Simul Bhuyan, Sayeed Mahmood Belal Haider, Mrityunjoy Kunda, Abid Husain, Enam Chowdhury, Venkatramanan Senapathi, K. Sivakumar, Manickam Elangovan
ABSTRACT. The current study was carried out at Rezu Khal to determine the ideal area for seaweed farming. Additionally, this investigation uncovered species of commercially productive and lucrative seaweed. Temperature, salinity, pH, dissolved oxygen (DO), conductivity, and Formazin Nephelometric Units (FNU) of surface water ranged from 20.9 to 26.2°C, 24 to 26.2‰, 6.45 to 8.5, 92 to 105%, 33,256 to 64,267 µS/cm, and 11.1 to 42.8, respectively. Phosphate-phosphorus concentrations in surface water were 2.6–7.6 mg/L, 0.04–0.12 mg/L for nitrate-nitrogen, 0.002–0.04 mg/L for nitrite-nitrogen, 0.15–0.83 mg/L for silica, and 0.13–0.28 mg/L for ammonia. Three seaweed species (Gracilaria lemaneiformis, Hypnea musciformes, and Sargassum oligocystum) were cultivated in the selected areas. Two methods (net and long-line) were used for the culture. In this study, 15–20 kg of G. lemaneiformis were harvested every 15 days using the net method. H. musciformes gained 4 to 12 kg every 15 days. Although S. oligocystum thrived nicely, it was challenging to maintain its viability. The findings of this study indicate that seaweed farming is feasible and coastal residents may participate in seasonal income-generating endeavours in coastal waters.
Keywords: culture; effects; physicochemical parameters; seaweed; Rezu Khal.
Cite
ALSE and ACS Style
Bhuyan, Md.S.; Haider, S.M.B.; Kunda, M.; Husain, Sk.A; Chowdhury, E.; Senapathi, V.; Sivakumar, K.; Elangovan, M. Experimental cultivation of seaweed on the coast of Cox’s Bazar, Bangladesh: identifying the effects of environmental parameters on seaweed growth. Journal of Applied Life Sciences and Environment 2023, 56 (3), 413-436.
https://doi.org/10.46909/alse-563108
AMA Style
Bhuyan MdS, Haider SMB, Kunda M, Husain SkA, Chowdhury E, Senapathi V, Sivakumar K, Elangovan M. Experimental cultivation of seaweed on the coast of Cox’s Bazar, Bangladesh: identifying the effects of environmental parameters on seaweed growth. Journal of Applied Life Sciences and Environment. 2023; 56 (3): 413-436.
https://doi.org/10.46909/alse-563108
Chicago/Turabian Style
Bhuyan, Md. Simul, Sayeed Mahmood Belal Haider, Mrityunjoy Kunda, Sk. Abid Husain, Enam Chowdhury, Venkatramanan Senapathi, K. Sivakumar, and Manickam Elangovan. 2023. “Experimental cultivation of seaweed on the coast of Cox’s Bazar, Bangladesh: identifying the effects of environmental parameters on seaweed growth” Journal of Applied Life Sciences and Environment 56, no. 3: 413-436.
https://doi.org/10.46909/alse-563108
View full article (HTML)
Experimental Cultivation of Seaweed on the Coast of Cox s Bazar, Bangladesh: Identifying the Effects of Environmental Parameters on Seaweed Growth
Md. Simul BHUYAN1,2*, Sayeed Mahmood Belal HAIDER3, Mrityunjoy KUNDA2, Sk. Abid HUSAIN4, Enam CHOWDHURY4, Venkatramanan SENAPATHI5, K. SIVAKUMAR5 and Manickam ELANGOVAN6
1Bangladesh Oceanographic Research Institute, Cox’s Bazar-4730, Bangladesh
2Sylhet Agricultural University, Sylhet, Bangladesh; email: kunda.arm@sau.ac.bd
3Bangladesh Fisheries Development Corporation (BFDC), Dhaka-1204, Bangladesh; email: belal_13th@yahoo.com
4Bangladesh Marine Fisheries Association, Dhaka, Bangladesh; email: abidbmfa@yahoo.com; enam@chowdhury.org
5Department of Geology, Alagappa University, Karaikudi- 630003, TN, India; email: venkatramanansenapathi@gmail.com; siva.karthi90@yahoo.com
6Department of Marine Biology, Sethupathi Government Arts College, Achundanvayel, Ramanathapuram, 623502 Tamil Nadu, India; email: marineelango@gmail.com
*Correspondence: simulbhuyan@gmail.com
Received: Sep. 06, 2023. Revised: Nov. 20, 2023. Accepted: Nov. 20, 2023. Published online: Dec. 08, 2023
ABSTRACT. The current study was carried out at Rezu Khal to determine the ideal area for seaweed farming. Additionally, this investigation uncovered species of commercially productive and lucrative seaweed. Temperature, salinity, pH, dissolved oxygen (DO), conductivity, and Formazin Nephelometric Units (FNU) of surface water ranged from 20.9 to 26.2°C, 24 to 26.2‰, 6.45 to 8.5, 92 to 105%, 33,256 to 64,267 µS/cm, and 11.1 to 42.8, respectively. Phosphate-phosphorus concentrations in surface water were 2.6–7.6 mg/L, 0.04–0.12 mg/L for nitrate-nitrogen, 0.002–0.04 mg/L for nitrite-nitrogen, 0.15–0.83 mg/L for silica, and 0.13–0.28 mg/L for ammonia. Three seaweed species (Gracilaria lemaneiformis, Hypnea musciformes, and Sargassum oligocystum) were cultivated in the selected areas. Two methods (net and long-line) were used for the culture. In this study, 15–20 kg of G. lemaneiformis were harvested every 15 days using the net method. H. musciformes gained 4 to 12 kg every 15 days. Although S. oligocystum thrived nicely, it was challenging to maintain its viability. The findings of this study indicate that seaweed farming is feasible and coastal residents may participate in seasonal income-generating endeavours in coastal waters.
Keywords: culture; effects; physicochemical parameters; seaweed; Rezu Khal.
INTRODUCTION
Seaweed is a multicellular macroscopic sea alga with no actual roots, stems, flowers, or leaves that are essential to the aquatic environment (Aasim et al., 2018; Bhuyan, 2023; Bhuyan et al., 2023; Salehi et al., 2019). Tropical, subtropical, and temperate regions have the greatest abundance of seaweed (Barrientos et al., 2021). They grow on rocks, coral, shells, sand, mud, and other plant bodies (Nedumaran, 2017). They often live below the high-water line, adhering to rocks or other hard surfaces, up to a depth of 118 m, in oceans, rivers, lakes, and other bodies of water where 0.1% photosynthetic light is available (Maberly, 2014; Pereira, 2015). Based on their natural colouring or pigmentation, they are typically classified as red (Rhodophyceae), brown (Phaeophyceae), or green (Chlorophyceae) seaweed (Rashad and El-Chaghaby, 2020; Yalçn et al., 2021).
Seaweed grows from November to March in Bangladesh, depending on turbidity, salinity, and temperature (Aziz and Alfasane, 2020; Sarker et al., 2021). By familiarising poor farmers with cost-effective technologies, seaweed culture can be introduced in places appropriate for its development (Siddiqui et al., 2019). Seaweed can be grown by utilising natural materials, such as bamboo and rope (Bokhtiar et al., 2022; Islam et al., 2021). Seaweed culture can be a good sector for coastal villages in Bangladesh because it involves little input, yields high returns, and employs a large number of people. From Cox’s Bazar to the Sundarbans (few locations), there are excellent locations for seaweed cultivation (Hossain et al., 2021; Siddiqui et al., 2019).
Seaweed is a versatile raw material used to make fertilisers, cosmetics, commercial gums, and compounds for the food, drug, and cosmetic sectors (Pati et al., 2016; Pradhan et al., 2022). Furthermore, the seaweed microbiome promotes seaweed growth and health by releasing development- and morphogenesis-promoting factors (Ghaderiardakani et al., 2019), even under abiotic stress (Ghaderiardakani et al., 2020; Hmani et al., 2023). For a long time, seaweed has been a staple diet in many other countries. Green seaweed, such as Enteromorpha sp., Ulva sp., Caulerpa sp., and Codium sp., are used solely as food sources (Dhargalkar, 2015; Pérez-Lloréns, 2019). These are frequently served as fresh salads or as cooked veggies with rice (Shafiuddin, 2019). Fish curry and beef meals, as well as soups and accompaniments, are made using Porphyra (Nori), Laminaria (Kombu), and Undaria (Wakame) (Khan and Satam, 2003). Additionally, seaweed is used in commercially produced burgers, juices, sandwiches, chocolate, ice cream, cakes, salads, biscuits, and chips (Sarkar et al., 2016). Seaweed can provide nutrients that are required for body growth. It might also be a lucrative source of foreign income. In addition to harvesting seafood, cultivating seaweed can provide an alternate source of income. It can be a profitable sector, especially for women (Makame and Shackleton, 2021; Msuya and Hurtado, 2017; Shafiuddin, 2019). It is possible to build a massive industry with endless potential. If industrial entrepreneurs from similar disciplines join forces with the government, they may be able to open the door to a new world in the blue economy, thus enriching the national economy. Increasing the production of non-traditional marine resources requires the employment of contemporary technology (Siddiqui et al., 2019).
Global aquaculture production of seaweed was reported to more than triple from 1995 to 2012, reaching 23.8 million tonnes per year, with China and Indonesia accounting for 81% of global production (FAO, 2014; Kotta et al., 2021). Due to environmental concerns, seaweed production in Europe is entirely dependent on the collection of natural stocks and has declined by about one-third from 2000 to 2012 to around 230,000 tonnes per year (Thomas et al., 2019). In the coming years, cultivation is likely to play a significant role in closing the growing gap between supply and demand. As research programmes and companies build the capacity to grow and process algal biomass, algal production has gained momentum (Ligtvoet et al., 2019; Sandquist et al., 2017). There are still many economic, political, and logistical challenges to overcome, many of which are region specific. Among these is the identification of suitable coastal areas that allow effective cultivation without interfering with current shipping operations (Jackson, 2018; Prutzer, 2019).
Farmers on the shore of Cox’s Bazar frequently cultivate seaweed using off-bottom net and long-line methods (Akhtar et al., 2022; Banik et al., 2023; Farhaduzzaman et al., 2023). Seaweed produced by the floating raft culture method has shown great success in producing seaweed (Sobuj et al., 2023; Yahya et al., 2020). Different environmental parameters affect seaweed growth. The development and composition of seaweed are influenced by salinity, and optimum salinity can promote growth (Kraan, 2018). According to Tresnati et al. (2021), Gracilaria changii growth is greatly impacted by excessive salinity. The measurement range for the pH water value of Eucheuma cottonii seaweed farming is 6.4–6.5 (Rahman et al., 2019). Bui et al. (2018) reported that a pH range of 6–9 is ideal for growing seaweed. Nurdin et al. (2020) stated that 3–7 mg/L of DO is the optimal range for seaweed cultivation. The water’s temperature fluctuates between 28 and 31°C. The production of E. cottonii is very good at temperatures between 27 and 30°C (Aslan, 1991). Water that is too warm is thought to cause seaweed to become unhealthily pale in colour (Sulu et al., 2004). In the meantime, the typical current velocity at seaweed cultivation sites is 1.5–10 cm/s. Current velocities of about 10 cm/s are adequate to support seaweed cultivation potential in nutrient-rich areas (Mustafa et al., 2017).
The natural abundance of seaweed has been documented in Bangladesh’s south-eastern region, and natural seaweed growth on St. Martin’s Island (SMI) is massive (Bhattacharjee and Islam, 2014; Islam et al., 2020, 2021). Within the coastal areas of Bangladesh, there are 138 seaweed species, 18 of which are commercially important (BFRI, 2019). Experiential seaweed cultivation is practical research aimed at developing a sustainable and profitable seaweed-growing method. The present study aimed to identify ideal locations for the cultivation of commercially important seaweed species. It also aimed to develop a sustainable and profitable culture technology for seaweed cultivation.
MATERIALS AND METHODS
Study area
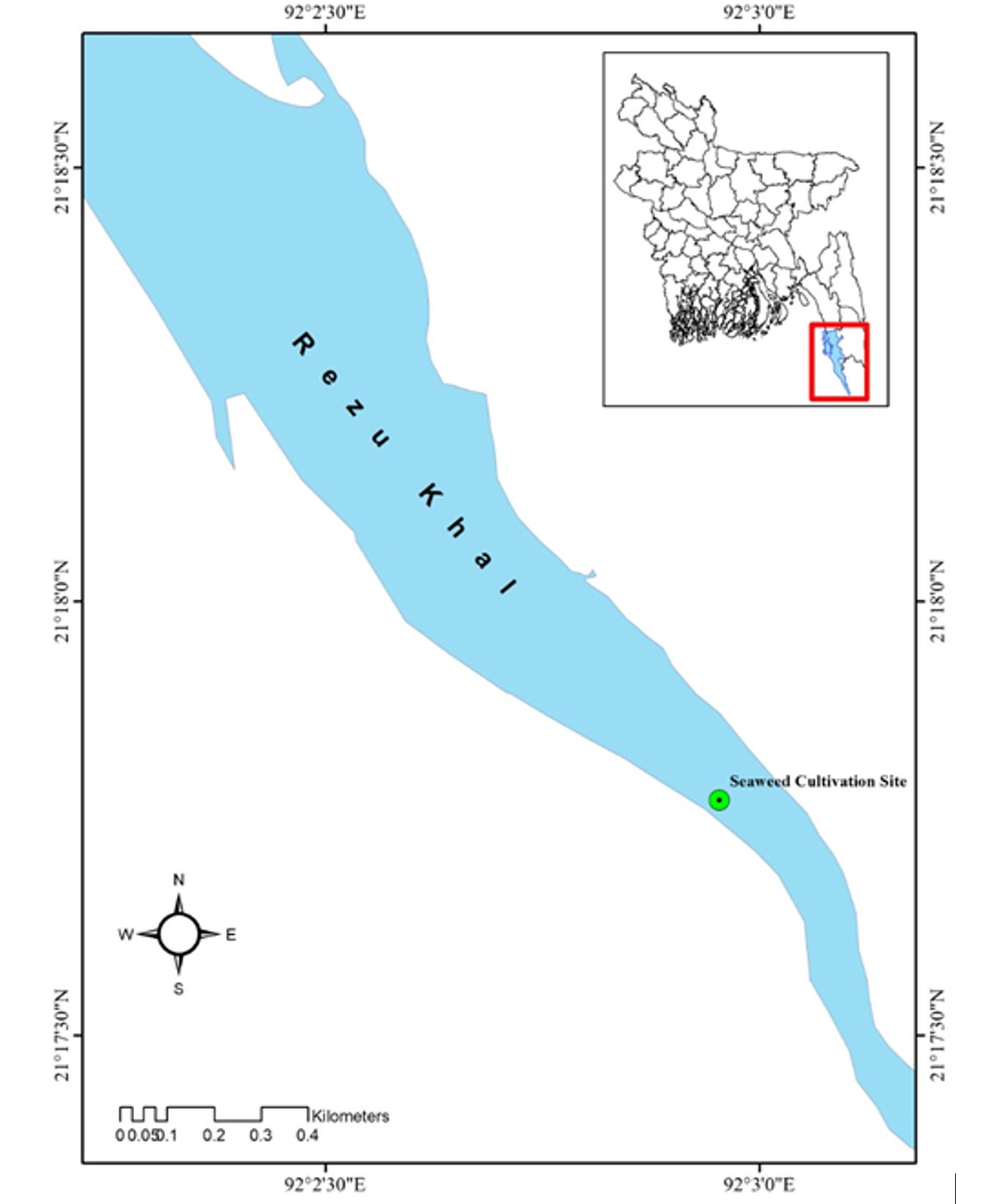
The present experimental seaweed culture was conducted in Rezu Khal along Cox’s Bazar coast (Figure 1). This culture area is very close to the coast, where the salinity remains 23–30 ppt. As a result, saline water enters the Khal. Near the cultivation site, there are several mangrove trees. Resort, in the east, dumps waste into the Khal every day. Hatchery in the west regularly discharges eutrophicated or chemically mixed water into the Khal. At the culture site, the soil is largely sandy, but the Khal bank is mostly muddy. As a result, the sedimentation rate is significant, and the water transparency is low. During both high and low tides, water movement is strong.
Selected seaweed species for culture
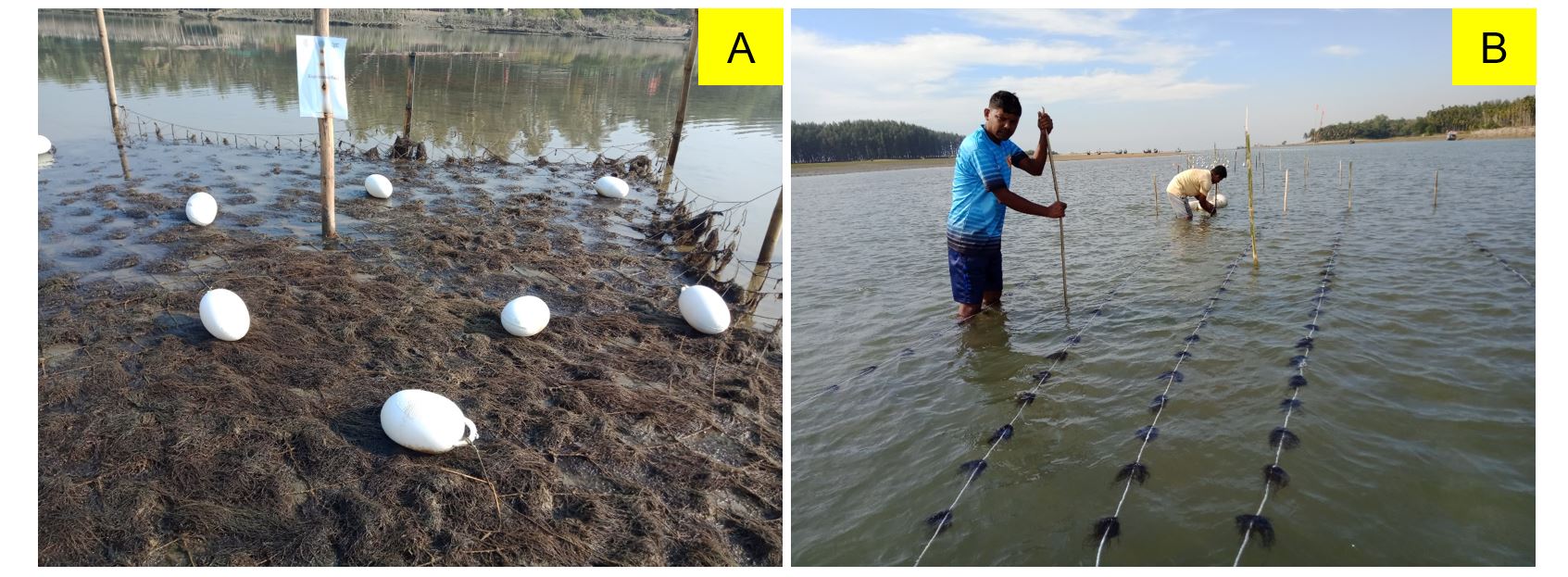
Three seaweed species were selected for culture: Gracilaria lemaneiformis, Hypnea musciformes, and Sargassum oligocystum (Figure 2, A-C). G. lemaneiformis and H. musciformes are red seaweed and have huge economic value. S. oligocystum, which is brown seaweed. These species are extremely valuable in terms of economics. These species can be used to make agar and carrageenan.
Experimental setup
In November, the infrastructure was built. Planting was completed in December. Bamboo was obtained from local residents. Ropes, knives, and other items were bought from a local market. The bamboo was cut and prepared for seaweed cultivation. The experimental plots were identified using identification leaflets. These identification plates helped identify the plot. A signboard was erected with project information. The experimental setup is detailed below.
Culture methods
Two culture methods (e.g., net and long-line) were applied (Figure 3 – A, B). G. lemaneiformis seed was planted using both the net and long-line methods. For the net method, 5 experiment plots (5 m × 5 m) were established for culture. For long-line culture, 5 ropes of 20 m long were used (5 × 2 ropes in two plots). H. musciformes and S. oligocystum were planted using the net method (4 m × 4 m). Two experimental plots were set for each species. Coir rope, jute rope, and bamboo mats were used as culture materials. For the net method, the net was fixed with a bamboo pillar in four corners, and another 4-bamboo pillar was placed in the middle of each side. This extra pillar was used so that the net could withstand strong currents. Another bamboo pillar was used in the middle of the net to create a strong structure. The rope was used to tighten the net with a bamboo pillar. In the long-line method, two bamboo pillars were used in each corner of the rope. Here, an extra bamboo pillar was used in the middle of the rope (Figure 3 – A, B).
Monitoring of parameters
Water quality parameters were measured to identify their effects on seaweed growth. Different parameters (temperature, salinity, pH, DO, conductivity) were analysed using a multiparameter (YSI Pro DSS, Made in USA). The sediment temperature and pH were measured with a thermometer and a Soil pH Tester (Takemura Electric Works Ltd., Tokyo, Japan). Nutrients were determined with a colorimeter (Nutrient Auto Analyzer, HACH, DR 900., Colorado, USA).
RESULTS AND DISCUSSION
In addition to being a source of nourishment, feed, and medication, seaweed is also a source of bioactive compounds that have nutritious and biological benefits (Lomartire et al., 2021; Yu-Qing et al., 2016). Seaweed cultivation has become increasingly popular around the world in recent years. For Bangladesh’s food, cosmetics, medicines, and fertiliser values in local and worldwide markets, seaweed cultivation has immense promise (Aktar et al., 2020; Shaika et al., 2022).
Growth of seaweed
Hypnea musciformis
In the present study, the production was 11 kg after 15 days of cultivation from 2 plots (each plot 4 × 4 m). This production increased to 13 kg in the next 15 days. The maximum production (22 kg) was recorded on the 45th day of cultivation. The seaweed was harvested every 15 days (Table 1). The high seaweed production found in this study could be due to favourable water factors at the culture site. Islam et al. (2021) reported that Cox’s Bazar coast is suitable for seaweed cultivation due to favourable environmental conditions. H. musciformis is tolerant of a variety of temperatures, salinities, and light levels (Durako and Dawes, 1980). On Cox’s Bazar coast, Islam et al. (2021) found that the biomass yield of H. musciformis was much higher than that of E. intestinalis and P. tetrastromatica. On SMI, Islam et al. (2017) found that the daily growth rate (DGR) of Hypnea sp. (3.21±0.01% day−1) was much higher than that of Inani (0.41±0.06% day−1).
Hoq et al. (2016) discovered the highest DGR of 3.21±0.01% day−1 on the 60th day at SMI and the lowest DGR of 0.41±0.11% day−1 on the 15th day at Inani. SMI produced much more biomass of Hypnea sp. (11.05±0.10 kg fresh wt. m−2) than Bakkhali and Inani. Bakkhali and Inani may be appropriate places for seaweed cultivation, adding a new dimension to Bangladesh’s mariculture prospects (Hoq et al., 2016).
During a 60-day culture period, Zafar (2007) used two types of culture systems and discovered growth rates of 1.06 and 0.95 cm day−1 for Hypnea sp. in SMI.
The growth of H. musciformis after 25 days of cultivation on long-line ropes in the bay of Krusadai Island, India, obtained a four-fold rise in biomass, which is consistent with the findings of Rao and Subbaramaiah (1986). In SMI, growth rates of 1.06 and 0.95 cm day−1 were recorded for Hypnea sp. after 60 days of farming using two methods (net and suspended rope, respectively) (Zafar, 2007). From July through January, a suitable season for H. musciformis cultivation was reported in the Gulf of Mannar, India, with a peak between August and September (Reddy et al., 2006). The growth rate was 2440 g (fresh weight) m−2 for Padina boergesenii after 90 days of cultivation on the Mandapam coast (Ganesan et al., 1999).
Table 1
Growth of Hypnea musciformes in net method cultured in Rezu Khal
|
Net Method (4/4m) |
|||||||
|
Time (Day) |
0th |
15th |
30th |
45th |
60th |
75th |
90th |
|
Experimental plot |
Growth (kg) |
||||||
|
1 |
4 |
5 |
7 |
10 |
12 |
10 |
11 |
|
2 |
4 |
6 |
6 |
12 |
12 |
11 |
10 |
Table 2
Growth of Gracilaria lemaneiformis in net method cultured in Rezu Khal
|
Net Method (5x5m) |
|||||||
|
Time (Day) |
0th |
15th |
30th |
45th |
60th |
75th |
90th |
|
Experimental plot |
Growth (kg) |
||||||
|
1 |
7 |
18 |
20 |
20 |
20 |
15 |
16 |
|
2 |
7 |
15 |
20 |
20 |
19 |
16 |
15 |
|
3 |
7 |
15 |
19 |
20 |
18 |
15 |
15 |
|
4 |
7 |
16 |
18 |
19 |
16 |
16 |
15 |
|
5 |
7 |
16 |
19 |
20 |
18 |
15 |
15 |
Table 3
Growth of Gracilaria lemaneiformis in long-line method cultured in Rezu Khal
|
Long-line Method (10m/5 rope) |
|||||||
|
Time (Day) |
0th |
15th |
30th |
45th |
60th |
75th |
90th |
|
Experimental plot |
Growth (kg) |
||||||
|
1 |
5 |
8 |
10 |
12 |
12 |
7 |
8 |
|
2 |
5 |
7 |
9 |
12 |
13 |
4 |
5 |
H. musciformis produced the lowest biomass production in January and the largest biomass yield in August (Ganesan et al., 2006). An enhanced seedling concentration increased the biomass output. The DGR of the 10 g fwm−1 initial seedling density was higher (7.6–10.9%) and differed substantially from the DGR of the other seedling densities (Ganesan et al., 2006). Summer and autumn were the peak seasons for H. musciformis development along the Moroccan Atlantic coast. The species remained productive virtually all year, with the highest levels (52–77%) in October and the lowest levels (0–2%) in April and June in 1997 and February and April (3–10%) in 1998 (Aziza et al., 2008).
The red alga H. musciformis dry weight levels in Florida’s Atlantic and Gulf of Mexico coastal sites were lowest in late winter and early spring, increased throughout the summer, and peaked in the fall (Durako and Dawes, 1980). In July and November, H. musciformis reached two doublings per day maxima of 0.12 and 0.09, respectively. In July, H. cornutu reached the highest specific growth rate (SGR) of 0.19 doublings day−1, the highest recorded (Friedlander and Zelikovitch, 1984).
Gracilaria lemaneiformis
In this study, 15–20 kg of G. lemaneiformis were harvested every 15 days using the net and long-line method (Table 2 and Table 3). Gracilaria tenuistipitata is an important seaweed species that can adapt to a high range of conditions and is a valuable raw material for making agar (Yarnpakdee et al., 2015). Gracilaria can withstand an extensive range of environmental conditions (Abreu et al., 2011; Raikar et al., 2001). An ideal atmosphere can promote the growth rate of algae by improving nutrient uptake (Hoq et al., 2016). Another element that influences the production of seaweed farming is harvesting time. Timely harvesting ensures a high-quality harvest and a high market value. Temperature, salinity, and light influence the growth and spread of Gracilaria spp. (Raikar et al., 2001). Gracilaria spp. are mainly grown in calm seas, especially in the water of the Gulf of Mannar, India (Reddy et al., 2006). Bokhtiar et al. (2022) reported that different physicochemical parameters (e.g., salinity, temperature, transparency, pH, and DO) were suitable for the cultivation of G. tenuistipitata on Cox’s Bazar coast.
Sargassum oligocystum
Sargassum oligocystum is a dominant species in marine ecology and plays an important ecological role. Compared to kelp, Sargassum has essential and non-essential amino acids, essential fatty acids, and minerals (Redmond et al., 2014). Sargassum also includes phycocolloids, bioactive chemicals, and polyphenols, all of which can be used in nutraceuticals and medicine (Álvarez-Viñas et al., 2019). Holdfast regeneration of the cut fronds allows for several harvests, allowing a seeded line to be produced for two to four years. The first year’s harvest occurs between December and January, whereas the second year’s harvest occurs between October and January (Redmond et al., 2014).
In all stages of cultivation, biofouling and predatory organisms can cause problems. Other algae and invertebrates can adhere to Sargassum and the culture lines, vying for space and resources (Redmond et al., 2014). Sargassum sp. peaks between November and April, when ocean temperatures are at their lowest (22–25°C). A temperature of 24°C is nearly optimal for the development of Sargassum embryos in culture tests. The timing of these different life history events is thought to be synchronised for maximal reproduction and resettlement, as well as providing ideal conditions for embryo growth (De Wreede, 1976). In the present study, there was a challenge to survival. It survived, and growth was good. There is huge potential for this species culture commercially.
Effects of physicochemical parameters on seaweed growth
External and internal variables in seaweed cultivation have a significant impact on the growth rate of seaweed (Gultom et al., 2019). Water quality is one of these crucial variables. In seaweed cultivation, optimal water quality factors are critical (Rusdi et al., 2017; Warnadi et al., 2017). DO levels and fluctuations in water temperature have a significant impact on the productivity rate of organic seaweed cultivation. Variations in temperature and DO caused by weather conditions or other natural factors are positively related to the cultivation rate and productivity of E. cottonii (Rahman et al., 2019). This is quite similar to the present study.
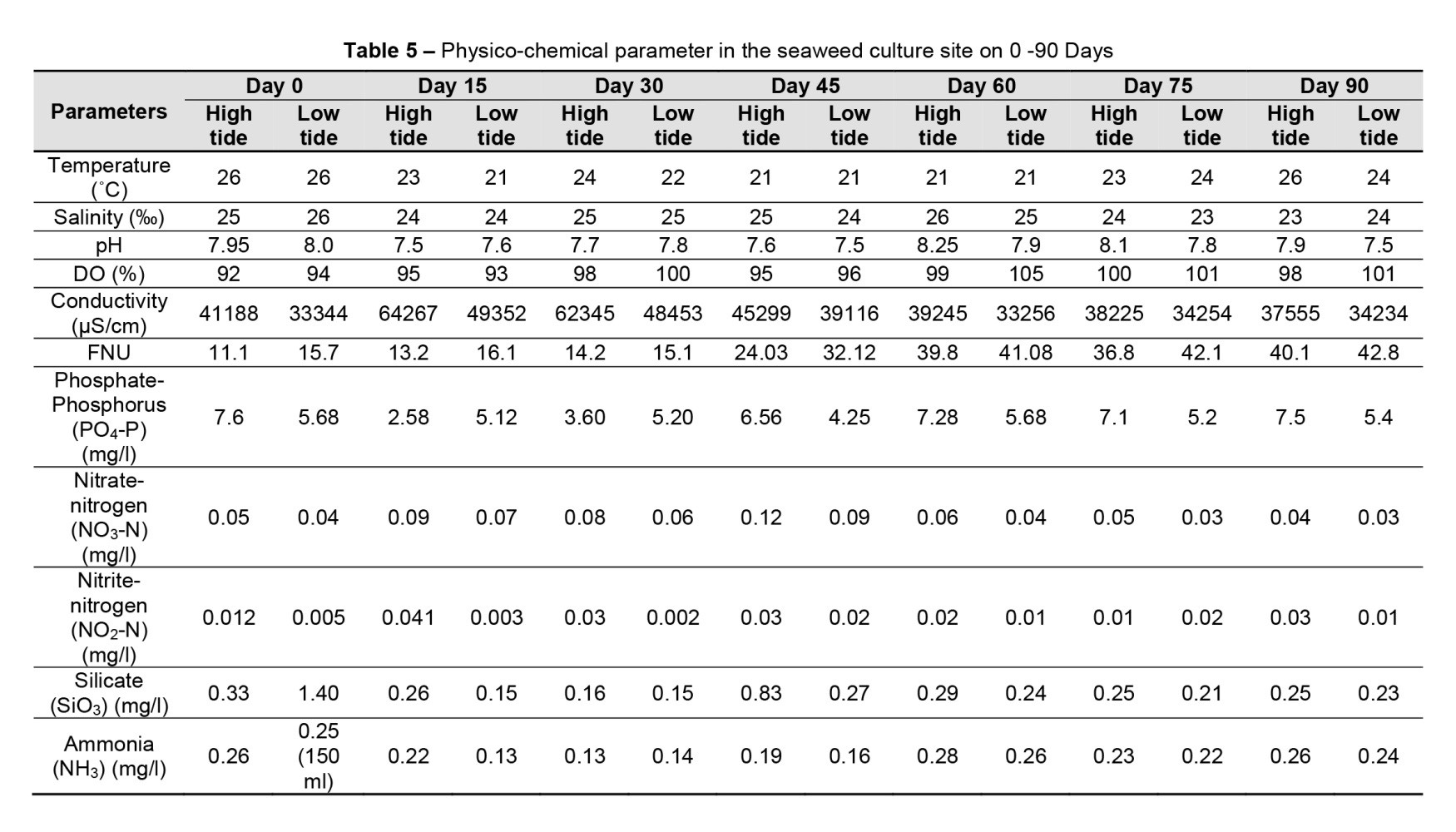
Temperature is crucial for seaweed growth (Eggert, 2012). In the present study, a temperature between 20 and 26°C was best for seaweed production. For this reason, seaweed production was high in the winter season (Table 1). The temperature of the water at the surface is influenced by precipitation, evaporation, moisture, air temperature, wind speed, and sunlight (Monteith, 1981). As a result, seasonal variations in surface temperatures are common (Morain, 1999). The water temperature for seaweed farming is between 27 and 30°C, with daily temperature fluctuations no more than 4°C. Temperature has both direct and indirect effects on photosynthesis in water (Sulistiawati et al., 2020). Temperature has a direct effect on photosynthesis because it regulates enzyme processes. The maximal rate of photosynthetic activity can rise with temperature, whereas changing the hydrological structure of the water column has an indirect effect on the distribution of phytoplankton (Carey et al., 2012).
Although the effect varies from species to species, temperature acts as a potential environmental variable that affects seaweed production. The colour of seaweed is said to turn pale and harmful when the water temperature is too high (Burdames and Ngangi, 2014). In Nuniachara, Cox’s Bazar Sadar, Bokhtiar et al. (2022) recorded a temperature of 22°C. Uddin (2019) measured temperatures ranging from 24.0 to 31.5°C at the Salimpur coast algae farming site. Temperatures of 28-31°C were reported by Rahman et al. (2019) at an Indonesian E. cottonii cultivation site. At seaweed culture sites in Sulawesi Province, Indonesia, Sulistiawati et al. (2020) found an average temperature of 30.5°C. Islam et al. (2017) recorded temperatures from 23.8 to 25.6°C at SMI, 21.7 to 24.1°C at Bakkhali, and 22.1 to 25.5°C at Inani. Because of the ideal temperature range, Hypnea sp. grew well at SMI (Islam et al., 2017). At SMI, Bakkhali, and Inani, Hoq et al. (2016) measured temperatures of 23–25, 25–28, and 23–25°C, respectively. Guist et al. (1982) discovered that maintaining the water temperature at 18–24°C boosted the biomass of H. musciformis by 20%. Ding et al. (2013) discovered that Hypnea grows rapidly at temperatures between 15 and 25°C (Table 4).
Temperatures varied between 20.9 and 26.2°C, which is ideal for culturing seaweed species G. arcuata and G. taxtorii from Japan, which grow best at 20°C. Different seaweed (G. venniculophylla, G. incwvata, G. foliifera, G. corticate) from Japan and India had optimum growth rates at 25°C, while G. edulis and G. lichenoides (from India and Malaysia) had optimal growth at 30°C. Gracilaria venniculophylla can tolerate high temperatures (up to 35°C), although the growth was highest when the temperature was between 18–24°C with continuous water flow and additional N and P (Guist et al., 1982; Raikar et al., 2001).
The salinity of the water varied according to depth; it was more variable at the surface than at the bottom. Because the waters of the research area are combined with freshwater from rivers during the measurement process, the recorded values reveal fairly different results. It plays a crucial role in the survival of aquatic organisms. The salinity levels vary spatiotemporally due to evaporation, sea ice freezing, precipitation, and freshwater inflow from upstream (Tomascik, 1997). Salinity plays a significant role in seaweed growth, and it has been reported that too high and too low salinity affect production. Fluctuations in salinity are caused by a variety of variables, one of which is season (Bricheno et al., 2021).
Salinity is an important determinant of osmotic balance; it is a prudent and potential factor for seaweed cultivation. According to Burdames and Ngangi (2014), the optimal salinity for E. cottonii growth is between 28 and 33 g/L. In field trials, seaweed grows best at hypohaline salinities (Van Ginneken, 2018). In Nuniachara, Cox’s Bazar Sadar, Bokhtiar et al. (2022) found an average value of 32‰. In the seaweed-growing area on the coast of Salimpur, Uddin (2019) found a salinity between 6 and 21‰. Rahman et al. (2019) found a salinity of 30–36‰ at an Indonesian E. cottonii production site. The salinity of seaweed culture sites in Sulawesi Province, Indonesia, ranged from 29.22 to 32.33‰ (Sulistiawati et al., 2020). At SMI, Bakkhali, and Inani, salinities ranged from 31.1 to 32.5, 27.1 to 29.9, and 29.5 to 30.5‰, respectively (Islam et al., 2017). At SMI, Bakkhali and Inani, Hoq et al. (2016) recorded salinities of 31–32, 27–29, and 29–30‰, respectively. Zafar (2007) recorded less Hypnea sp. production at <24‰ and high production at >30‰ at SMI. The summer biomass yield of H. musciformis was reduced by the high salinity of water on the Florida coast (Durako and Dawes, 1980). In the current study, salinity in the Rezu Khal ranged from 24 to 26.2‰, and algal growth was good at this salinity. Therefore, this study showed that a critical variable for the best biomass yield in Rezu Khal was constant and moderate salinity. All Gracilaria spp. have different growth rates depending on salinity, although most of them reach their maximum growth rates at a standard salinity of 35‰ (Raikar et al., 2001).
Osmotic balance (determined by salinity) stimulates nutrient accumulation from water, although the range of optimal salinity varies depending on the species. Phosphate (PO4) is an important nutrient for aquatic plants and has a significant impact on primary productivity. Variations in the PO4 concentration are generally triggered by changes in measurement time and sampling site. Furthermore, PO4 concentration variations can be caused by an increase in organic material in the form of domestic trash (detergents), agricultural waste, or the breakdown of phosphorous rock by water movement. Effendi (2003) reported that most PO4 comes from organic material from the land, e.g., industrial or household waste (detergents). The total PO4 values measured show that the waters of the sampling area with total PO4 values of 0.051–0.1 mg/L are still suitable and optimal for seaweed production (Rugebregt et al., 2020).
Coastal waters with a nitrate (NO3) value of 0.008 mg/L are ideal for aquaculture (Sulistiawati et al., 2020). Excess amounts of NO3 can result from liquid waste from agriculture and plantation activities, as well as inputs from soil and adjacent activities (Indriani and Suminarsih, 2003). The presence of biotic communities that allow NO3 to enter water bodies leads to changes in average NO3 concentrations. NO3 is required more than PO4 for the optimal growth of phytoplankton in water. Electrical storms, N-fixing organisms, and bacteria that consume ammonium (NH4) are all potential sources of NO3. The decomposition of plant or animal waste causes an increase in the NH4 concentration (Effendi, 2003). PO4 and NO3 levels were 0.10–0.21 and 0.22–0.44 mg/L at SMI, 0.22–0.33 and 0.74–1.1 mg/L at Bakkhali, and 0.19–0.26 and 0.25–0.64 mg/L at Inani, respectively, according to Islam et al. (2017). In this investigation, 2.6–7.6 mg/L PO4-P, 0.04–0.12 mg/L NO3-N, and 0.002–0.04 mg/L NO2-N were found at the Rezu Khal seaweed culture site (Table 5).
In Nuniachara, Cox’s Bazar Sadar, Bokhtiar et al. (2022) found an average of 0.632 mg/L NO3-N and 0.443 mg/L NO2-N. In an algae growth site on the Salimpur coast, Uddin (2019) found 0.56 to 0.69 mg/L NO3-N, 0.18 to 0.47 mg/L NO2-N, and 0.90 to 1.10 mg/L PO4-P. In the surface water of a Kappaphycus alvarezii and Spinosum sp. cultivation site in Sulawesi Province, Indonesia, Sulistiawati et al. (2020) found 0.008 to 0.09 mg/L PO4. In the surface water of a seaweed culture site in Sulawesi Province, Indonesia, NO3 levels range from 0.005 to 0.06 mg/L (Sulistiawati et al., 2020). Hoq et al. (2016) found 0.73, 0.67, and 0.51 mg/L NO3-N, while NO2-N were detected ND, 0.45 and 0.23 mg/L at SMI, Bakkhali, and Inani, respectively.
DO is required for aquatic organisms for both metabolic and respiration processes. For all living creatures, DO is a limiting element. DO is a basic requirement for the survival of aquatic organisms.
Table 4
Water quality criteria for seaweed cultivation (Source: Zafar, 2005, 2007)
|
Parameters |
Unsuitable |
Suitable |
Strongly suitable |
|
Depth (m) |
<2 or >15 |
1-2 |
2-15 |
|
Wave (cm) |
0.10->0.40 |
0.10-<0.20 |
0.20-0.30 |
|
Water Transparency (cm) |
<30 |
30-100 |
>100 |
|
Salinity (ppt) |
<4 or >37 |
24-37 |
28-34 |
|
Water Temperature (°C) |
<20 or >30 |
20-24 |
24-2 S |
|
pH |
<6.5 or>8.5 |
6.5-<7.5 |
7.5-8.5 |
|
DO (mg/l) |
<4 or >7 |
6.1-7 |
4-6 |
|
Alkalinity (ppm) |
<45 or >130 |
80-120 |
100-130 |
The difference in DO content is caused by the movement and mixing of water. DO is a natural condition in open water; hence, water conditions that are weak or low in DO are uncommon (Kannel et al., 2007). DO levels ranged from 5.5 to 6.2 mg/L at SMI, 4.1 to 4.9 mg/L at Bakkhali, and 5.0 to 6.1 mg/L at Inani (Islam et al., 2017). DO levels in the Rezu Khal seaweed culture site varied from 92 to 105% in this study (Table 5).
In Nuniachara, Cox’s Bazar Sadar, Bokhtiar et al. (2022) found an average of 7.2 mg/L. In an algae growth site on the Salimpur coast, Uddin (2019) found 3.8 to 5.8 mg/L DO. In an Indonesian E. cottonii-growing site, Rahman et al. (2019) found 5–6.5 mg/L DO. In the surface water of a K. alvarezii and Spinosum sp. culture site in Sulawesi Province, Indonesia, the DO ranges from 5.50 to 7.41 mg/L (Sulistiawati et al., 2020). At SMI, Bakkhali, and Inani, Hoq et al. (2016) found values of 6.9–7.2, 5.8–6.8, and 6.1–7.2 mg/L, respectively. The concentration of DO in SMI was much greater than in Bakkhali and Inani. This is another reason for the increased Hypnea production at SMI.
One of the most important criteria in assessing water stability is acidity or pH. Since each biota has different pH thresholds, changes in water pH affect the longevity of the biota. The input of waste from upstream into the aquatic ecosystem leads to an upsurge in pH from the river mouth to the open ocean.
The pH of marine and coastal waters is usually constant and has a narrow range, mostly between 7 and 8 (Akib et al., 2015). Buffer capacity, i.e., the presence of carbonate and bicarbonate salts, influences pH (Kautsari and Ahdiansyah, 2015). In Nuniachara, Cox’s Bazar Sadar, Bokhtiar et al. (2022) found an average pH of 8.0. In an algal growth site on the coast of Salimpur, Uddin (2019) reported a pH of 7.2–8.4. In an Indonesian E. cottonii-growing site, Rahman et al. (2019) found a pH of 6.4–6.5. The surface water pH at a seaweed-growing site in Sulawesi Province, Indonesia, ranged from 7.71 to 8.10 (Sulistiawati et al., 2020). At SMI, Bakkhali, and Inani, Hoq et al. (2016) found a pH of 8.0–8.5, 7.4–7.5, and 7.6–8, respectively. The lower salinity could explain the significantly lower pH in Bakkhali. A concentration of 3–8 mg/L DO is required to support the seaweed cultivation industry (Tuwo et al., 2020).
Soil quality parameters
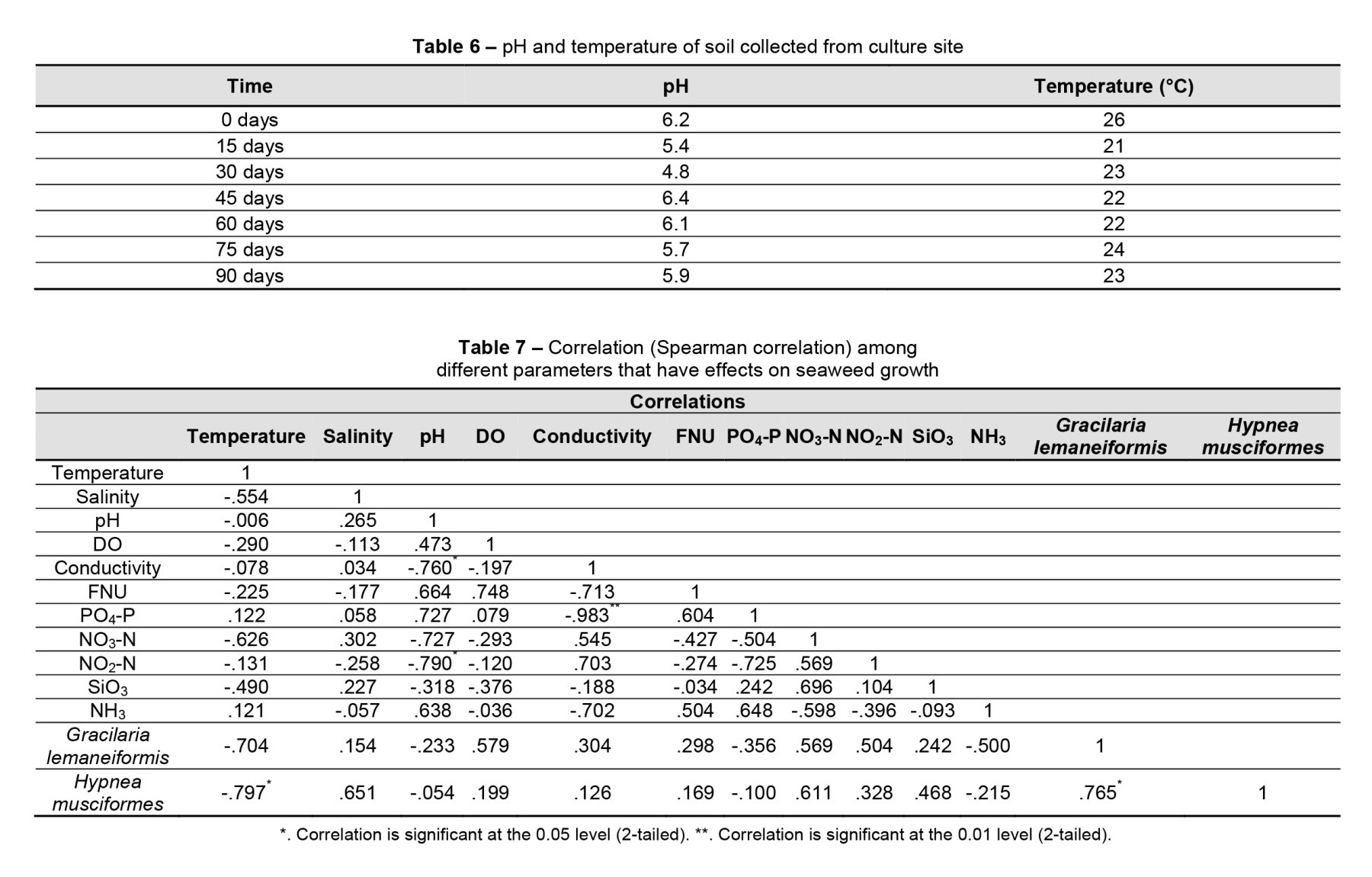
Seaweed cultures alone tend to lower the pH of the system, whereas animal waste is acidic. A good pH balance can be achieved with care (Colt and Huguenin, 2002). The ideal pH range for seaweed cultivation is between 7.0 and 8.5 (Rosyida et al., 2021). The depositional soils on SMI have pH values ranging from 5.23-6.23 (Salam, 2020). Uddin (2019) measured soil pH at a Catenella nipae growing site on the Salimpur coast and found it to be 5.9–6.7. Grant (1981) recorded the inter-tidal soil pH of North Inlet, South Carolina, USA, which ranged from 7.6 to 8.1. In the bottom silt taken from the lower Meghna River estuary, Islam (2016) found pH values ranging from 6.35 to 6.85. The availability of phosphorus is affected by soil pH and organic matter degradation rates. The soil pH at the culture site in this study ranged from 4.8 to 6.4 (Table 6). The maximum pH (6.4) was observed after 45 days of culture, whereas the lowest pH was recorded after 30 days. At the seaweed culture site, soil temperatures ranged from 21 to 26°C. The maximum temperature was recorded early in the culture period, and the minimum temperature was discovered after 15 days of seaweed culture (Table 6).
Multivariate analysis
Variation in water quality parameters
One-way analysis of variance (ANOVA) results (SPSS Analysis) indicates that, during the study period, temporal variation of different parameters such as temperature, salinity, pH, DO, conductivity, FNU, PO4-P, NO3-N, NO2-N, SiO3, and NH3 was normal. There was no significant variation in these parameters in terms of month or tide. For example, temperature (F = 2.173 and p = 0.270), salinity (F = 0.065 and p = 0.975), and NO3-N (F = 0.431 and p = 0.812) had no prevalent effect on seaweed growth since the culture was carried out in winter.
Correlation matrix (CM)
Correlation is a method of determining a possible two-way linear relationship between two continuous variables (Altman, 1990). The strength of the relationship can fall between −1 and +1 and the stronger the correlation, the closer the correlation coefficient comes to ±1 (Mukaka, 2012). Directly related variables give a coefficient with a positive number, while inversely related variables give a coefficient with a negative number (Hinkle et al., 2003). In this study, the Spearman correlation was executed to determine the relationship between seaweed spp. and water parameters. There were some correlations between the water parameters and seaweed (Table 7). G. lemaneiformis had a positive correlation with salinity (r = 0.154), DO (r = 0.579), NO3-N (r = 0.569), and NO2-N (r = 0.504). H. musciformes also had a positive relationship with these parameters (Table 7).
Canonical correspondence analysis (CCA)
The Redundancy Analysis (RDA) triplot was used to describe the preferred abiotic environmental factors for seaweed and indicated the influence of the parameters (Figure 4).

Figure 4 – (A) The redundancy analysis triplot displaying the ecological Relationship between physico-chemical parameters and seaweed growth
Based on the CCA, salinity and pH had a good impact on seaweed growth. Different nutrients (e.g., NO2-N, PO4-P, SiO3) had a good role in seaweed growth.
Hence, a detailed study on the interrelations among the seaweed species and environmental factors was performed, and the triplot in the RDA was supportive by both visualising all the data points plotted in the coordinate system and identifying the interrelationships among species and environmental factors.
CONCLUSIONS
In the present study, Rezu Khal was identified as a suitable site for seaweed cultivation. The growth of G. lemaneiformis and H. musciformes was massive. S. oligocystum survived and grew well but was not found in sufficient numbers. If coastal people become involved in the seaweed culture in Rezu Khal, they can make money. Seaweed cultivation has great potential to contribute to the national economy. It can be a great source of income for coastal dwellers. It can be an alternative source of income for fishermen during a period when fishing is prohibited. Women can play a crucial role in the cultivation and processing of seaweed. Seaweed can change the economic structure of coastal dwellers by improving their livelihoods. The national economy can be transformed by meeting local demand and exports. A few challenges to having a good growth rate were found. A few problems were minimised, and the rest could also be minimised. To fulfil the dream of the Bule economy, seaweed can be an important component.
Author Contributions: M.S.B.: Conceptualisation, Methodology, Statistical analysis, Writing – original draft, Supervision, Funding acquisition, S.M.B.H., M.K.: Supervision, Software, Data curation, Editing, S.A.H., E.C., S.K.: review & editing, V.S.: – Investigation, Visualisation, Writing – review & editing, M.E.: Investigation, Visualisation, Literature survey.
Data availability: This study’s findings are supported by the data presented in the report.
Acknowledgements: The authors offer special thanks to the Business Promotion Council (BPC), Ministry of Commerce, for financial support and to the Biological Oceanography Lab, Bangladesh Oceanographic Research Institute, for technical support. The authors express heartfelt thanks to Bangladesh Marine Fisheries Association (BMFA) for this research facility.
Conflicts of Interest: The authors declare no competing interests.
REFERENCES
Aasim, M.; Bakhsh, A.; Sameeullah, M.; Karataş, M.; Khawar, K.M. Aquatic Plants as Human Food. In Global Perspectives on Underutilized Crops Ozturk, M., Hakeem, K., Ashraf, M., Ahmad, M. (eds). Springer, Cham, 2018, pp. 165-187. http://dx.doi.org/10.1007/978-3-319-77776-4_6.
Abreu, M.H.; Pereira, R.; Buschmann, A.H.; Sousa-Pinto, I.; Yarish, C. Nitrogen uptake responses of Gracilaria vermiculophylla (Ohmi) Papenfuss under combined and single addition of nitrate and ammonium. Journal of Experimental Marine Biology and Ecology. 2011, 407, 190-199. https://doi.org/10.1016/j.jembe.2011.06.034.
Akib, A.; Litaay, M.; Ambeng, A.; Asnady, M. Kelayakankualitas air untukkawasanbudidaya Eucheuma cottoniberdasarkanaspekfisika, kimia dan biologi di Kabupaten Kepulauan Selayar. Jurnal Pesisir dan Laut Tropis. 2015, 3, 25-36. https://doi.org/10.35800/jplt.3.1.2015.9203.
Aktar, S.; Rahman, K.; Hoque, A. Seaweed Cultivation in Bangladesh and Its Role in The Blue Economy. Bangladesh Journal. 2020, 349.
Akhtar, A.; Khan, M.S.; Hasan, M.; Farhaduzzaman, A.M.; Osman, M.H.; Shovon, M.N.H. Baseline Survey Report on Seaweed Cultivation, Processing, and Marketing for Employment Generation in Bangladesh’s Coastal Poor Communities. International Journal of Innovative Science and Research Technology. 2022, 7, 1679-1687. https://doi.org/10.5281/zenodo.7478867.
Altman, D.G. Practical Statistics for Medical Research. (1st Edition). Chapman & Hall/CRC, 1990, pp 624.
Álvarez-Viñas, M.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H. Successful approaches for a red seaweed biorefinery. Marine drugs. 2019, 17, 620. https://doi.org/10.3390/md17110620.
Aslan, L.M. Budidaya rumput laut. Kanisius. Yoyakarta, 1991, 95 hlm.
Aziz, A.; Alfasane, M.A. New records of seaweeds from southeastern coasts of Cox’s Bazar district, Bangladesh. Bangladesh Journal of Plant Taxonomy. 2020, 27, 335-343. http://dx.doi.org/10.3329/bjpt.v27i2.50672.
Aziza, M.; Givernaud, T.; Chikhaoui-Khay, M.; Bennasser, L. Seasonal variation of the growth, chemical composition and carrageenan extracted from Hypnea musciformis (Wulfen) Lamouroux harvested along the Atlantic coast of Morocco. Scientific Research and Essays. 2008, 3, 509-514.
Banik, U.; Mohiuddin, M.; Wahab, M. A.; Rahman, M. M.; Nahiduzzaman, M.; Sarker, S.; et al. Comparative performances of different farming systems and associated influence of ecological factors on Gracilaria sp. seaweed at the south-east coast of the Bay of Bengal, Bangladesh. Aquaculture. 2023, 574, 739675. https://doi.org/10.1016/j.aquaculture.2023.739675.
Barrientos, S.; Zarco-Perello, S.; Piñeiro-Corbeira, C.; Barreiro, R.; Wernberg, T. Feeding preferences of range-shifting and native herbivorous fishes in temperate ecosystems. Marine Environmental Research. 2021, 172, 105508. https://doi.org/10.1016/j.marenvres.2021.105508.
BFRI. Bangladesh Fisheries Research Institute. Seaweeds of Bangladesh coast. Bangladesh Fisheries Research Institute, 2019, 152 p.
Bhattacharjee, S.; Islam, G.M.R. Seaweed antioxidants as novel ingredients for better health and food quality: Bangladesh prospective. Proceedings of the Pakistan Academy of Sciences. 2014, 51, 215-233.
Bhuyan, M.S. Ecological risks associated with seaweed cultivation and identifying risk minimization approaches. Algal Research. 2023, 102967. http://dx.doi.org/10.1016/j.algal.2022.102967.
Bhuyan, M.S.; Haider, S.M.B.; Kundu, M.; Husain, S.K.A.; Chowdhury, E. Challenges in Cultivation of Seaweed: A Recent Experience from RezuKhal, Cox’s Bazar Coast, Bangladesh. Journal of Oceanography & Marine Environmental System. 2023, 7, 01-09. https://doi.org/10.5829/idosi.jomes.2023.01.09.
Bokhtiar, S.M.; Ali, M.A.; Chowdhury, M.A.Z.; Ahmed, K.U.; Hassan, M.K.; Ahmed, M.; et al. Yield improvement of Gracilaria tenuistipitata by optimizing different aspects in coast of cox’s bazar, Bangladesh. Scientific Reports. 2022, 12, 1-8. https://doi.org/10.1038/s41598-022-08040-3.
Bricheno, L.M.; Wolf, J.; Sun, Y. Saline intrusion in the Ganges-Brahmaputra-Meghna megadelta. Estuarine, Coastal and Shelf Science. 2021, 252, 107246. https://doi.org/10.1016/j.ecss.2021.107246.
Bui, H.T.T.; Luu, T.Q.; Fotedar, R. Effects of temperature and pH on the growth of Sargassum linearifolium and S. podacanthum in potassium-fortified inland saline water. American Journal of Applied Sciences. 2018, 15, 186-197. https://doi.org/10.3844/ajassp.2018.186.197.
Burdames, Y.; Ngangi, E.L.N.L. Kondisilingkunganperairanbudidayarumputlaut di DesaArakan, KabupatenMinahasa Selatan. e-Journal BUDIDAYA PERAIRAN. 2014, 2. https://doi.org/10.35800/bdp.2.3.2014.5706.
Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water research. 2012, 46, 1394-1407. https://doi.org/10.1016/j.watres.2011.12.016.
Colt, J.; Huguenin, J.E. Design and operating guide for aquaculture seawater systems. Elsevier, Second Edition, 2002.
De Wreede, R.E. The phenology of three species of Sargassum (Sargassaceae, Phaeophyta) in Hawaii. Phycologia. 1976, 15, 175-183.
Dhargalkar, V. Uses of seaweeds in the Indian diet for sustenance and well-being. Environmental Science, Agricultural and Food Sciences, Medicine. 2015, 80, 192-202.
Ding, L.; Ma, Y.; Huang, B.; Chen, S. Effects of seawater salinity and temperature on growth and pigment contents in Hypnea cervicornis J. Agardh (Gigartinales, Rhodophyta). BioMed research international. 2013, 594308. https://doi.org/10.1155/2013/594308.
Durako, M.J.; Dawes, C. A comparative seasonal study of two populations of Hypneamusciformis from the East and West Coasts of Florida, USA I. Growth and chemistry. Marine Biology. 1980, 59, 151-156.
Eggert, A. Seaweed Responses to Temperature. In Seaweed Biology, Wiencke, C., Bischof, K. (eds). Ecological Studies, vol 219. Springer, Berlin, Heidelberg, 2012, 47-66. https://doi.org/10.1007/978-3-642-28451-9_3.
Effendi, H. Study of water quality for the management of aquatic resources and environments. Yogyakarta, Kanisius, Indonesia, 2003.
FAO. Fisheries and Aquaculture Information and Statistics Service – 16/03/2014, 2014.
Farhaduzzaman, A.M.; Khan, M.S.; Hasan, M.; Islam, R.; Osman, M.H.; Shovon, M.N.H.; et al. Seaweed Culture, Post-Harvest Processing, And Market Generation for Employment of Coastal Poor Communities in Cox’s Bazar. Journal of Applied Life Sciences and Environment. 2023, 56, 231-244. https://doi.org/10.46909/alse-562098.
Friedlander, M.; Zelikovitch, N. Growth rates, phycocolloid yield and quality of the red seaweeds, Gracilaria sp., Pterocladia capillacea, Hypnea musciformis, and Hypnea cornuta, in field studies in Israel. Aquaculture. 1984, 40, 57-66.
Ganesan, M.; Rao, P.V.; Mairh, O.P. Culture of marine brown alga Padina boergesenii (Dictyotales, Phaeophyta) at Mandapam coast, southeast coast of India. Indian Journal of Marine Science. 1999, 28, 461-463.
Ganesan, M.; Thiruppathi, S.; Jha, B. Mariculture of Hypnea musciformis (Wulfen) Lamouroux in South east coast of India. Aquaculture. 2006, 256, 201-211. https://doi.org/10.1016/j.aquaculture.2006.01.039.
Ghaderiardakani, F.; Califano, G.; Mohr, J. F.; Abreu, M. H.; Coates, J. C.; Wichard, T. Analysis of algal growth-and morphogenesis-promoting factors in an integrated multi-trophic aquaculture system for farming Ulva spp. Aquaculture Environment Interactions. 2019, 11, 375-391. https://doi.org/10.3354/aei00319.
Ghaderiardakani, F.; Quartino, M. L.; Wichard, T. Microbiome-dependent adaptation of seaweeds under environmental stresses: a perspective. Frontiers in Marine Science. 2020, 7, 575228. https://doi.org/10.3389/fmars.2020.575228.
Grant, J. Factors affecting the occurrence of intertidal amphipods in reducing sediments. Journal of experimental marine biology and ecology. 1981, 49, 203-216.
Guist Jr, G.G.; Dawes, C.J.; Castle, J.R. Mariculture of the red seaweed, Hypnea musciformis. Aquaculture. 1982, 28, 375-384. https://doi.org/10.1016/0044-8486(82)90079-5.
Gultom, R.C.; Dirgayusaa, I.G.N.P.; Puspithaa, N.L.P.R. PerbandinganLajuPertumbuhanRumputLaut (Eucheuma cottonii) DenganMenggunakanSistemBudidaya Ko-kultur dan Monokultur di Perairan Pantai Geger, Nusa Dua, Bali. JMRT. 2019, 2, 8-16.
Hmani, I.; Ghaderiardakani, F.; Ktari, L.; El Bour, M.; Wichard, T. High-temperature stress induces bacteria-specific adverse and reversible effects on Ulva (Chlorophyta) growth and its chemosphere in a reductionist model system. Botanica Marina. 2023. https://doi.org/10.1515/bot-2023-0053.
Hoq, M.E.; Haque, M.A.; Islam, M.M. Feasibility of seaweed culture in Inani and Bakkhali coast of Cox’s Bazar, Bangladesh. Pakistan Journal of Marine Sciences. 2016, 25, 27-36.
Hossain, M.S.; Sharifuzzaman, S.M.; Nobi, M.N.; Chowdhury, M.S.N.; Sarker, S.; Alamgir, M.; et al. Seaweeds farming for sustainable development goals and blue economy in Bangladesh. Marine Policy. 2021, 128, 104469. https://doi.org/10.1016/j.marpol.2021.104469.
Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences 5th ed. Houghton Mifflin, Boston. 2003, 756pp.
Indriani, H.; Suminarsih, E. Seaweed Cultivation, Processing and Marketing (in Bengali), Jakarta, Penebar Swadaya, 2003, p. 4-8, 11-2.
Islam, M.M.; Hasan, J.; Ali, M.Z.; Hoq, M.E. Culture of Three Seaweed Species in Cox’s Bazar Coast, Bangladesh. Bangladesh Journal of Zoology. 2021, 49, 47-56. https://doi.org/10.3329/bjz.v49i1.53681.
Islam, M.M.; Hoq, M.E.; Ali, M.Z.; Hasan, S.J.; Sharif, A.S.M.; Bhuyan, M.S. Diversity and occurrence of seaweeds from the south-eastern coast of Bangladesh. Indian Journal of Geo Marine Sciences. 2020, 49, 1-10.
Islam, M.M.; Khan, M.S.K.; Hasan, J.; Mallick, D.; Hoq, M.E. Seaweed Hypnea sp. culture in Cox s Bazar coast, Bangladesh. Bangladesh Journal of Zoology. 2017, 45, 37-46. https://doi.org/10.3329/bjz.v45i1.34192.
Islam, S.N. Deltaic floodplains development and wetland ecosystems management in the Ganges–Brahmaputra–Meghna Rivers Delta in Bangladesh. Sustainable Water Resources Management. 2016, 2, 237-256. https://doi.org/10.1007/s40899-016-0047-6.
Jackson, K. The pharmaceuticals industry in Japan: current trends and emerging business opportunities for EU-based small-and medium-sized enterprises. 2018. On line at: https://www.eu-japan.eu/sites/default/files/2018-04-the-pharmaceuticals-industry-in-japan-jackson-eubij.pdf.
Kannel, P.R.; Lee, S.; Lee, Y.S.; Kanel, S. R.; Khan, S.P. Application of water quality indices and dissolved oxygen as indicators for river water classification and urban impact assessment. Environmental monitoring and assessment. 2017, 132, 93-110. https://doi.org/10.1007/s10661-006-9505-1.
Kautsari, N.; Ahdiansyah, Y. DayaDukung dan Kesesuaian Lahan Perairan Labuhan Terata, Sumbawa untukPengembanganBudidayaRumputLaut. Indonesian Journal of Marine Sciences/IlmuKelautan. 2015, 20.
Khan, S.I.; Satam, S.B. Seaweed mariculture: scope and potential in India. Aquaculture Asia. 2003, 8, 26-29.
Kotta, J.; Jänes, H.; Paalme, T.; Peterson, A.; Kotta, I.; Aps, R.; et al. GoA 2.1. Assessing the PanBaltic potential of macroalgae cultivation and of harvesting wild stocks. 2021, Available online: https://www.submariner-network.eu/images/grass/outputs/GRASS_OA21_pan-Baltic_map_depicting_potential_of_macroalgal_cultivation_and_harvesting.pdf (accessed on 10 November 2021).
Kraan, W. The influence of nutrient availability, salinity, temperature and the underwater light climate on the growth of seaweed (MSc Thesis), University of Groningen the Netherlands, 12/05/2018.
Ligtvoet, A.; Maier, F.; Sijtsma, L.; van den Broek, L.A.M.; Safi, C.; Doranova, A.; et al. Blue Bioeconomy Forum: Highlights: Summary of the roadmap and a selection of viable and innovative projects. European Commission. 2019. https://doi.org/10.2826/234088.
Lomartire, S.; Marques, J.C.; Gonçalves, A.M. An overview to the health benefits of seaweeds consumption. Marine Drugs. 2021, 19, 341. https://doi.org/10.3390/md19060341.
Maberly, S.C. The fitness of the environments of air and water for photosynthesis, growth, reproduction and dispersal of photoautotrophs: an evolutionary and biogeochemical perspective. Aquatic Botany. 2014, 118, 4-13. http://dx.doi.org/10.1016/j.aquabot.2014.06.014.
Makame, M.O.; Shackleton, S. Coping with and Adapting to Climate and Non-Climate Stressors Within the Small-Scale Farming, Fishing and Seaweed Growing Sectors, Zanzibar. 2021.
Monteith, J.L. Evaporation and surface temperature. Quarterly Journal of the Royal Meteorological Society. 1981, 107, 1-27.
Morain, S.A. GIS solutions in natural resource management: Balancing the technical-political equation (No. 910.285 G58). Santa Fe: Onword press. 1999.
Msuya, F.E.; Hurtado, A.Q. The role of women in seaweed aquaculture in the Western Indian Ocean and South-East Asia. European journal of phycology. 2017, 52, 482-494. https://doi.org/10.1080/09670262.2017.1357084.
Mukaka, M.M. Statistics Corner: A guide to appropriate use of Correlation coefficient in medical research. Malawi Medical Journal. 2012, 24, 69-71.
Mustafa, A.A.; Hasnawi, T.; Radiarta, I.N. Karakteristik, kesesuaian, dan daya dukung perairan untuk budidaya rumput laut di Kabupaten Kepulauan Sangihe, Sulawesi Utara. Jurnal Riset Akuakultur. 2017, 12, 187-196. http://dx.doi.org/10.15578/jra.12.2.2017.187-196.
Nedumaran, T. Seaweed: A fertilizer for sustainable agriculture. In Sustainable Agriculture towards Food Security. Springer, Singapore. 2017, 159-174.
Nurdin, N.; Setiawan, R.Y.; Helmi, M.; Maslukah, L.; Agus, A.; AS, M.A.; et al. Spatial water quality and plastic buoy of seaweed culture in coastal area, Indonesia. In SPIE Future Sensing Technologies. 2020, 11525, 231-243. https://doi.org/10.1117/12.2576385.
Parsons, T.R. A manual of chemical & biological methods for seawater analysis. Elsevier. 2013.
Pati, M.P.; Sharma, S.D.; Nayak, L.A.K. S.H.M.A.N.; Panda, C.R. Uses of seaweed and its application to human welfare: A review. Int. J. Pharm. Pharm. Sci. 2016, 8, 12-20.
Pereira, L. Seaweed flora of the european north atlantic and mediterranean. In Springer handbook of marine biotechnology. Springer, Berlin, Heidelberg. 2015, pp. 65-178.
Pérez-Lloréns, J.L. Seaweed consumption in the Americas. Gastronomica. 2019, 19, 49-59.
Pradhan, B.; Bhuyan, P.P.; Patra, S.; Nayak, R.; Behera, P.K.; Behera, C.; et al. Beneficial effects of seaweeds and seaweed-derived bioactive compounds: Current evidence and future prospective. Biocatalysis and Agricultural Biotechnology. 2022, 39, 102242.
Prutzer, E.S. Ecologies of digital mapping: Open-source communities and grassroots maps (Doctoral dissertation, University of Illinois at Urbana-Champaign), 2019.
Rahman, I.S.; Fadjar, M.; Tjahjono, A. The Relationship Water Physical-Chemical Parameters In Seaweed Cultivation Eucheuma Cottonii With Long-Line System. International Journal of Scientific & Technology Research. 2019, 8, 1448-1452.
Raikar, S.V.; Iima, M.; Fujita, Y. Effect of temperature, salinity and light intensity on the growth of Gracilaria spp. (Gracilariales, Rhodophyta) from Japan, Malaysia and India. Indian Journal of Marine Science. 2001, 30, 98-104.
Rao, R.; Subbaramaiah, R. A technique for the field cultivation of Hypnea musciformsis (Wulf.) Lam. In Proc. Symp. Coastal Aquaculture. 1986, 1190-1192.
Rashad, S.; A El-Chaghaby, G. Marine Algae in Egypt: distribution, phytochemical composition and biological uses as bioactive resources (a review). Egyptian Journal of Aquatic Biology and Fisheries. 2020, 24, 147-160.
Reddy, C.R.K.; Rao, P.S.; Ganesan, M.; Eswaran, K.; Zaidi, S.H.; Mantri, V. A. The seaweed resources of India. World Seaweed Resources. Eti Information Services Ltd., Wokingham, Berkshire, UK, 2006, 25pp.
Redmond, S.; Kim, J.K.; Yarish, C.; Pietrak, M.; Bricknell, I. Culture of Sargassum in Korea. NOAA Sea Grant, Orono Maine, 12, 2014.
Rosyida, E.; Masyahoro, A.; Putera, F.H.A.; Natsir, S. The utilization of seaweed-based liquid organic fertilizer to stimulate Gracilaria verrucosa growth and quality. International Journal of Environmental Science and Technology. 2021, 18, 1637-1644.
Rugebregt, M.J.; Arfah, H.; Pattipeilohy, F. Correlation between macroalgae diversity and water quality in Southwest Maluku waters. Marine Research in Indonesia. 2020, 45, 25-32.
Rusdi, W.A.; Agus, T.; Bambang, S. Analysis of water quality parameters for seaweed (Eucheuma cottonii) farming site suitability in Mandar Bay, West Sulawesi, Indonesia. Russian Journal of Agricultural and Socio-Economic Sciences. 2017, 72.
Salam, M.A. Potential of seaweed culture on St. Martin’s Island, Bangladesh. 2020. https://doi.org/10.1079/ac.68674.20203483386.
Salehi, B.; Sharifi-Rad, J.; Seca, A.M.; Pinto, D.C.; Michalak, I.; Trincone, A.; et al. Current trends on seaweeds: Looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules. 2019, 24, 4182. https://doi.org/10.3390%2Fmolecules24224182.
Sandquist, J.; Skjermo, J.; McDillan, J.D. Macroalgae for Higher Value Products and Liquid Fuels. State of Technology Review–Algae Bioenergy: an IEA Bioenergy Inter-Task Strategic Project. 2017.
Sarkar, M.S.I.; Kamal, M.; Hasan, M.M.; Hossain, M.I. Present status of naturally occurring seaweed flora and their utilization in Bangladesh. Research in Agriculture Livestock and Fisheries. 2016, 3, 203-216. https://doi.org/10.3329/ralf.v3i1.27879.
Sarker, S.; Akter, M.; Rahman, M.S.; Islam, M.M.; Hasan, O.; Kabir, M. A.; Rahman, M.M. Spatial prediction of seaweed habitat for mariculture in the coastal area of Bangladesh using a Generalized Additive Model. Algal Research. 2021, 60, 102490. https://doi.org/10.1016/j.algal.2021.102490.
Shafiuddin, M. Sustainable Livelihood of Coastal Communities Through Seaweed Cultivation and Utilization for South-Eastern Coast of Bangladesh. Social Change (YPSA). 2019, 9, 126-140.
Shaika, N.A.; Ritu, J.R.; Khan, S.; Rao, A.R. Prospects and Challenges in Commercialization of Seaweeds in Bangladesh. In Sustainable Global Resources of Seaweeds. Springer, Cham, 2022, 1, 225-247.
Siddiqui, A.A.M.; Kashem, M.A.; Mondal, M.A.I.; Shafiuddin, M. Commercially important seaweed cultivation and its potentials for the coastal areas of Cox’s Bazar, Bangladesh. International Journal of Fisheries and Aquatic Studies. 2019, 7, 463-470.
Sobuj, M.K.A.; Mostofa, M.G.; Islam, Z.; Rabby, A.F.; Rahman, T.; Sonia, S. S.; et al. Floating raft culture of Gracilariopsis longissima for optimum biomass yield performance on the coast of Cox’s Bazar, Bangladesh. Scientific Reports. 2023, 13, 2308. https://doi.org/10.1038/s41598-023-28675-0.
Sulistiawati, D.; Ya’la, Z.R.; Mubaraq, D.Z. Water quality study in several seaweeds culture sites in the post-earthquake-tsunami Palu Central, Sulawesi Province. In Journal of Physics: Conference Series. 2020, 1434, 012035. https://doi.org/10.1088/1742-6596/1434/1/012035.
Sulu, R.; Kumar, L.; Hay, C.; Pickering, T. Kappaphycus seaweed in the Pacific: review of introductions and field-testing proposed quarantine protocols. Secretariat of the Pacific Community, Noumea, 2004, 85.
Thomas, J.B.E.; Ramos, F.S.; Gröndahl, F. Identifying suitable sites for macroalgae cultivation on the Swedish West Coast. Coastal Management. 2019, 47, 88-106. http://dx.doi.org/10.1080/08920753.2019.1540906.
Tomascik, T. The ecology of the Indonesian seas. Oxford University Press. 1997.
Tresnati, J.; Yasir, I.; Bestari, A.D.; Yanti, A.; Aprianto, R.; Tuwo, A. Effect of salinity on the growth of seaweed Gracilaria changii (Xia and Abbott, 1987). IOP Conference Series: Earth and Environmental Science. 2021, 763, 012030. https://doi.org/10.1088/1755-1315/763/1/012030.
Tuwo, A.; Samawi, M.F.; Aprianto, R.; Tresnati, J. Feasibility study of seaweed farming Kappaphycus alvarezii in Sub-District North PulauLaut and Sub-District East PulauLaut Kota baru Regency, South Borneo, Indonesia. IOP Conference Series: Earth and Environmental Science. 2020, 564, https://doi.org/012026. 10.1088/1755-1315/564/1/012026.
Uddin, M.R. Water and sediment parameters at the algae culture area of Salimpur coast Chittagong. MOJ Ecology & Environmental Sciences. 2019, 4, 39-42.
Warnadi, S.; Setyaningsih, A.I.; Kasih, W.A. Water Quality and It’s Effect on Seaweed Cultivation in Pari Island, KepulauanSeribu DKI Jakarta. IOP Conference Series: Earth and Environmental Science. 2018, 145, 012145. https://doi.org/10.1088/1755-1315/145/1/012145.
Yahya, B.; Yahya, S.; Mmochi, A.; Jiddawi, N. Comparison of seaweed growth, fish abundance and diversity in deep water floating raft with tubular nets and shallow water off-bottom lines seaweed farms. Tanzania Journal of Science. 2020, 46, 840-850.
Yalçın, S.; Karakaş, Ö.; Okudan, E.Ş.; Başkan, K.S.; Çekiç, S.D.; Apak, R. HPLC Detection and antioxidant capacity determination of brown, red and green algal pigments in seaweed extracts. Journal of Chromatographic Science. 2021, 59, 325-337. https://doi.org/10.1093/chromsci/bmaa107.
Yarnpakdee, S.; Benjakul, S.; Kingwascharapong, P. Physico-chemical and gel properties of agar from Gracilaria tenuistipitata from the lake of Songkhla, Thailand. Food Hydrocolloids. 2015, 51, 217-226. https://doi.org/10.1016/j.foodhyd.2015.05.004.
Yu-Qing, T.; Mahmood, K.; Shehzadi, R.; Ashraf, M.F. Ulva lactuca and its polysaccharides: Food and biomedical aspects. Journal of Biology, Agriculture and Healthcare. 2016, 6, 140-151.
Zafar, M. Seaweed culture in Bangladesh holds promise. INFOFISH International. 2005, 1, 18-10.
Zafar, M. Seaweed culture (Hypnea sp.) in Bangladesh- Seaweed Culture Manual-1. Institute of Marine Science and Fisheries, University of Chittagong, Chittagong, Bangladesh. 2007, 14 p.
Academic Editor: Dr. Isabela Maria Simion
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.
Bhuyan Simul, Chowdhury Enam, Elangovan Manickam, Haider Sayeed Mahmood Belal, Husain Abid, Kunda Mrityunjoy, Senapathi Venkatramanan, Sivakumar K.