can become favourable for the transmission of the virus (Hubalek and Halouzka, 1999). Irrigation in agriculture plays an important role in the risk of the occurrence of WNV epidemics (Gates and Boston, 2009).
In Romania, the monitoring and control of vectors, as well as vector-borne diseases, is not done, which is why we set out to establish the influence of the local climate on the development of mosquitoes as well as the emergence of West Nile outbreaks.
We projected the average climatological temperatures favourable for the development of the mosquito population as a vector for the spread of WNV. We also predicted the optimal conditions for the extrinsic incubation of the WNV based on two bioclimatic indices capable of predicting future thermal periods to identify the geographical areas in Romania favourable for the occurrence of WNV outbreaks. Bioclimatic indices calculate the thermal periods required for Culex mosquito egg hatching and WNV infection to understand the direction of the development of ecoclimatic conditions.
MATERIALS AND METHODS
The study aims to establish the specific climatic influence on the biological cycle of mosquitoes and the multiplication of the virus inside vectors created on the basis of previously published laboratory experiments. Thus, temperatures favourable to the hatching of mosquito eggs were used as an index of the conditions favourable for the development of vector populations, and temperatures favourable for the replication of the virus within the vector were used to define periods suitable for virus transmission.
Measurement of climatological parameters
Air temperature was measured, at the time of observation (Ti), in a meteorological shelter at a height of 2 m, and precipitation was recorded at a height of 1.5 m using a rain gauge, according to the standards of the World Meteorological Organization (UN) (IMO, 1950). The measurements were carried out by the National Meteorological Agency (ANM) (www.anm.ro), these data being entered into the European Climate Assessment and Dataset (Klein Tank et al., 2002). The measurements were performed in meteorological stations distributed throughout Romania in areas favourable for the development of mosquitoes (Figure 2).
Temperature projection for the year 2100
Forecasting weather conditions during succeeding decades is one of the major challenges in climatology. In recent years, several physical climate models and socioeconomical scenarios have been developed to estimate temperatures in the long term. The physical climate models are based on historical data and weather conditions, ensuring good short-term results. Long-term temperature projections depend on climatologic conditions and other variables such as the chemical composition of the air, which depends on anthropic activities. The models are, however, limited because the evolution of the chemical composition of air depends on the public policies regarding greenhouse gas (GHG) emissions implemented by all countries. In the last years, weather conditions were estimated using socio-economical scenarios (Kunkel et al., 2013).
The scenarios described in the fifth assessment report (AR5) of the specific Working Group III of the Intergovernmental Panel on Climate Change (IPCC) offer the most common ways to project temperatures over a longer period. Models that followed “representative concentration pathways” (RCPs) predict changes in GHG concentrations depending on the policies that states will implement in the years to come. According to this approach, the forecasts are based on nine scenarios which take into consideration both climatological conditions and socioeconomic pathway (SSP).
The complex model of projections (CMIP5) (Kunkel et al., 2013) considers the impact of anthropogenic activities on the greenhouse gases which affect the atmosphere, changing the natural radiative forcing of Earth. Radiative forcing and the history of climatic parameters are used as input into physical models which provide an output able to assess the impacts, adaptation and vulnerability to climatic changes.
We simplified the implementation of the climatic model assuming that the CMIP5 is valid. We therefore kept the same initial conditions and only adapted the model to the radiative forcing and temperature at regional conditions. As it is a mathematical model, we used the pattern scaling technique, in which the estimated temperature is determined as a product between a scalar term and response pattern of temperature. In our regional circulation approach, the annual average temperature at time t was used as scalar, and the response pattern of temperature was related to radiative forcing. This term was computed based on a linear least squares regression for temperature sequences.
This approach was used to estimate the possible daily temperature in 2100 in all regions of this study. The results obtained were applied to determine the most probable values for the bioclimatic indices already described.
In the present approach, we considered only the two extreme scenarios: 1) Low Scenario (LS) is the optimistic case in which the temperature increase on the ground surface will be kept below 2°C according to the Paris Agreement.This scenario is consistent with the Coupled Model Intercomparison Project Phase 5 (CMIP5) scenario SSP1-1.9 showing an increase in Northern Hemisphere temperatures of about 1.5 C on average in the context of drastic mitigation measures of GHG emissions; 2) High Scenario (HS) is the most pessimistic scenario, which shows an average temperature increase of 4.5C compared to the pre-industrial era (Lyon et al., 2022; O`Neill et al., 2016). This scenario will occur if actions to control GHG emissions are not taken or implemented effectively.
The simulations were performed for the two extreme scenarios because they have the advantage that they cover the entire possible temperature range, which encompasses most oscillations.
The above approach was used to forecast the temperatures in the relief forms of Romania which are prone to the development of mosquito populations. The average daily temperatures were simulated for the year 2100 to forecast temperature changes compared to the climatological reference period of 1991–2020. The global temperature would increase linearly until 2100 in all scenarios of CMIP5 (Kunkel et al., 2013). Projecting the temperature for 2100 is important because it provides an easy method to identify the temperature at any time before and after this year by using mathematical extrapolation.
Bioclimatic indices
Identifying which conditions can cause WNV outbreaks in Romania is necessary to prevent future epidemics. To this end, we studied the climatic changes in the Romanian regions where cases of WNV infection have been reported. We proposed two indices to predict the periods with temperatures favourable for the evolution of mosquito populations involved in virus transmission and the incubation time of the WNV. The bioclimatic indices were used to estimate how the development conditions of the WNV and its vectors spatially or temporally change in relation to temperature as the main environmental factor. We propose two indices to estimate how global warming will influence the conditions in Romania for the hatching of Culex mosquito eggs and the periods of WNV transmission.
Hatching potential index of Culex mosquito eggs – MPI
In this study, the annual index representing the hatching potential (MPI) of mosquito eggs of the genus Culex (according to laboratory studies – Norbert Becker) was used as an indicator of the periods in which mosquitoes could develop depending on temperatures. It adds all monthly periods (MPIm) which are thermally favourable for mosquito larvae to reach maturity. This index is useful to identify the months favourable for the development of mosquitoes because the spring and autumn months have temperatures close to the minimum biological threshold, whereas the temperatures in the summer months can exceed the maximum biological ceiling. Moreover, the speed of development depends on the optimum temperature range. The monthly hatching potential index (MPIm) of the Culex mosquito eggs is calculated the number of periods in each month with thermal conditions favourable for hatching. The size of the thermal periods depends on the average temperatures of each day. We calculated MPIm by considering the sequences of consecutive days with favourable conditions, using three temperatures (10°C, 20°C and 30°C). The favourable condition was considered only if at least one sequence of days with average temperatures above an established threshold was identified. The annual hatching potential index was calculated as the sum of the monthly hatching indices (Equation 1):

This bioclimatic index determines whether, from a thermal point of view, the hatching conditions for mosquito eggs are met. The monthly hatching periods were calculated based on three temperature levels because the mosquito eggs develop at different rates, depending on the heat accumulated. Based on this statement, we calculated the potential hatching ranges for three temperature levels (10oC, 20oC and 30oC). The MPI counts the periods within a month which are favourable for hatching, based on the number of days in which the temperature theoretically is at least above the threshold level. The longest development period is near the low-threshold temperature (10°C) but often also includes days with higher temperatures, which shortens the interval proportional to the temperature value. The monthly hatching potential (MPIm) is calculated as follows (Equation 2):
![]()
The MPIm of the Culex mosquito eggs is calculated for the three temperature values; based on the result, the annual hatching potential index is determined.
As eggs hatch after 1 day at 30°C, the potential hatching periods at this temperature ( is the number of days in which the average daily temperature in month m exceeds 30°C. At 20°C, they hatch after 3 days , at 10°C after 10 days (, and below 7°C, embryonic development is blocked (. For the calculation of the possible hatches per year, the MPI is important, under local temperature conditions.
Establishing the potential conditions for the hatching of mosquito eggs allows us to evaluate how the temperature conditions will evolve at the end of the century, which is necessary for the estimation of the population at this time.
Potential WNV infestation index – PII
We defined the second index as annual, calculating the maximum possible period from the moment female mosquitoes are infected until they become capable of transmitting WNV. This index is calculated similarly to the previous one, only that it considers the times which mosquito females need to transmit the virus, depending on temperature. In this calculation, we considered the maximum number of possible periods in a month during which mosquitoes can infect people. This index was correlated with the MPI index to ensure that there were theoretical conditions for the mosquito population to exist.
The maximum possible annual periods were calculated taking into account the change in post-infection duration according to the temperature, as documented in the laboratory by Norbert Becker for Cx. pipiens pipiens (2010). The index calculates the possible periods in which female mosquitoes who live at 14°C are able to transmit the virus. At this temperature, they transmitted the virus for the first time 36 days after infection. Females kept at 18°C transmitted the virus for the first time at 22 days post-infection , and at temperatures from 26°C to 30°C, they were able to transmit the virus at 5 days post-infection (Reisen et al., 2006). The potential WNV infestation index (PII) is calculated as follows (Equation 3):

The monthly potential WNV infestation index (PIIm) is the number of days in which the virus can be transmitted within a month and is calculated using the following equation:
![]()
where PIIm considers the maximum possible intervals in which the virus can be transmitted based on the most relevant three temperature levels (14oC, 18oC and 26oC). The PIIm is more selective than the MPI because it identifies the potential temperature windows required for the infestation until the virus can be transmitted.
These proposed indices are theoretical, but they are useful to analyse whether the conditions which favour the occurrence of possible outbreaks will change significantly in the context of global warming (DeGroote and Sugumaran, 2012).
RESULTS
Insects, as poikilothermic animals, are directly affected by environmental parameters, with temperature playing an important role in egg hatching and development. In Romania, the monitoring of WNV outbreaks began after the epidemic in 1996. This event attracted the attention of specialists in infectious diseases and public authorities because until then, it had been considered that in the temperate continental climate specific to mid-latitudes, mosquito populations of the genus Culex could not transmit the WNV. The distribution of WNV cases recorded from 1997 to 2021 shows that most were reported in low-lying areas in all regions of the country. The climatic conditions in the south of Romania are favourable for the development of Cx. pipiens mosquito populations, which explains why most cases were recorded for the plain areas of this region, where the temperatures favour the replication of the virus inside the mosquitoes for a longer time of the year (NCSCCD, 2023).
During the period from 1996–2021, 1,043 cases of West Nile fever were reported in Romania, with the peak of cases in 1996, when the virus was reported for the first time in humans. This was surprising as the Romanian climate was not considered favourable for the transmission of this virus through the mosquito vector (Figure 1). A similar outbreak was recorded in 2018, which was inexplicable from a climatic point of view and requires a complex understanding of the factors involved in the transmission of the WNV. A much higher number of cases was reported in Europe in the transmission season of 2018 compared to previous years. Thus, the total number of local infections reported in 2018 (n = 2,083) far exceeded that of the past 7 years (n = 1,832). Compared to the previous transmission season in 2017, there was an increase by 7.2 times (Popescu et al., 2018).
In addition, in 2018, the last case diagnosed in the transmission season in the EU was reported at the end of November in France, with an onset date in week 46, which was particularly late as compared to previous transmission seasons, when the last onset date was between weeks 39 and 42 (O`Neill et al., 2016). In this study, we also compared the climatic conditions between the years 1996 and 2018 in regions favourable for the development of mosquitoes in Romania (Figure 2), with the aim to determine the factors affecting WNV transmission. These regions only included the lowlands (Danube Meadow, plains, hills and plateaus).
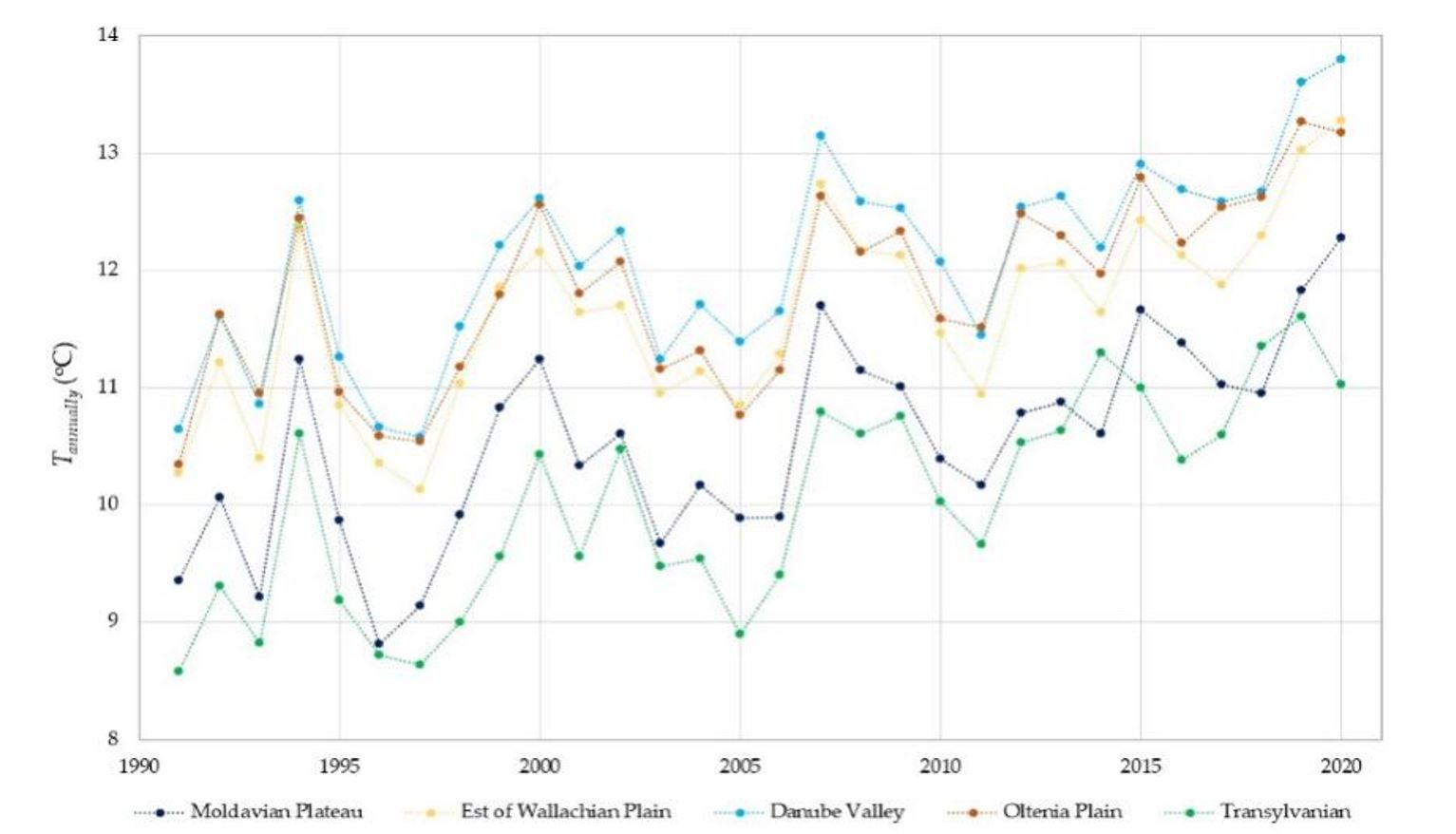
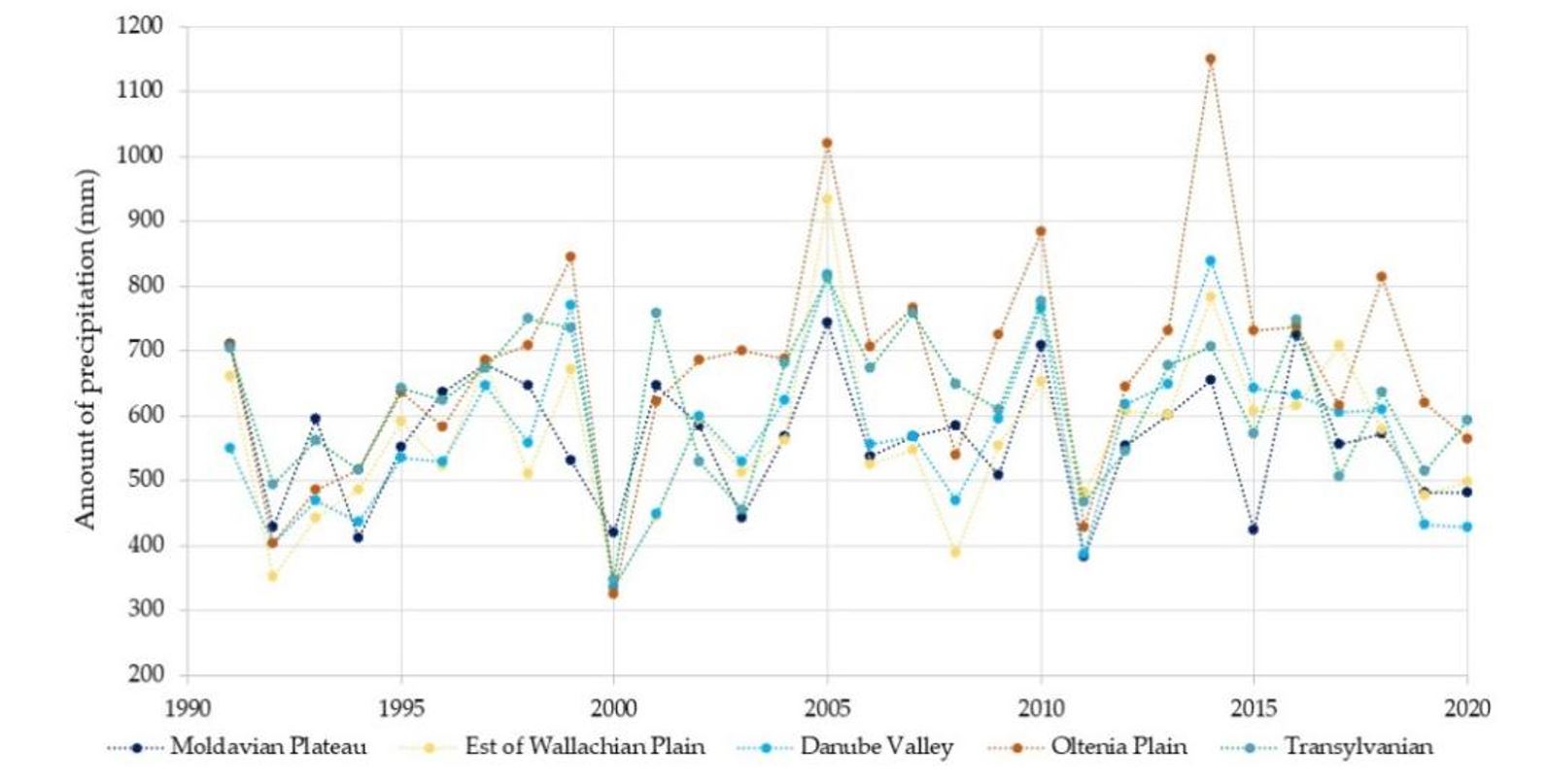
Distribution of WNV cases in Romania differed among different regions, with 56%being recorded in the East of the Romanian Plain (also known as Wallachian Plain), 14% in the Oltenia Plain and an average of 9% in the narrow area of the Danube Valley. The Danube Valley is a favourable environment for the development of Culex mosquito populations because the optimal temperature-humidity conditions are met. Compared to the area and the number of inhabitants, the percentage of West Nile cases was highest in the Wallachian Plain (Figure 3). Average temperatures recorded at meteorological stations in Romania from 1961–2020 increased constantly after 1991 (Figure 4). Record temperatures were registered in the decade 2011–2020.
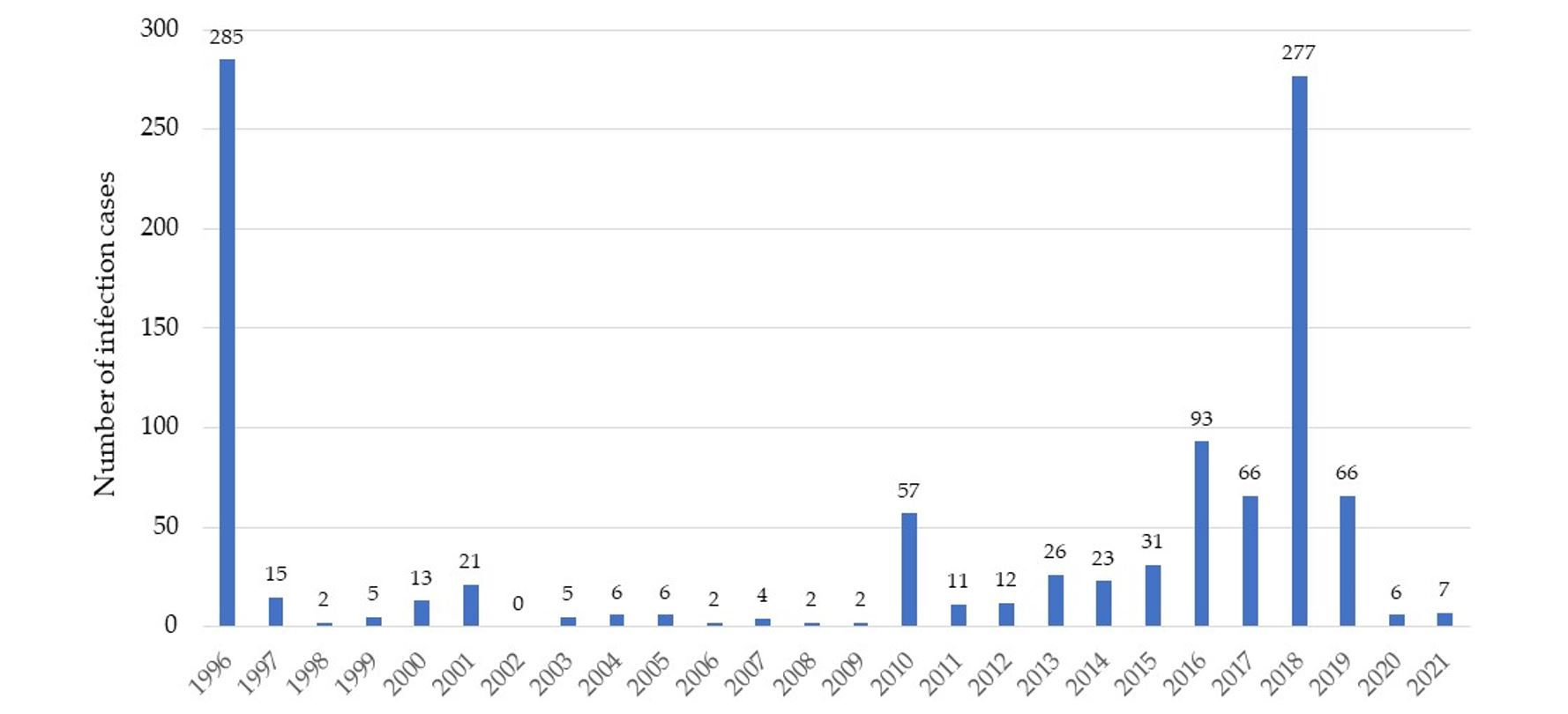
Figure 1 – West Nile Virus cases in Romania recorded between 1996 and 2021 [Database of the Romania National Centre for Surveillance and Control of Communicable Diseases (NCSCCD) within the Institute of Public Health (IPH)]
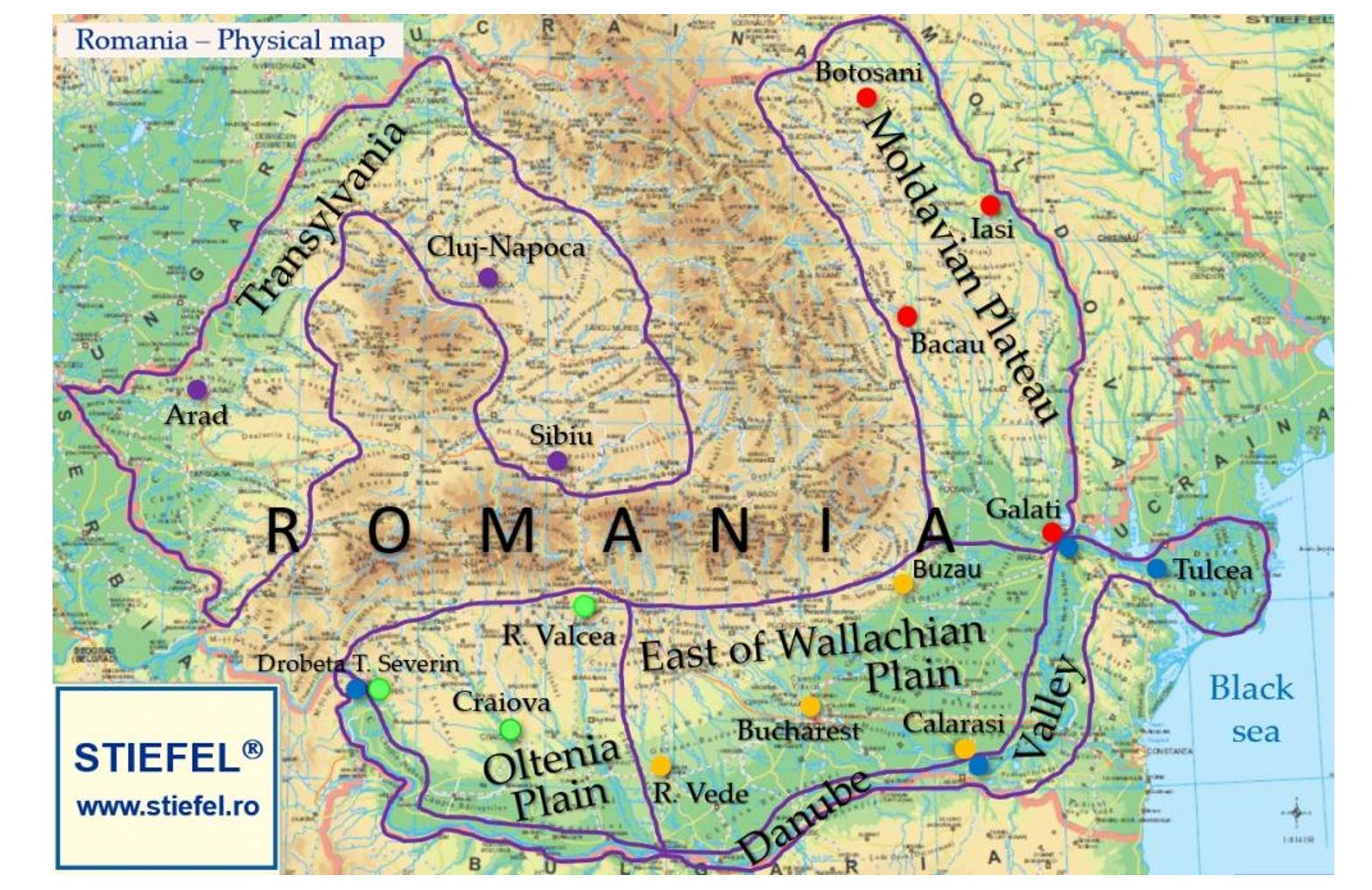
Figure 2 – Regions in Romania where potential West Nile virus outbreaks may appear (Meteorological stations: ● – Moldavian Plateau (Botoșani, Bacau, Iasi, Galati); ● – East of Wallachian Plain (Rosiori de Verde, Bucharest-Baneasa, Buzau, Calarasi) ● – Oltenia Plain (Ramnicu Valcea, Craiova, Drobeta Turnu Severin) ● – Danube Valley (Calarasi, Tulcea, Galati) ● Transylvania (Sibiu, Arad, Cluj-Napoca) [The physical map used as background was provided by www.stiefel.ro]
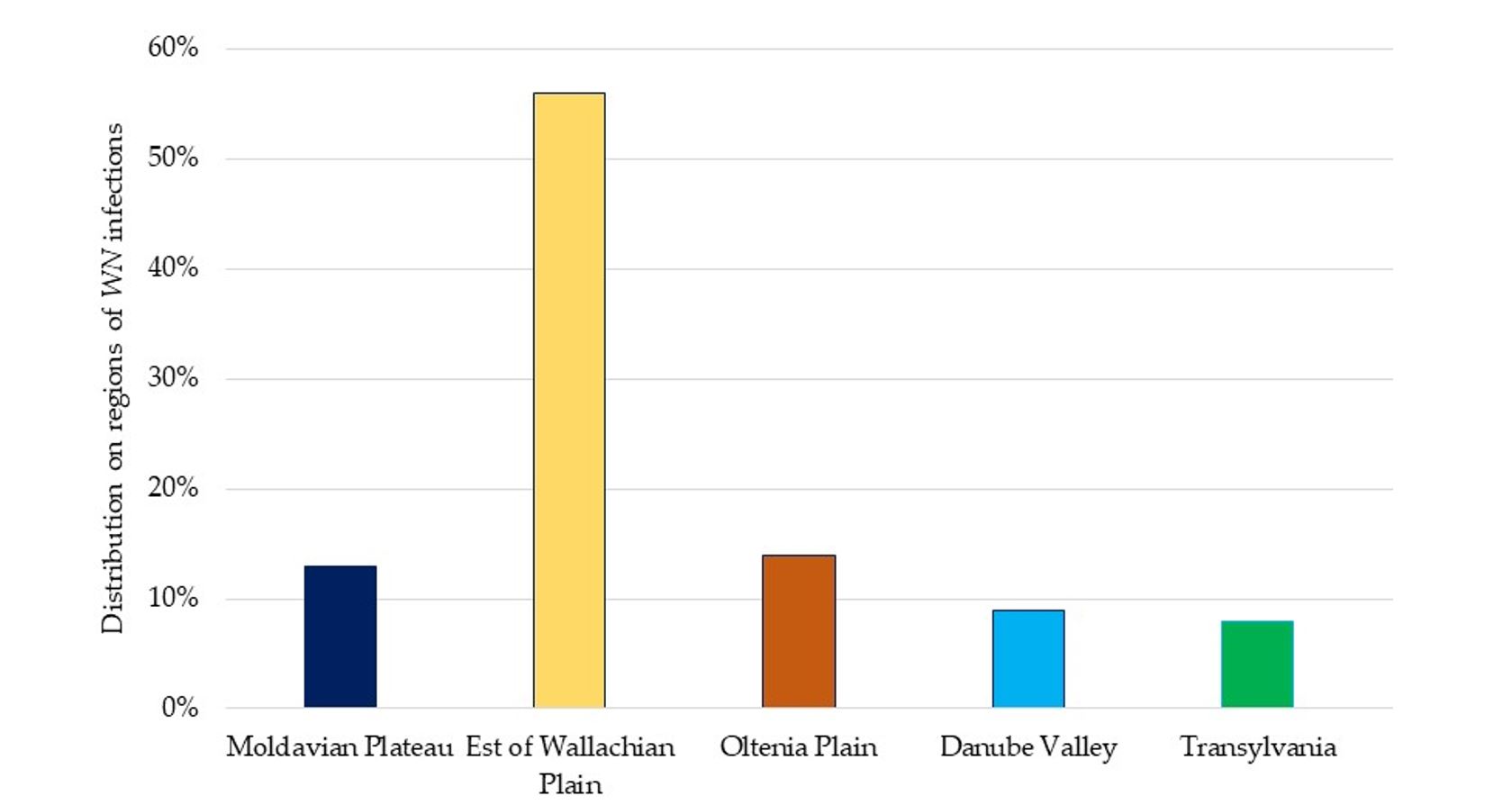
Figure 3 – Distribution of West Nile virus cases from 1997 to 2021 in Romanian regions favourable for mosquito development [Database of the Romania National Centre for Surveillance and Control of Communicable Diseases (NCSCCD) within the Institute of Public Health (IPH)]
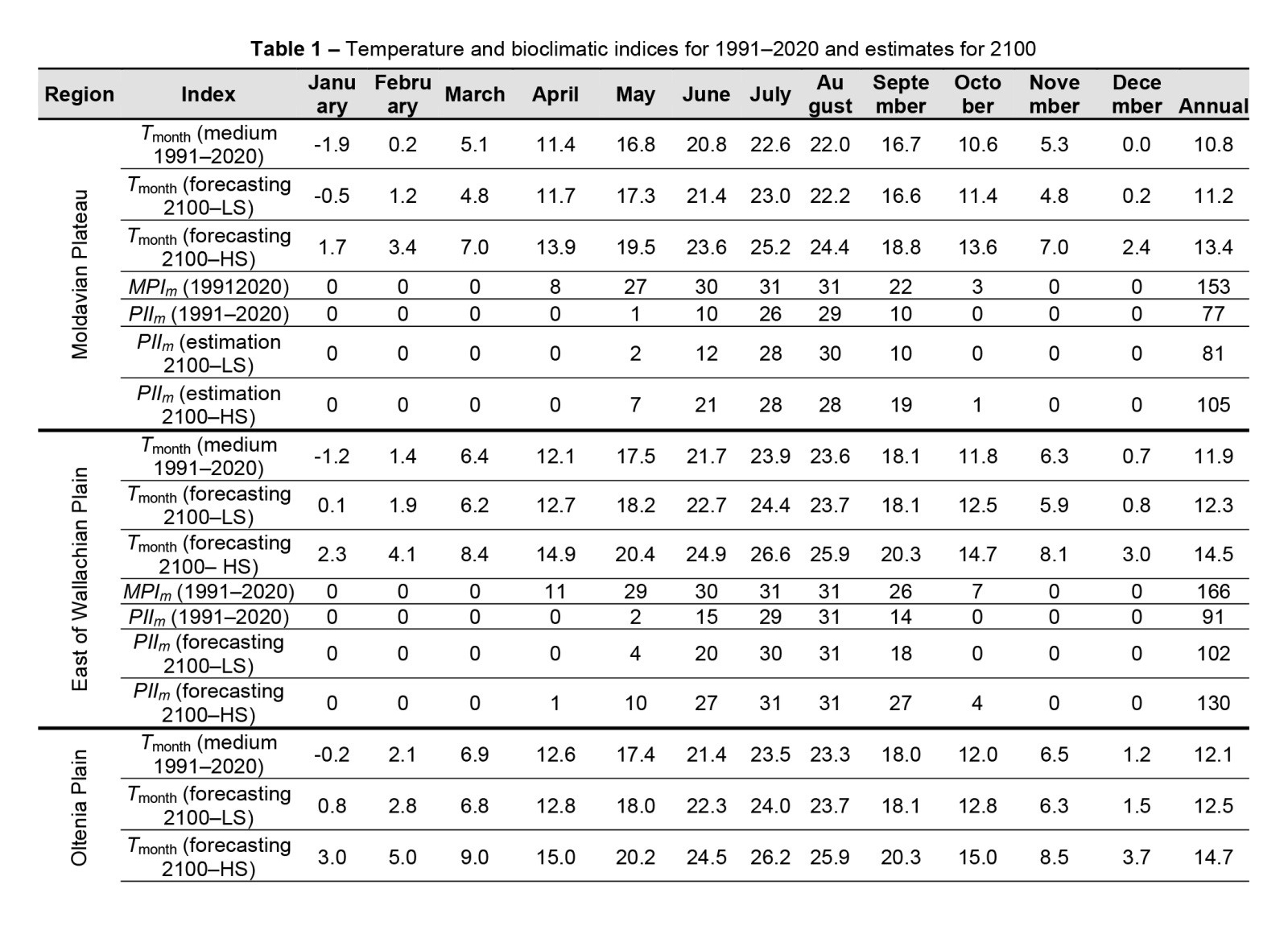
In the low regions of Romania, from a thermal point of view, mosquito hatching and development are possible until the first week of November and the development of the WNV inside the mosquito can occur until October (Table 1). The simulations under the conditions of climate change for the year 2100 show that in the coming decades, these periods may be extended by another month, which favours the development of mosquito populations transmitting the virus in all low-lying regions of Romania throughout the year (Table 1).
As shown in Figure 5, between 2012 and 2020, in Oltenia and Wallachian Plains, mosquito eggs could hatch at up to 190 days a year. Figure 6 shows that in 2018, in Oltenia and Wallachian Plains, there were 130 days with ideal conditions for mosquito vectors to transmit the WNV; at these temperatures, virus replication is rapid.
The WNV survives inside the diapause vector during the winter, but this vector is unable to transmit the virus, even though species such as Cx. pipiens might also feed in winter. Temperatures between 2°C and 6°C recorded in winter represented one of the most important predictors of WNV infections per year; an explanation for this result could be the successful wintering of infected adult mosquitoes (probably Cx. pipiens) being responsible for the intensity of outbreaks the following year. Monthly calculations of average temperatures indicate that in Moldova, Wallachia and Transylvania, there are 2 months (January and February) with average temperatures below 2°C and only one month (January) in the remaining regions under study. Temperature simulations for 2100 show that the time intervals with temperatures below 2°C will decrease significantly over the next 80 years.
According to our calculations, if drastic a series of measures are carried out to limit GHG emissions in the Moldovan area, compared to the 1990s, temperatures will increase by 0.4°C by 2100 (Low Scenario – LS). In contrast, it compliant actions to control GHG emissions are not taken or not implied effectively, temperatures will heat up 2.6°C (High Scenario – HS). Under LS conditions, there is a total of 81 days, from May to October, during which the WNV can replicate inside the vector. Under HS conditions, this period spans 105 days, from May to September October; in comparison, under current conditions, this period only lasts for 77 days.
In the Wallachia Plain, temperature will increase by 0.4°C under LS and by 2.6°C under HS by the year 2100, with an extension of the virus replication period from May to September, with a total of 130 days per year under HS and 102 days under LS, compared to 91 days at present.
In the Oltenia region, the temperature change will be similar, with an increase to 131 days per year, indicating a higher risk of virus transmission.
In the Danube Valley, temperature is predicted by increase by 0.3°C under LS and by 2.5°C under HS, with a virus transmission period of 133 days in 2100 compared to 96 days at present, also resulting in a longer risk period.
The temperature will increase by 0.2°C, in Transylvania, under LS and by 2.4°C under HS by the year 2100, with 87 days favourable for virus transmission under HS, compared to 57 days at present. The Culex hatching period, forming a new mosquito population, currently lasts from April to October in Moldova, the Wallachian Plain, Oltenia Plain and Transylvania and from April to November in the Danube Valley (Table 1).
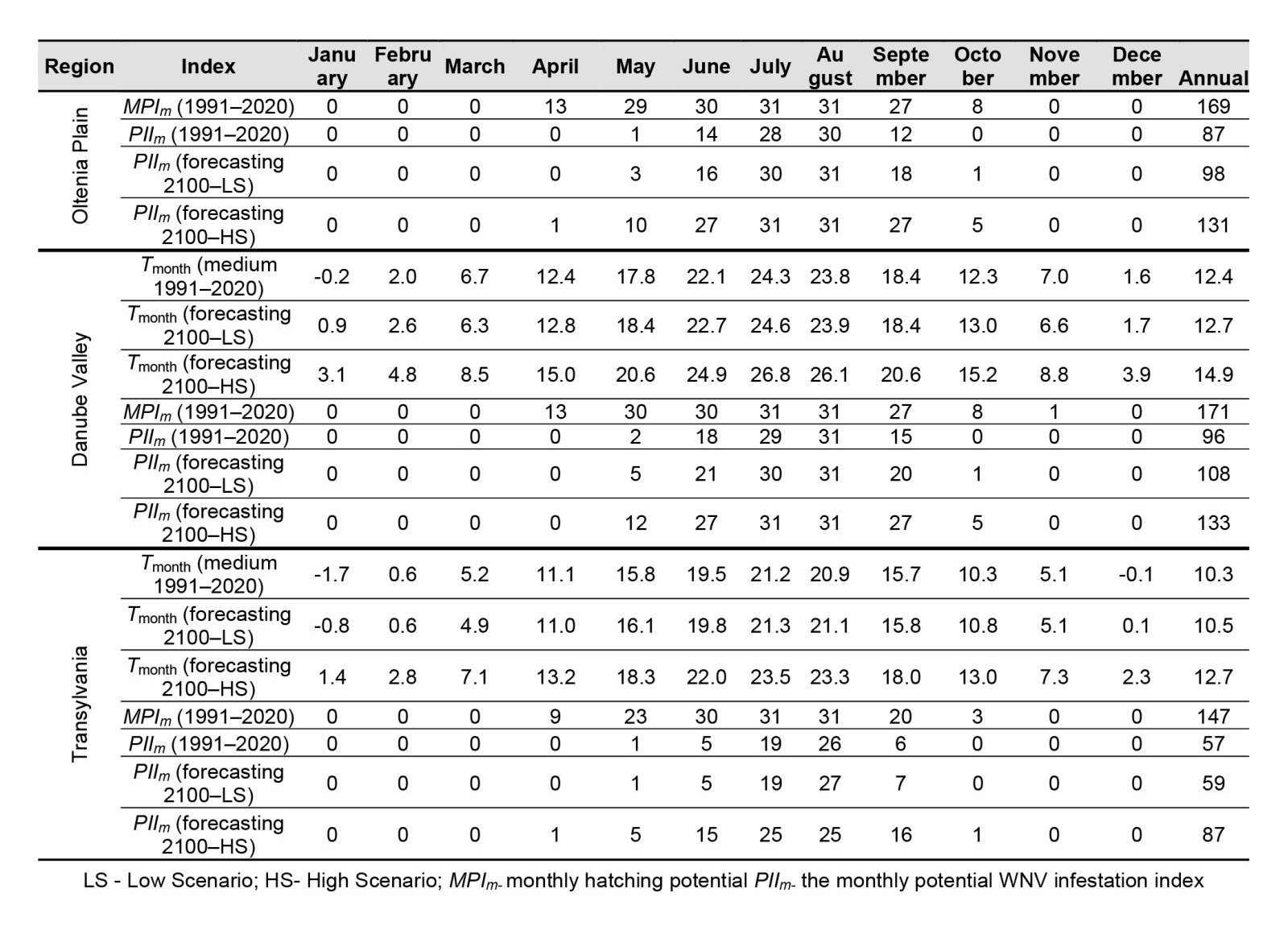
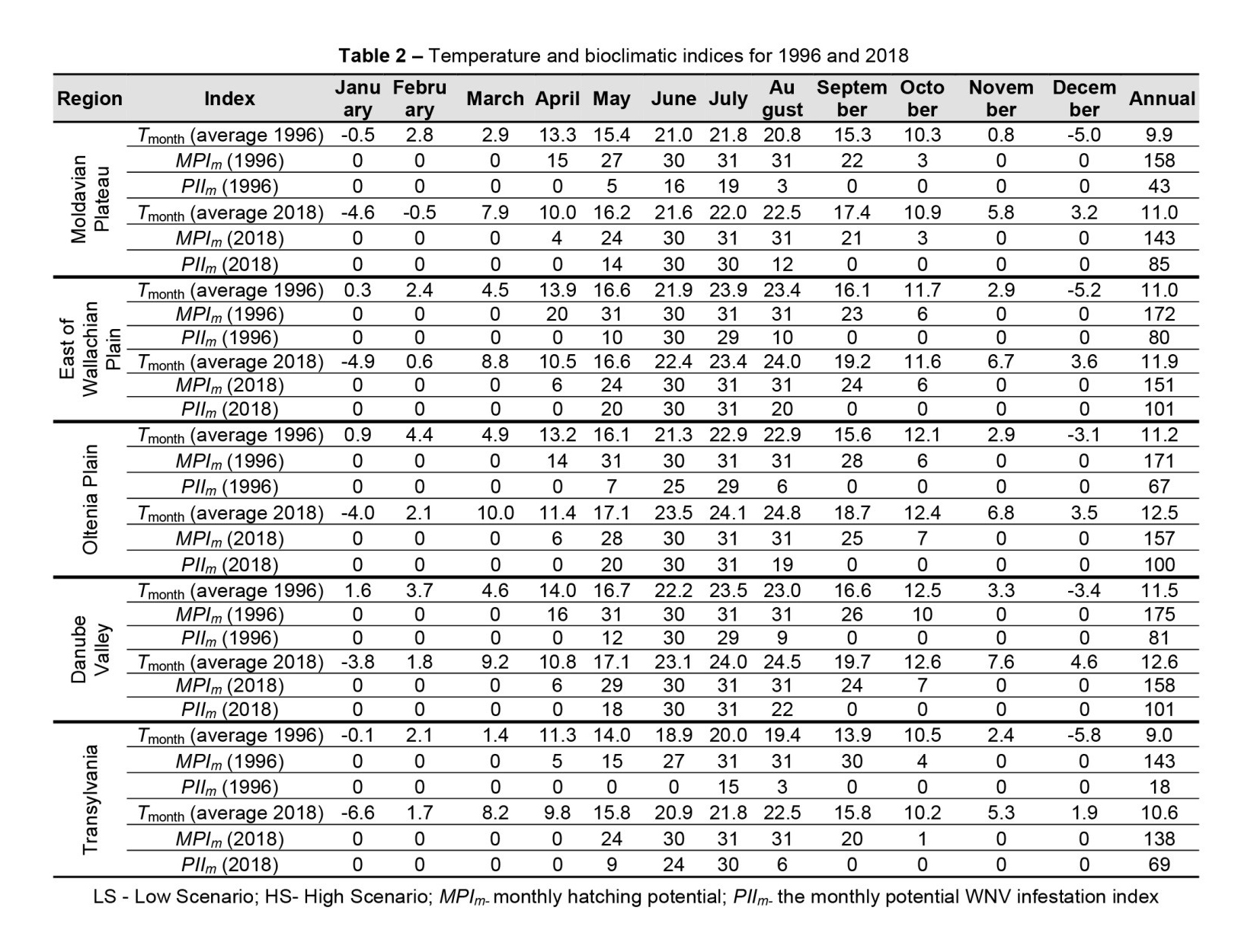
Regarding the influence of temperature on the two major outbreaks of West Nile fever recorded in 1996 and 2018, the risk of WNV transmission was higher in 2018. In Moldova, there were 85 days favourable for WNV replication inside the vector and 143 days favourable for hatching, compared to 43 days for PII and 158 days for MPI. This leads us to infer that a higher number of mosquito populations per year may be a more important factor in the occurrence of WNV outbreaks, and emphasis should therefore be placed on the control of mosquito populations. Similar conditions were observed for the Wallachian Plain, Oltenia Plain, Danube Valley and Transylvania regions, where 172, 171, 175 and 143 days, respectively, favourable for the hatching of mosquito eggs were recorded in 1996, compared to 151, 157, 158 and 138 days, respectively, in 2018, with a difference of approximately 3 weeks.
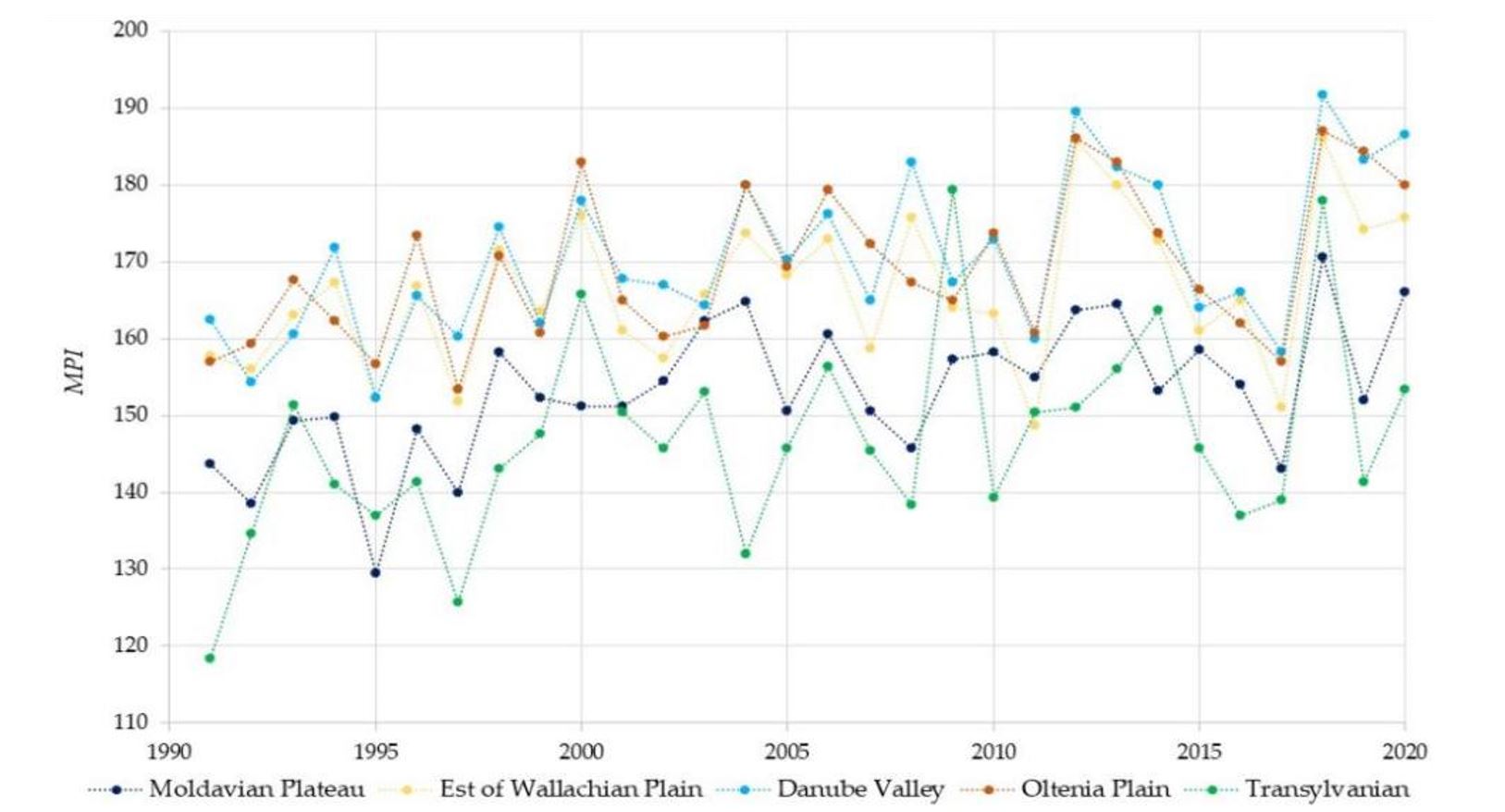
Figure 5 – Hatching potential index of Culex mosquito eggs (MPI) in Romanian regions between 1990 and 2020
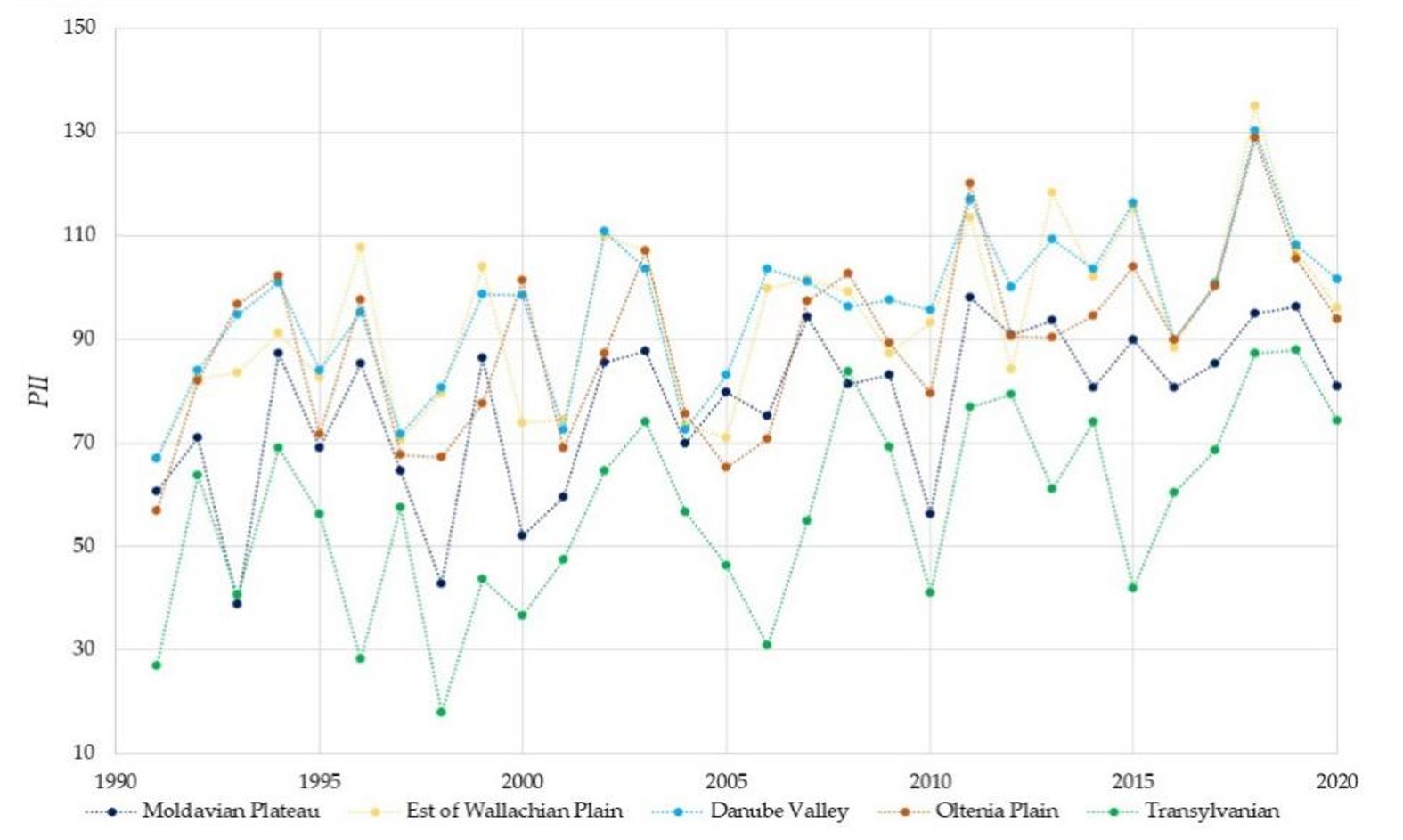
Figure 6 – Potential West Nile virus infestation index (PII) of Romanian regions between 1990 and 2020
This indicates that in 1996, the number of mosquito populations was extremely high, which led to WNV outbreaks. However, in 2018, the number of days favourable for the replication of the virus inside the mosquito was greater. Thus, in 2018, 85, 101, 100, 101 and 69 favourable days, respectively, were observed, compared to 43, 80, 67, 81 and 18 days in 1996 for the Moldova Plateau, Wallachian Plain, Oltenia Plain, Danube Valley and Transylvania.
When comparing the years with large numbers of West Nile fever cases, it is mandatory to constantly calculate not only the periods with risk of virus transmission but also those with conditions conducive to the hatching of mosquito eggs, which suggests the number of populations; both factors are extremely important in preventing WNV epidemics. In addition, in 1996, the winter temperatures were milder, which confirms the data in the literature, according to which high temperatures during winter are an important indicator of WNV outbreaks in the following year as the virus can survive inside the mosquitoes. This indicator must therefore also be taken into account when developing monitoring, prevention and control strategies (Table 2).
The second set of climatological parameters analysed is based on the humidity conditions necessary for the growth and development of mosquito populations. For this reason, we analysed the monthly amounts of precipitation and the number of days in which precipitation occurred in the five regions from which infections were reported.
Although drought can be a predictor for WNV outbreaks, when analysing the amount of precipitation in the years when large numbers of cases were recorded, i.e., 1996 and 2018, these were not dry years, with precipitation values between 520 and 720 mm (1 mm = 1 L/m²) in 1996 and between 520 and 800 mm in 2018 (Figure 7). Regarding the precipitation amount recorded, the years with major outbreaks of WNV were neither dry nor rainy, i.e., the conditions did not favour the development of the formation of large mosquito populations. Our results do not indicate that the atmospheric precipitation played an important role in the occurrence of WNV outbreaks, but it is important for maintaining the humidity of the soil and, especially, of the humid areas conducive to the development of mosquitoes. The number of days with precipitation varied between 110 and 130 days in 1996 and between 115 and 135 days in 2018, without showing ideal conditions for virus replication or for hatching (Figure 8). Our results suggest that the period of precipitation, indicated by days within 1 year, does not represent an important indicator for the prediction of WNV outbreaks.
DISCUSSION
The economic situation of a country is highly important because it indicates resources available for infrastructure, surveillance and intervention. In Romania, the rural area does not benefit from air conditioning, tap water and adequate drainage, making people more exposed to mosquito bites.
Several studies have demonstrated the nexus between the number of West Nile cases and a number of socio-economic and demographic factors, such as income, sanitation and population density (DeGroote and Sugumaran, 2012; Lyon et al., 2022; Watts et al., 2021). Higher economic status may favor people’s contact with mosquitoes, for example, owners of houses with green space, swimming pools and ponds or those who may have access to recreational areas, which constitute habitat for mosquito reproduction. (Downling et al., 2013). The best solution remains the implementation of mosquito control by public services, actions which again are directly related to the economic status of the country.
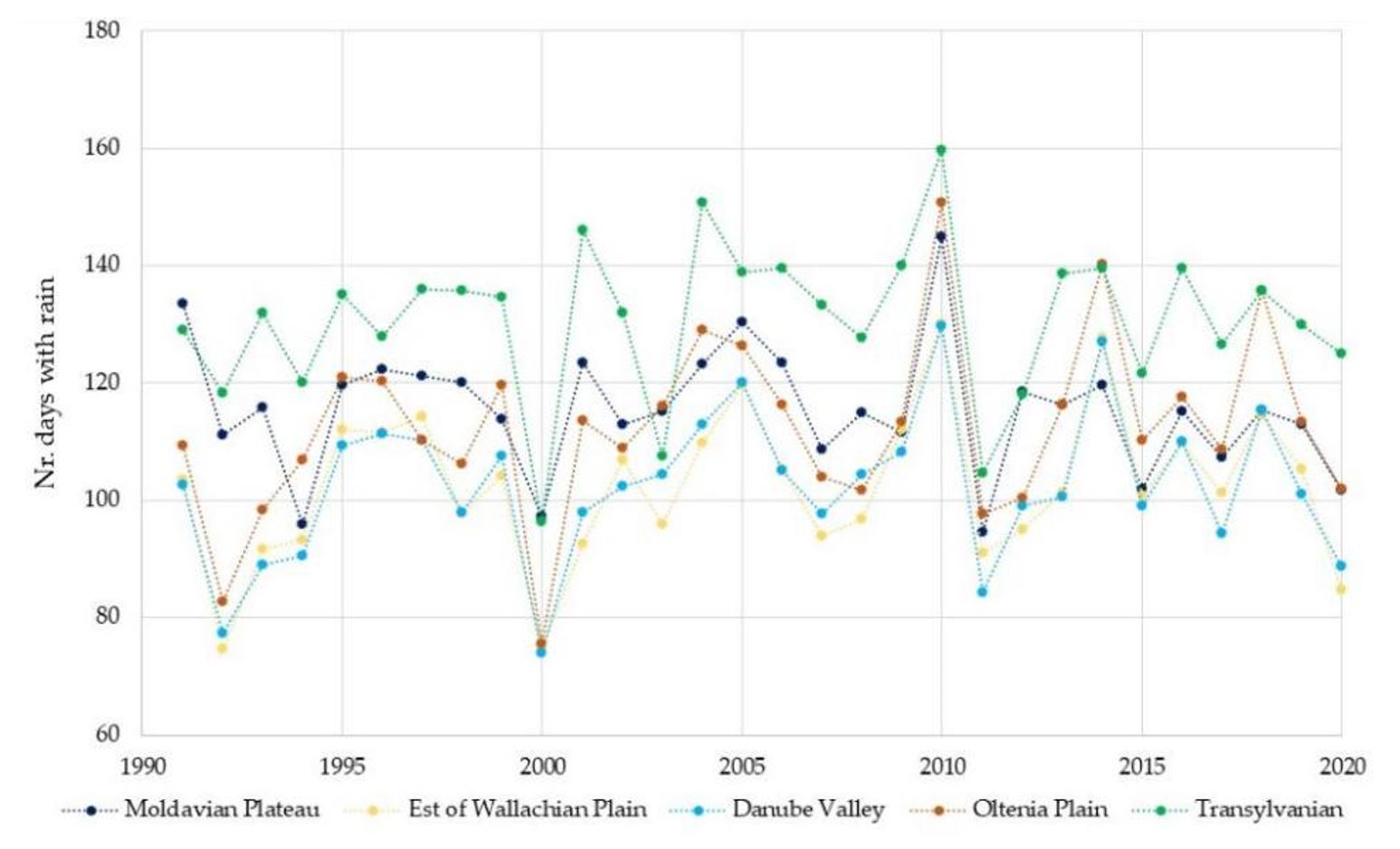
Figure 8 – Numbers of days with precipitation in the low relief forms of Romania between 1991 and 2020
Analysing a study conducted in Romania by Popescu et al. (2018) regarding West Nile cases from 2012 to 2017, there was a lack of laboratory diagnosis of WNV infection; thus, all recorded cases, except for one, were from south-eastern Romania (from areas close to the capital), with 42.6% residing in the capital city, Bucharest. Most cases were diagnosed at ʺVictor Babesʺ Hospital in Bucharest – a hospital for infectious and tropical diseases – indicating that WNV infection is underdiagnosed in Romania. In 2016 and 2017, the highest numbers of cases were also recorded in Bucharest, although from the standpoint of climatic factors, the conditions were more favourable for transmission in Tulcea, both regarding the development of mosquitoes and virus replication inside mosquitoes.
In Europe, including Romania, in 2018, there was an increase in WNV neuroinfection outbreaks in humans (Becker et al., 2012; Farooq et al., 2022; ECDC, 2022; Marini et al., 2020; Nicolescu et al., 2016; Popescu et al., 2018).
In Romania, we compared the 2 years in which major outbreaks of West Nile occurred, namely 1996 and 2018. In these years, the conditions for the development of mosquito populations were favourable, with a high number of favourable days for egg hatching in 1996 and favourable conditions for the transmission of the WNV, relative to the number of days it can replicate inside the mosquito, in the year 2018. However, in terms of climatic factors, there were excessive heat anomalies in the years with WNV outbreaks, which could explain such a large number of cases.
Our study took into account the main vector of the WNV in Romania, showing its activity from March to November.
To complete their life cycle, Culex mosquitoes require the presence of surface water, and generally, over time, epidemics have occurred after hurricanes because of the creating of egg-laying sites. However, in the case of the Culex mosquito, it seems that its bioecology is complicated, with a series of studies showing that outbreaks of West Nile infections occurred under conditions with low rainfall or that rain events did not play any role in the intensity of the outbreaks (Becker et al., 2012; Cotar et al., 2016; Soh and Aik, 2021). Temperature has been the most consistent variable for predicting Cx. pipiens dynamics in southern Europe directly with simple linear models; temperature correlations were higher than those for relative humidity, and precipitation showed much less consistent results and weaker correlations. Temperatures above 28°C negatively affect mosquito populations (Chaskopoulou et al., 2016; Gangoso et al., 2020).
Determining the role of precipitation in this type of study is laborious, and we therefore identified the correlation between precipitation and local warming as well as that between precipitation and the number of WN cases in Romania (Pearson´s correlation and principal components analysis). So far, the statistical results do not reveal a link between humidity and the evolution of mosquito populations in the analysed regions. The forecast for 2100 for Romania, using the two scenarios LS and HS, shows in both cases a favourable scenario for the evolution of mosquitoes, with the increase in the number of days per year favourable for egg hatching and for virus replication inside the mosquito. Under HS, there is an extension of the egg hatching period until November, with the risk that adult mosquitoes are active throughout the year, ensuring a high survival rate of the virus into the mosquito, which can lead to outbreaks in the following spring. In addition, under HS, the virus transmission period is extended from April to October, which underlines the need to establish monitoring and control programmes for both mosquito populations and the spread of the virus among animal and human populations.
So far, there are no surveillance programmes underway in Romania, and the recorded cases are only those with symptoms of neuroinfection. Thus, it is an underdiagnosed disease, making it difficult to correlate the number of recorded cases with climatic factors favourable to the development of mosquito vectors as well as the development of the virus inside the mosquito.
Consequently, for a programme to monitor West Nile cases, the factors involved in the development of mosquito populations must be calculated, in addition to those necessary for the sufficient replication of the virus to produce the disease since they can lead to outbreaks of neuroinfection induced by the WNV.
Previous studies have shown that certain climatic conditions, such as precipitation and temperature anomalies, WNV transmission can be predicted. In Romania, the data we obtained for 2018 do not indicate temperature anomalies which would explain the West Nile epidemic in humans, which underlines the fact that the prevention and control of West Nile outbreaks are much more complex. Therefore, besides the continuous monitoring of climatic factors, the monitoring of the virus in birds, horses and humans is also mandatory.
There is an emphasis on the need for a One Health-type approach in the control and prevention of vector-borne diseases, which should follow the monitoring of mosquito populations involved in virus transmission. In the case of Culex, monitoring the presence of the pathogen inside the vector and its presence in the human and animal population is important, and these factors are related to climatic factors, temperature and precipitation.
CONCLUSIONS
Based on our results, we conclude that not all factors involved in the risks of WNV outbreaks are known and that sustained and in-depth research is required not only at the national but also at the continental level. In 2018, in Romania, the weather conditions were not favourable for WNV outbreaks, yet numerous cases were reported throughout Europe.
The difference between residential and non-residential areas is large, which makes it difficult to establish favourable periods for the growth of mosquito populations and the development of the WNV within the vector, with temperature remaining the most consistent predictor which may favour the development of West Nile vectors regardless of rainfall and humidity. Thus, calculating the conditions favourable to the development of Culex mosquito populations must be done in restricted areas, taking into account relief, the degree of human occupation and irrigation. The monitor the risk of West Nile outbreaks, periodic serological screening should also be done among horse and bird populations, in addition to the human population. For 2100, the hatching period is predicted to last until November, ensuring a high survival rate of the virus inside the mosquito and leading to outbreaks the following spring. Also, the transmission period of the virus is extended from April to October, emphasising the need for year-round mosquito vector surveillance programmes as well as population testing for the WNV.
We need to understand that WNV transmission and maintenance in nature are highly complex, emphasising the need for in-depth studies and the permanent monitoring of each factor contributing to disease outbreaks. The model proposed here can be improved by the introduction of more calculation parameters, such as the population of mosquitoes, birds and horses, and a clear distinction must be made among urban, rural and agricultural areas. The study draws attention to the need to introduce tailored programmes to monitor and control vectors as well as the pathogens transmitted by them, with the aim to predict the risk of outbreaks and initiate timely intervention. However, the mathematical model used by us can allow the establishment of favourable periods for the appearance of mosquito populations as well as the risk of WNV transmission.
Author Contributions: Conceptualisation, L.M. and L.M.I.; methodology, I.B., L.M. and L.M.I.; software, I.B.; validation, L.M., L.M.I. and I.B.; formal analysis, S.M.; investigation, R.M., I.L. and S.M.; resources, G.M., R.M., S.M.; data curation, L.M.I., I.B.; writing – original draft preparation, L.M., I.B. and L.M.I.; writing – review and editing, L.M., I.B. and L.M.I.; visualisation, L.M. and I.B.; supervision, L.M.; project administration, L.M.; funding acquisition, R.M. and G.M. L.M., L.M.I. and G.M. contributed equally to this work and share first authorship All authors have read and agreed to the published version of the manuscript. L.M. and L.M.I. contributed equally to this work and share first authorship.
Funding: There was no external funding for this study.
Acknowledgements: The authors would like to thank the Stiefel Eurocart SRL, www.stiefel.ro (Oșorhei 417360 Bihor County, Romania), for providing the physical map of Romania (Agreement nr. 55/31.01.2023).
Conflicts of Interest: There is no conflict of interest.
REFERENCES
Anderson, S.L.; Richards, S.L.; Tabachnick, W.J.; Smartt, C.T. Effects of West Nile Virus dose and extrinsic incubation temperature on temporal progression of vector competence in Culex Pipiens Quinquefasciatus. Journal of the American Mosquito Control Association. 2010, 26, 103-107. https://doi.org/10.2987%2F09-5926.1.
Becker, N.; Jöst, A.; Weitzel, T. The Culex Pipiens Complex in Europe. Journal of the American Mosquito Control Association. 2012, 28, 53-67. https://doi.org/10.2987/8756-971X-28.4s.53.
Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, second edition.; Springer Heidelberg: Dordrecht, New York. 2010, pp.13.
Bravo-Barriga, D.; Parreira, R.; Almeida, A.P.G.; Calado, M.; Blanco-Ciudad, J.; Serrano-Aguilera, F.J.; Pérez-Martín, J.E.; Sánchez-Peinado, J.; Pinto, J.; Reina, D.; Frontera, E. Culex Pipiens as a Potential Vector for Transmission of Dirofilaria Immitis and Other Unclassified Filarioidea in Southwest Spain. Veterinary Parasitology. 2016, 223, 173-180. https://doi.org/10.1016/j.vetpar.2016.04.030.
Brugman, V.A.; Hernández-Triana, L.M.; Medlock, J.M.; Fooks, A.R.; Carpenter, S.; Johnson, N. The Role of Culex Pipiens L. (Diptera: Culicidae) in Virus Transmission in Europe. International Journal of Environmental Research and Public Health. 2018, 15, 389. https://doi.org/10.3390/ijerph15020389
Chaskopoulou, A.; L’Ambert, G.; Petric, D.; Bellini, R.; Zgomba, M.; Groen, T.A.; Marrama, L.; Bicout, D.J. Ecology of West Nile Virus across Four European Countries: Review of Weather Profiles, Vector Population Dynamics and Vector Control Response. Parasites Vectors. 2016, 9, 482. https://doi.org/10.1186/s13071-016-1736-6.
Ciota, A.T.; Ehrbar, D.J.; Matacchiero, A.C.; Van Slyke, G.A.; Kramer, L.D. The Evolution of Virulence of West Nile Virus in a Mosquito Vector: Implications for Arbovirus Adaptation and Evolution. BMC Evolutionary Biology. 2013, 13, 71. https://doi.org/10.1186/1471-2148-13-71.
Cotar, A.I.; Falcuta, E.; Prioteasa, L.F.; Dinu, S.; Ceianu, C.S.; Paz, S. Transmission Dynamics of the West Nile Virus in Mosquito Vector Populations under the Influence of Weather Factors in the Danube Delta, Romania. EcoHealth. 2016, 13, 796-807. https://doi.org/10.1007/s10393-016-1176-y.
Crivei, L.A.; Moutailler, S.; Gonzalez, G.; Lowenski, S.; Crivei, I.C.; Porea, D.; Anita, D.C.; Ratoi, I.A.; Zientara, S.; Oslobanu, L.E.; Tomazatos, A.; Savuta, G.; Lecollinet, S. Detection of West Nile Virus Lineage 2 in Eastern Romania and First Identification of Sindbis Virus RNA in Mosquitoes Analyzed using High-Throughput Microfluidic Real-Time PCR. Viruses. 2023, 15, 186. https://doi.org/10.3390/v15010186.
DeGroote, J.P.; Sugumaran, R. National and Regional Associations Between Human West Nile Virus Incidence and Demographic, Landscape, and Land Use Conditions in the Coterminous United States. Vector-Borne and Zoonotic Diseases. 2012, 12, 657-665. https://doi.org/10.1089/vbz.2011.0786.
Di Pol, G.; Crotta, M.; Taylor, R.A. Modelling the temperature suitability for the risk of West Nile Virus establishment in European Culex pipiens populations. Transbound Emerg Dis 69. 2022, e1787-e1799. https://doi.org/10.1111/tbed.14513.
Dowling, Z.; Ladeau, S.L.; Armbruster, P.; Biehler, D.; Leisnham, P.T. Socioeconomic Status Affects Mosquito (Diptera: Culicidae) Larval Habitat Type Availability and Infestation Level. Journal of Medical Entomology. 2013, 50, 764-772. https://doi.org/10.1603/ME12250.
Ebi, K.L.; Lindgren, E.; Suk, J.E.; Semenza, J.C. Adaptation to the Infectious Disease Impacts of Climate Change. Climatic Change. 2013, 118, 355-365. https://doi.org/10.1007/s10584-012-0648-5.
EPA. Climate Change Indicators: West Nile Virus. https://www.epa.gov/climate-indicators/climate-change-indicators-west-nile-virus (acecsed on 10 June 2023).
ECDC. Epidemiological Update: West Nile Virus Transmission Season in Europe. https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2018 (accessed on 02 December 2022).
Farajollahi, A.; Fonseca, D. M.; Kramer, L. D.; Marm Kilpatrick, A. “Bird Biting” Mosquitoes and Human Disease: A Review of the Role of Culex Pipiens Complex Mosquitoes in Epidemiology. Infection, Genetics and Evolution. 2011, 11, 1577-1585. https://doi.org/10.1016/j.meegid.2011.08.013.
Farooq, Z.; Rocklöv, J.; Wallin, J.; Abiri, N.; Sewe, M.O.; Sjödin, H.; Semenza, J.C. Artificial Intelligence to Predict West Nile Virus Outbreaks with Eco-Climatic Drivers. The Lancet Regional Health – Europe 2022, 17. https://doi.org/10.1016/j.lanepe.2022.100370.
Fay, R.L.; Keyel, A.C.; Ciota, A.T. Chapter Three – West Nile Virus and Climate Change. In Advances in Virus Research; Roossinck, M. J., Ed.; Viruses and Climate Change; Academic Press, 2022; Vol. 114, pp 147–193. https://doi.org/10.1016/bs.aivir.2022.08.002.
Fritz, M.L.; Walker, E.D.; Miller, J.R.; Severson, D.W.; Dworkin, I. Divergent Host Preferences of Above- and below-Ground Culex Pipiens Mosquitoes and Their Hybrid Offspring. Medical and Veterinary Entomology. 2015, 29, 115-123. https://doi.org/10.1111/mve.12096.
Gangoso, L.; Aragonés, D.; Martínez-de la Puente, J.; Lucientes, J.; Delacour-Estrella, S.; et al. Determinants of the Current and Future Distribution of the West Nile Virus Mosquito Vector Culex Pipiens in Spain. Environmental Research. 2020, 188, 109837. https://doi.org/10.1016/j.envres.2020.109837.
Gates, M.C.; Boston, R.C. Irrigation Linked to a Greater Incidence of Human and Veterinary West Nile Virus Cases in the United States from 2004 to 2006. Preventive Veterinary Medicine. 2009, 89, 134-137. https://doi.org/10.1016/j.prevetmed.2008.12.004.
Haba, Y., McBride, L. Origin and status of Culex pipiens mosquito ecotypes. Current Biology. 2022, 32, R237–R246. https://doi.org/10.1016/j.cub.2022.01.062.
Hoover, K.C.; Barker, C.M. West Nile Virus, Climate Change, and Circumpolar Vulnerability. WIREs Climate Change. 2016, 7, 283–300. https://doi.org/10.1002/wcc.382.
Hotez, P.J. Southern Europe’s Coming Plagues: Vector-Borne Neglected Tropical Diseases. PLOS Neglected Tropical Diseases. 2016, 10, e0004243. https://doi.org/10.1371/journal.pntd.0004243.
Hubálek, Z.; Halouzka, J. West Nile Fever–a Reemerging Mosquito-Borne Viral Disease in Europe. Emerging Infectious Diseases. 1999, 5, 643-650.
IMO. (International Meteorological Organization). In Provisional Guide to Meteorological Instrument and Observing Practice, Chapters 1 to 10; IMO Publication: Imprimerie La Concorde: Lausanne, 1950; Vol. 78.
Kampen, H.; Tews, B.A.; Werner, D. First Evidence of West Nile Virus Overwintering in Mosquitoes in Germany. Viruses. 2020, 13, 2463. https://doi.org/10.3390/v13122463.
Klein Tank, A. M. G.; Wijngaard, J. B.; Können, G. P.; Böhm, R.; Demarée, G.; Gocheva, A.; et al. Daily Dataset of 20th-Century Surface Air Temperature and Precipitation Series for the European Climate Assessment. International Journal of Climatology. 2002, 22, 1441-1453. https://doi.org/10.1002/joc.773.
Kunkel, K.E.; Stevens, L.E.; Stevens, S.E.; Sun, L. Regional Climate Trends and Scenarios for the U.S. National Climate Assessment Part 4. Climate of the U.S. Great Plains.
Lyon, C.; Saupe, E.E.; Smith, C.J.; Hill, D.J.; Beckerman, A.P.; Stringer, L.C.; Marchant, R.; McKay, J.; Burke, A.; O’Higgins, P.; Dunhill, A. M.; Allen, B.J.; Riel-Salvatore, J.; Aze, T. Climate Change Research and Action Must Look beyond 2100. Global Change Biology. 2022, 28, 349-361. https://doi.org/10.1111/gcb.15871.
Marini, G.; Calzolari, M.; Angelini, P.; Bellini, R.; Bellini, S.; Bolzoni, L.; Torri, D.; Defilippo, F.; Dorigatti, I.; Nikolay, B.; Pugliese, A.; Rosà, R.; Tamba, M.A. Quantitative Comparison of West Nile Virus Incidence from 2013 to 2018 in Emilia-Romagna, Italy. PLOS Neglected Tropical Diseases. 2020, 14, e0007953. https://doi.org/10.1371/journal.pntd.0007953.
Martínez-de la Puente, J.; Ferraguti, M.; Ruiz, S.; Roiz, D.; Soriguer, R.C.; Figuerola, J. Culex Pipiens Forms and Urbanization: Effects on Blood Feeding Sources and Transmission of Avian Plasmodium. Malaria Journal. 2016, 15, 589. https://doi.org/10.1186/s12936-016-1643-5.
Naish, S.; Dale, P.; Mackenzie, J.S.; McBride, J.; Mengersen, K.; Tong, S. Climate Change and Dengue: A Critical and Systematic Review of Quantitative Modelling Approaches. BMC Infectious Diseases. 2014, 14, 167. https://doi.org/10.1186/1471-2334-14-167.
NCSCCD. (Database of Romania National Centre for Surveillance and Control of Communicable Diseases) within the Institute of Public Health (IPH). https://www.cnscbt.ro/ (accessed on 21 March 2023).
Nicolescu, G.M.; Ciulacu, V.S.P.; Vladimirescu, A.; Coipan, E.C.; Petrisor, A.I.; Dumitrescu, G.; Saizu, D.; Savin, E.; Sandric, I.; Mihai, F. Emergence Risk and Surveillance of West Nile Virus Infections in Romania. International Journal of Infectious Diseases 2016, 53, 158-159. https://doi.org/10.1016/j.ijid.2016.11.387.
O’Neill, B.C.; Tebaldi, C.; van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.-F.; Lowe, J.; Meehl, G.A.; Moss, R.; Riahi, K.; Sanderson, B.M. The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6. Geoscientific Model Development. 2016, 9, 3461-3482. https://doi.org/10.5194/gmd-9-3461-2016.
Olesen, O.F.; Ackermann, M. Increasing European Support for Neglected Infectious Disease Research. Computational and Structural Biotechnology Journal. 2017, 15, 180-184. https://doi.org/10.1016/j.csbj.2017.01.007.
Ometto, T.; Durigon, E.L.; de Araujo, J.; Aprelon, R.; de Aguiar, D.M.; Cavalcante, G.T.; et al. West Nile Virus Surveillance, Brazil, 2008–2010. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2013, 107, 723-730. https://doi.org/10.1093/trstmh/trt081.
Patz, J.A.; Epstein, P.R.; Burke, T.A.; Balbus, J.M. Global Climate Change and Emerging Infectious Diseases. JAMA. 1996, 275, 217–223. https://doi.org/10.1001/jama.1996.03530270057032.
Patz, J.A.; Martens, W.J.; Focks, D.A.; Jetten, T.H. Dengue Fever Epidemic Potential as Projected by General Circulation Models of Global Climate Change. Environmental Health Perspectives. 1998, 106, 147-153. https://doi.org/10.1289/ehp.98106147.
Paz, S. Climate Change Impacts on West Nile Virus Transmission in a Global Context. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015, 370, 20130561. https://doi.org/10.1098/rstb.2013.0561.
Paz, S.; Semenza, J.C. Environmental Drivers of West Nile Fever Epidemiology in Europe and Western Asia-A Review. International Journal of Environmental Research and Public Health. 2013, 10, 3543-3562. https://doi.org/10.3390/ijerph10083543
Petrović, T.; Šekler, M.; Petrić, D.; Vidanović, D.; Debeljak, Z.; Lazić, G.; Lupulović, D.; Kavran, M.; Samojlović, M.; Ignjatović Ćupina, A.; Tešović, B.; Lazić, S.; Kolarević, M.; Labus, T.; Djurić, B. Intensive West Nile Virus Circulation in Serbia in 2018—Results of Integrated Surveillance Program. Pathogens. 2021, 10, 1294. https://doi.org/10.3390/pathogens10101294.
Popescu, C.P.; Florescu, S.A.; Cotar, A.I.; Badescu, D.; Ceianu, C.S.; Zaharia, M.; Tardei, G.; Codreanu, D.; Ceausu, E.; Ruta, S.M. Re-Emergence of Severe West Nile Virus Neuroinvasive Disease in Humans in Romania, 2012 to 2017–Implications for Travel Medicine. Travel Medicine and Infectious Disease. 2018, 22, 30-35. https://doi.org/10.1016/j.tmaid.2018.03.001.
Reisen, W.K.; Fang, Y.; Martinez, V.M. Effects of Temperature on the Transmission of West Nile Virus by Culex Tarsalis (Diptera: Culicidae). Journal of Medical Entomology. 2006, 43, 309-317. https://doi.org/10.1093/jmedent/43.2.309.
Sauer, F.G.; Grave, J.; Lühken, R.; Kiel, E. Habitat and Microclimate Affect the Resting Site Selection of Mosquitoes. Medical and Veterinary Entomology 2021, 35, 379-388. https://doi.org/10.1111/mve.12506.
Savini, G.; Capelli, G.; Monaco, F.; Polci, A.; Russo, F.; Di Gennaro, A.; Marini, V.; Teodori, L.; Montarsi, F.; Pinoni, C.; Pisciella, M.; Terregino, C.; Marangon, S.; Capua, I.; Lelli, R. Evidence of West Nile virus lineage 2 circulation in Northern Italy. Veterinary Microbiology. 2012, 158, 267-273. https://doi.org/10.1016/j.vetmic.2012.02.018.
Soh, S.; Aik, J. The Abundance of Culex Mosquito Vectors for West Nile Virus and Other Flaviviruses: A Time-Series Analysis of Rainfall and Temperature Dependence in Singapore. Science of The Total Environment. 2021, 754, 142420. https://doi.org/10.1016/j.scitotenv.2020.142420.
Tabachnick, W. J. Challenges in Predicting Climate and Environmental Effects on Vector-Borne Disease Episystems in a Changing World. Journal of Experimental Biology. 2010, 213, 946-954. https://doi.org/10.1242/jeb.037564.
Tabachnick, W.J. Climate Change and the Arboviruses: Lessons from the Evolution of the Dengue and Yellow Fever Viruses. Annual Review of Virology. 2016, 3, 125-145. https://doi.org/10.1146/annurev-virology-110615-035630.
Tackett, J.; Charnigo, R.; Caldwell, G. Relating West Nile Virus Case Fatality Rates to Demographic and Surveillance Variables. Public Health Reports. 2006, 121, 666-673. https://doi.org/10.1177/003335490612100606.
Tesh, R.B.; Parsons, R.; Siirin, M.; Randle, Y.; Sargent, C.; Guzman, H.; Wuithiranyagool, T.; Higgs, S.; Vanlandingham, D.L.; Bala, A.A.; Haas, K.; Zerinque, B. Year-Round West Nile Virus Activity, Gulf Coast Region, Texas and Louisiana. Emerging Infectious Diseases. 2004, 10, 1649-1652. https://doi.org/10.3201/eid1009.040203.
Tsioka, K.; Gewehr, S.; Kalaitzopoulou, S.; Pappa, S.; Stoikou, K.; Mourelatos, S.; Papa, A. Detection and molecular characterization of West Nile virus in Culex pipiens mosquitoes in Central Macedonia, Greece, 2019–2021. Acta Tropica. 2022, 230, 106391. https://doi.org/10.1016/j.actatropica.2022.106391.
Tolle, M.A. Mosquito-Borne Diseases. Current Problems in Pediatric and Adolescent Health Care. 2009, 39, 97-140. https://doi.org/10.1016/j.cppeds.2009.01.001.
Tran, A.; Sudre, B.; Paz, S.; Rossi, M.; Desbrosse, A.; Chevalier, V.; Semenza, J.C. Environmental Predictors of West Nile Fever Risk in Europe. International Journal of Health Geographics. 2014, 13, 26. https://doi.org/10.1186/1476-072X-13-26.
Turell, M.J.; Dohm, D.J.; Sardelis, M.R.; O’guinn, M.L.; Andreadis, T.G.; Blow, J.A. An Update on the Potential of North American Mosquitoes (Diptera: Culicidae) to Transmit West Nile Virus. Journal of Medical Entomology. 2005, 42, 57-62. https://doi.org/10.1093/jmedent/42.1.57.
Vogels, C.B.F.; van de Peppel, L.J.J.; van Vliet, A.J.H.; Westenberg, M.; Ibañez-Justicia, A.; Stroo, A.; Buijs, J.A.; Visser, T.M.; Koenraadt, C.J.M. Winter Activity and Aboveground Hybridization Between the Two Biotypes of the West Nile Virus Vector Culex pipiens. Vector-Borne and Zoonotic Diseases. 2015, 15, 619-626. https://doi.org/10.1089/vbz.2015.1820.
Watts, M.J.; Sarto i Monteys, V.; Mortyn, P.G.; Kotsila, P. The Rise of West Nile Virus in Southern and Southeastern Europe: A Spatial–Temporal Analysis Investigating the Combined Effects of Climate, Land Use and Economic Changes. One Health. 2021, 13, 100315. https://doi.org/10.1016/j.onehlt.2021.100315.
Academic Editor: Prof. Dr. Daniel Simeanu
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Bodale Ilie, Ivănescu Larisa Maria, Martinescu Gabriela-Victoria, Mătiuț Simona, Mîndru Raluca, Miron Liviu Dan