Constantin Spataru, Mihaela-Claudia Spataru
ABSTRACT. Guinea pigs (Cavia porcellus) are rodents that feed on grassy plants, buds and sprouts, with cheek teeth having specialised abrasive surfaces for plant grinding. In analysing the prehension and trituration ways of guinea pigs, many differences concerning mandible conformation, the positioning of cheek teeth and the morphology of the masticatory muscles compared to other rodents were found. Masticatory muscles of guinea pigs are predominant compared to the mimetic muscles which are reduced. Compared to other rodents, in guinea pigs, inside the tendon thickness of the superficial part of the masseter muscle there are two rounded cartilaginous structures such as sesamoids. The dorsal one is larger, measuring about 3–4 mm in diameter having the role of reducing pressure on the tendon when it passes over the mandible ridge. The other is ventrally placed, about 2–3 mm in size and protects the tendon of the superficial part of the masseter muscle when it passes over the ventral tubercle of the mandible.
Keywords: guinea pig; mandible; masseter; masticator muscles; skull.
Cite
ALSE and ACS Style
Spataru, C.; Spataru, M.-C. Peculiarities of the masticatory apparatus of guinea pigs (Cavia porcellus). Journal of Applied Life Sciences and Environment 2023, 56 (1), 127-138.
https://doi.org/10.46909/alse-561090
AMA Style
Spataru C, Spataru M-C. Peculiarities of the masticatory apparatus of guinea pigs (Cavia porcellus). Journal of Applied Life Sciences and Environment. 2023; 56 (1): 127-138.
https://doi.org/10.46909/alse-561090
Chicago/Turabian Style
Spataru, Constantin, Mihaela-Claudia Spataru. 2023. “Peculiarities of the masticatory apparatus of guinea pigs (Cavia porcellus)” Journal of Applied Life Sciences and Environment 56, no. 127-138.
https://doi.org/10.46909/alse-561090
View full article (HTML)
Peculiarities of the Masticatory Apparatus of Guinea Pigs (Cavia porcellus)
Constantin SPATARU1 and Mihaela-Claudia SPATARU2,*
1Department of Preclinics, Faculty of Veterinary Medicine, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 8, Mihail Sadoveanu Alley, 700489, Iasi, Romania; email: cspataru@yahoo.com
2Department of Public Health, Faculty of Veterinary Medicine, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 8, Mihail Sadoveanu Alley, 700489, Iasi, Romania
*Correspondence: mspataru@yahoo.com
Received: Jun. 14, 2023. Revised: Jul. 10, 2023. Accepted: Jul. 15, 2023. Published online: Jul. 19, 2023
ABSTRACT. Guinea pigs (Cavia porcellus) are rodents that feed on grassy plants, buds and sprouts, with cheek teeth having specialised abrasive surfaces for plant grinding. In analysing the prehension and trituration ways of guinea pigs, many differences concerning mandible conformation, the positioning of cheek teeth and the morphology of the masticatory muscles compared to other rodents were found. Masticatory muscles of guinea pigs are predominant compared to the mimetic muscles which are reduced. Compared to other rodents, in guinea pigs, inside the tendon thickness of the superficial part of the masseter muscle there are two rounded cartilaginous structures such as sesamoids. The dorsal one is larger, measuring about 3–4 mm in diameter having the role of reducing pressure on the tendon when it passes over the mandible ridge. The other is ventrally placed, about 2–3 mm in size and protects the tendon of the superficial part of the masseter muscle when it passes over the ventral tubercle of the mandible.
Keywords: guinea pig; mandible; masseter; masticator muscles; skull.
INTRODUCTION
Guinea pigs or domestic guinea pigs (Cavia porcellus), also known as cavy or domestic cavy, are a species of Hystricomorpha rodent belonging to the family Caviidae (Álvarez and Pérez, 2019; Álvarez et al., 2023; Graur et al., 1991; Spotorno et al., 2005). Guinea pigs are diurnal, being most active during dawn and dusk. The guinea pig’s food by diet consists of grassy plants, buds and sprouts (Noguchi et al., 1994; Spotorno et al., 2005). The cheek teeth have specialised occlusion surfaces for grinding both by propulsion/retropulsion and lateral movements (Noguchi et al., 1994; Spataru and Spataru, 2019).
Guinea pigs are the most publicised experimental animal. Louis Pasteur and Robert Koch used them in investigations of infectious diseases, the same contributing to the work of several Nobel Prize awards research. Since the nineteenth century, guinea pigs have been used in research in the field of physiology, dermatology, pharmacological tests, metabolism (Westropp and Buffington, 2002, West and Fernindez, 2004), asthma (Ricciardolo et al., 2008), study of foetal and placental evolution and aspects of birth (Carter et al., 2007; Mitchell and Taggart, 2009), in the diagnosis of some diseases such as tuberculosis, diphtheria (Dharmadhikari and Nardell, 2008; Obregon-Henao et al., 2011) or in infectious diseases with common, nosocomial causes, such as Staphylococcus aureus (Padilla-Carlin et al., 2008), etc. For example, guinea pigs share many similarities to humans, both hormonally, immunologically and physiologically. Unlike other rodents, but similar to humans, guinea pigs are prone to scurvy if they do not receive vitamin C in their diet (Clarke et al., 1980).
Guinea pigs are monophyodont, the teeth erupting continuously (hypsodontic) (Harkness et al., 2002), the lower incisors being longer than the upper ones, and the molars have no cusps presenting a groove or deep indentation (Cooper and Schiller, 1975; Pereira et al., 2020). In all mammals , the muscles directly involved in mastication are the masseter, temporalis and pterygoideus (Cooper and Schiller, 1975; Myers et al., 2006). Some differences have been identified in the terms of skull conformation and masticatory apparatus among Myomorpha (mice and rats), Scuiromorpha (squirrels), Hystricomorpha (South American caviomorph rodents and porcupines) and Protogomorpha (mountain beaver), based on differences in the muscles of mastication and other characteristics of the masticatory apparatus (Druzinsky, 2010; Vassalo and Verzi, 2001; Hautier et al., 2012; Pereira et al., 2020; Álvarez et al., 2023).
MATERIALS AND METHODS
The study of the skull and head muscles was performed on five corpses of specimens of adult guinea pigs. The muscles were highlighted using layer by layer dissections, following the place of origin and insertion of each muscle and the direction of fibres and analysing the role of each muscle in the mastication process. The skulls were prepared by scraping the adjacent tissues and through light boiling. The identified features were photographed, processed and interpreted, being compared to other information from the specific literature (Woods, 1972; Popesko et al., 1992; Constantinescu, 2018; Álvarez and Pérez, 2019; Álvarez et al., 2023).
RESULTS AND DISCUSSION
Viewed from the side, the guinea pig’s skull fits into a rectangle with a small side of about 2 cm and a large one of 6.5 cm. The viscerocranium represents 2/3 of the entire skull, being the dominant portion of the skull (Pereira et al., 2020; Spataru et al., 2013).
In guinea pigs, the premaxillary bones (os incisivum) are very developed, showing a curved appearance laterally. Each of them has a narrow incisor alveolus, the incisors having continuous growth (Cooper and Schiller, 1975; Druzinsky, 2010; Harkness et al., 2002; Isotupa and Rönning, 1977; Spataru, 2016; Stan, 2014). Each alveolar process of the incisive bone is deep, reaching the base of the alveolar processes of the maxillary bone, thus increasing the resistance of the incisors. The incisors have a chisel appearance, being shorter and triangular in cross-section and slightly curved ventrally, reaching the plane of the root of the molars.
From each maxilla, a zygomatic process in the form of a triangular blade is laterally detached, giving attachment point for the masseter muscle (Figure 1).
The maxillary bone has one premolar and three molar alveolar processes. The dental formula is 2x (I1/1, C 0/0, P 1/1, M 3/3), with a total of 20 teeth showing an open rooted aspect (hypsodont) (Isotupa and Rönning, 1977; Kruska and Steffen, 2013; Spataru and Spataru, 2019; Stan, 2014).
In guinea pigs, the oblique rostro-aboral position of the molars is easily noticeable. The alveolar processes of the premolar and the first molars are close to the median plane of the oral cavity, being separated only by the median intermaxillary suture. The rest of the molars have a divergent position, widening the oral cavity. The molars have no cusps, the abrasion surface being formed by two transverse ridges each; taken as a whole, the abrasion surface being oblique mid-laterally. In guinea pigs, all cheek teeth have short and uniform dental crowns, while the crowns decrease from the premolar to the last lower molar (Boivin et al., 2022; Butler, 1980, 1985; Clarke et al., 1980; Harkness et al., 2002; Isotupa and Rönning, 1977; Stan, 2014).
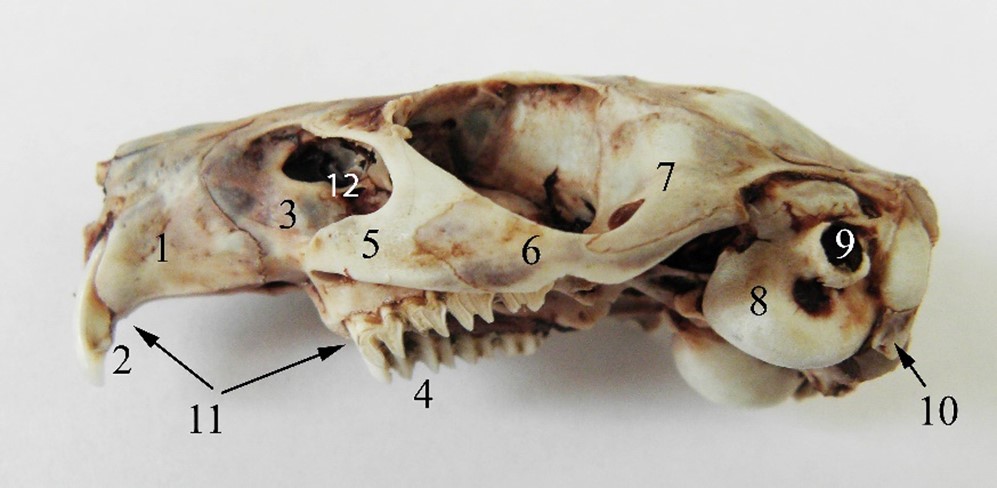
Figure 1 – The lateral aspect of the guinea pig skull 1. os incisivum, 2. dentes incisive superiores, 3. os maxillare, 4. dentes premolaris et molares superiores, 5. processus zygomaticus ossis maxillaris, 6. os zygomaticum, 7. processus zygomaticus ossis temporalis, 8. bulla tympanica, 10. processus paracondylaris, 11. diastema, 12. fossa infraorbitalis
On the other hand, the oblique position of the molars and the slight mid-lateral obliquity of their abrasion surface show that, in addition to the propulsion and retropulsion movements of the mandible, lateral movements are also used for chewing food (Figure 2, Figure 3, Figure 8) (Álvarez and Pérez, 2019; Álvarez et al., 2023; Byrd, 1981; Spataru, 2016; Stan, 2014).
In guinea pigs, the mandible is impressive by its length, being as long as the cranium. If in most rodents the caudal angle of the mandible has the appearance of a trident, being formed by the three obvious processes: the coronoid process, the articular condyle and the angular process. In guinea pigs a reduction in height of the coronoid process that appears as a triangular bony blade is notable, being 2–3 mm high or sometimes absent (Anthwal et al., 2012, 2015; Cox et al., 2012; Spataru et al., 2013). The condyle has a lamellar appearance, being thin (Figure 3, Figure 4) (Spataru, 2016).
The mandibular condyle is placed in the plane of the lower molar level, similar to carnivores, being slightly deviated medially, an aspect that increases the mandible`s resistance to mechanical stress (Spataru, 2016). The angular process is lamellar and exceeds the aboral plane of the skull, with a wide pterygoid fossa on its medial face (Figure 3). During resting, the tip of the incisors and the mandibular condyle are placed in a plane that passes dorsally to the mandibular molar level. This indicates a powerful force of the incisors in sectioning food (Butler, 1980, 1985; Christiansen and Adolfssen, 2005; Boivin et al., 2022). The masticatory muscles in guinea pigs are predominant, being represented by strong muscles. The mimetic muscles are reduced and simpler, being related to precise oronasal movements and regional sensitivity, with only the malaris and buccinator muscles being more prominent, which are involved in chewing the bolus (Álvarez et al., 2023). The masseter, temporal and pterygoid muscles have strong bellies formed by muscle bundles, being distributed in different directions, increasing the muscle strength and the sectioning force in chewing food between the dental plates (Byrd, 1981; Cox and Jeffery, 2011).
Within the masticatory muscles, the masseter muscle is more developed in guinea pigs, formed by three parts consisting of fibers distributed in different planes and angles (Cox and Jeffery, 2011; Spataru, 2019; Álvarez et al., 2023;). The superficial part has an aponeurotic aspect and forms a strong and wide tendon which originates on the facial tubercle (Figure 4, Figure 5, Figure 6). The musculo-aponeurotic fibres have an accentuated ventro-aboral obliquity, which then merges into a massive muscular belly that is briefly inserted on the angle and the ventral edge of the angular process of the mandible (Figure 4, Figure 5). Thus, Cox and Jeffery (2011) indicated that this part represents about 45% of the masticatory musculature, compared to about 30% in the squirrel and rat.
In adult guinea pigs, there are two cartilaginous thickenings, similar to sesamoids, in the thickness of the tendon. The first is larger, about 3–4 mm in length, protecting the tendon when it passes over the mandibular ridge. The second is placed ventrally to the first, about 2–3 mm in length, protecting the tendon of the superficial part of the masseter muscle when it passes over the ventral tubercle of the mandible.
During mastication, the propulsion of the mandible is mainly produced by the deep part of the masseter muscle. It originates on the zygomatic arch being inserted over a wide area of the mandible, from the rostral limit of the premolar and the first molar, the whole angular process ventrally towards the ridge that delineates both the masseter and the pterygoid fossa.
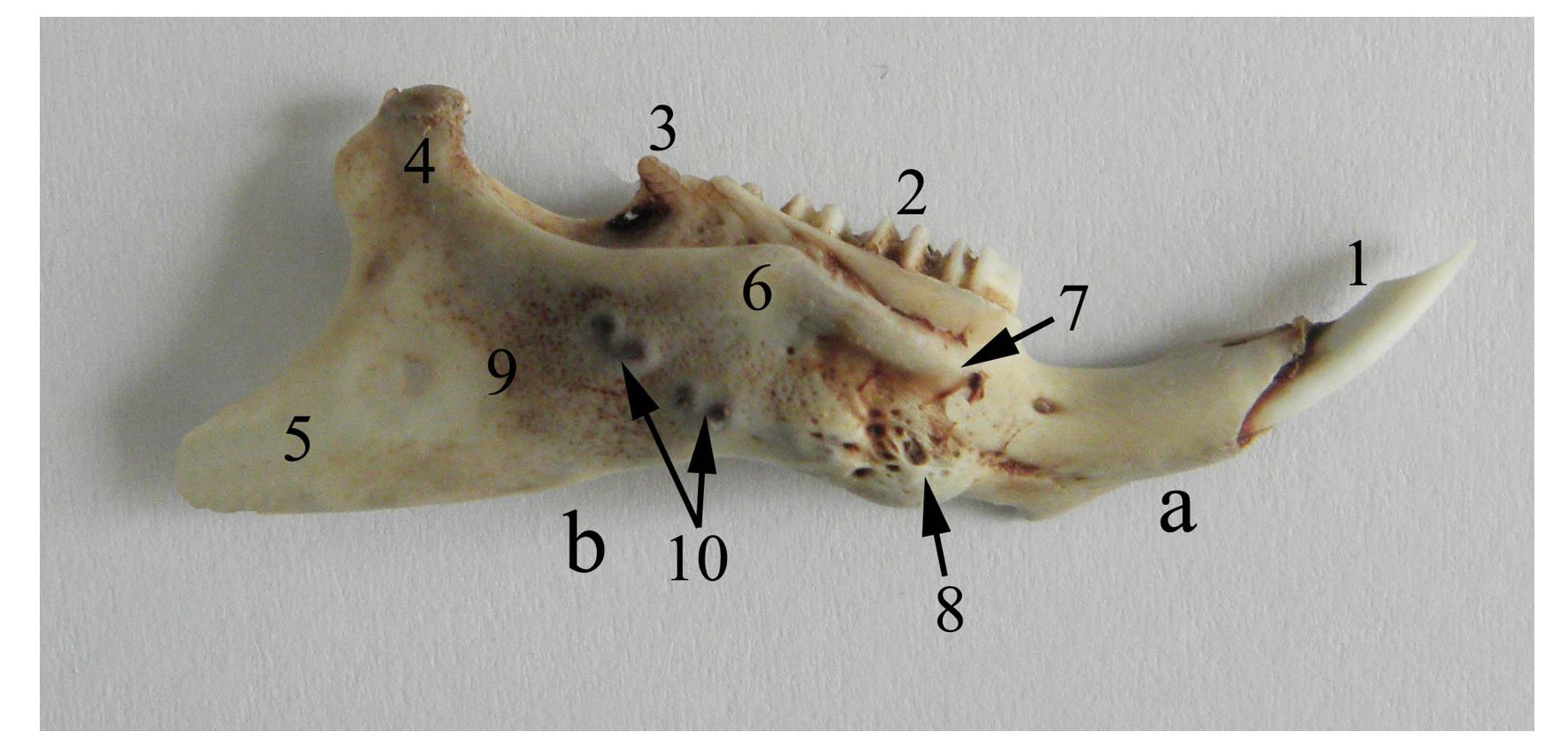
Figure 2 – Lateral aspect of the right mandible of the guinea pig a. corpus mandibulae, b. ramus mandibulae, 1. dentes incisivi, 2. dentes molares, 3. processus coronoideus, 4. processus condylaris, 5. processus angularis, 6. crista mandibularis lateralis (mandible ridge), 7. tuberculum mandibularis dorsalis, 8. tuberculum mandibularis ventralis, 9. fossa masseterica, 10. alveolae molares
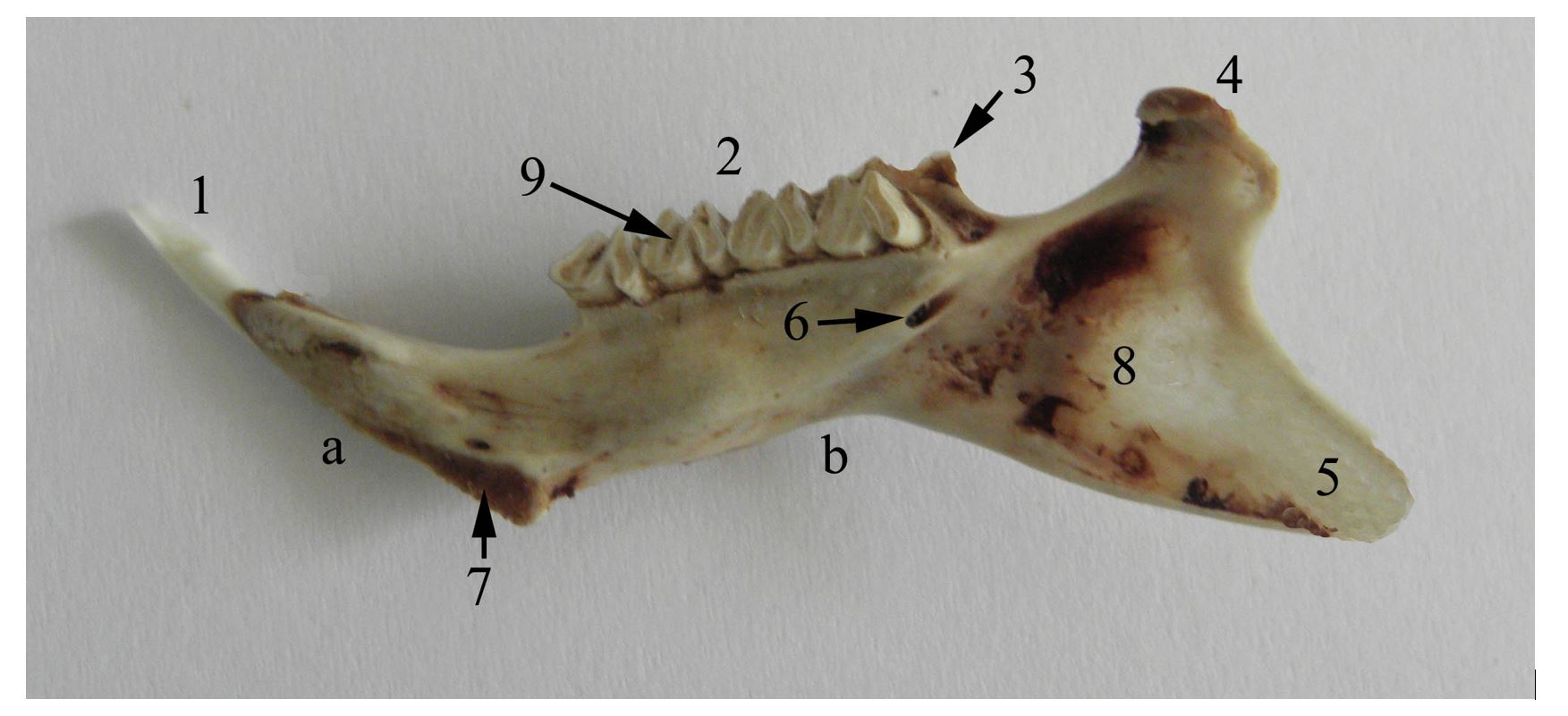
Figure 3 – Medial aspect of the right mandible of the guinea pig a. corpus mandibulae, b. ramus mandibulae, 1. dentes incisivi, 2. dentes molares, 3. processus coronoideus, 4. processus condylaris, 5. processus angularis, 6. canalis mandibulae, 7. articulatio intermandibularis, 8. fossa pterygoidea, 9. facies
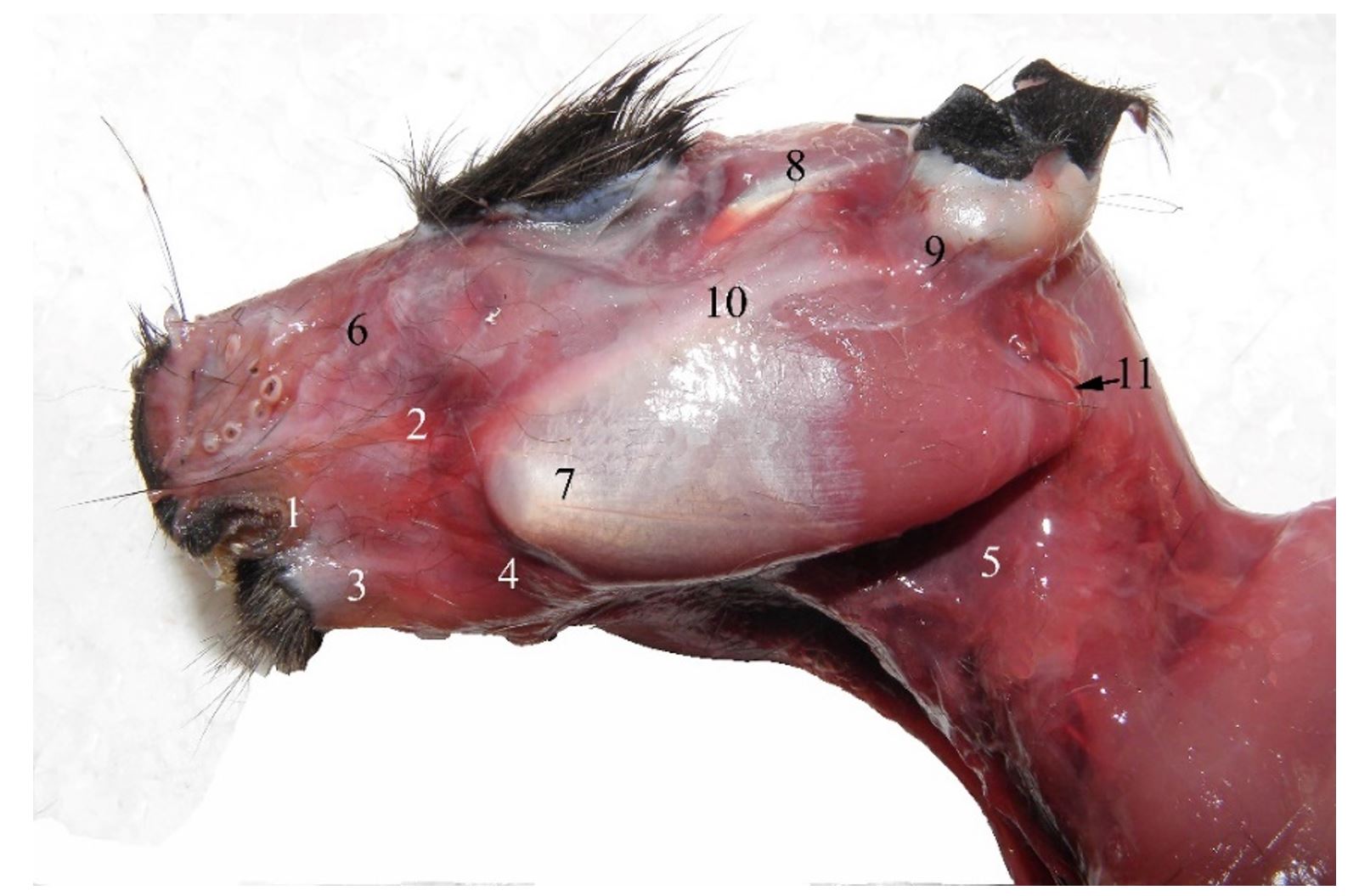
Figure 4 – Left lateral aspect of the superficial head muscles in the guinea pig 1. rima oris, 2. m. zygomaticus, 3. m. depressor labii inferioris et m. depressor anguli oris, 4, 5. platysma, 6. m. levator nasolabialis, 7. m. masseter, 8. m. temporalis, 9. concha auricularis, 10. arcus zygomaticus, 11. margo aboralis processus angularis mandibulae
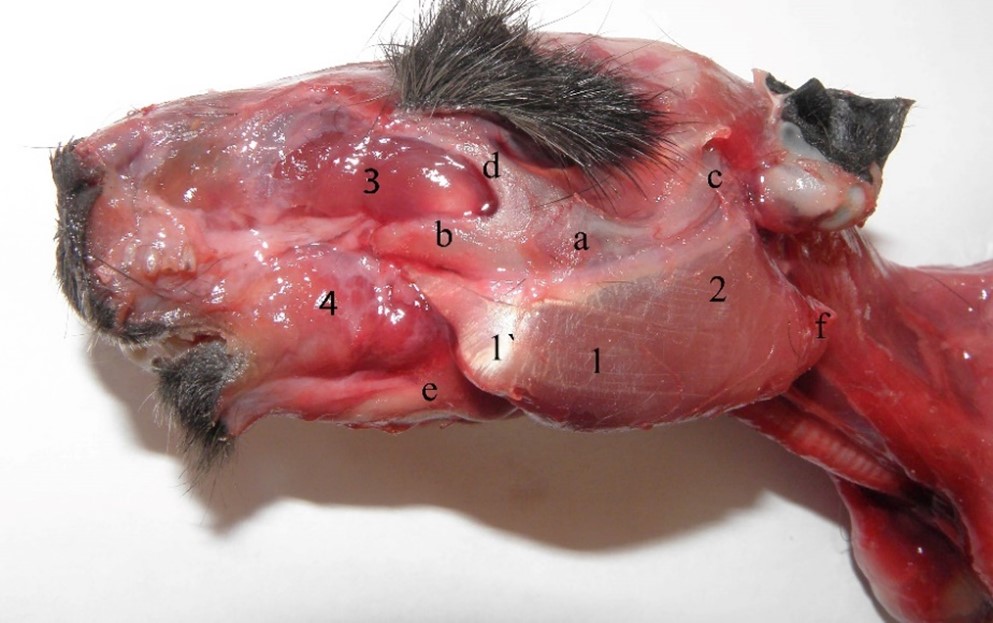
Figure 5 – Superficial part of the masseter muscle 1. m. masseter, pars superficialis, 1`. sesamoid placed into the thickness of the superficial part of the masseter muscle, 2. m. masseter, pars profunda (pars zygomatica), 3. m. masseter, pars infraorbitalis, 4. m. buccinatorius, a. Os zygomaticus, b. processus zygomaticus ossis maxillaris, c. processus zygomaticus ossis temporale, d. processus zygomaticus ossis frontalis, e. corpus mandibulae, f. processus angularis mandibulae
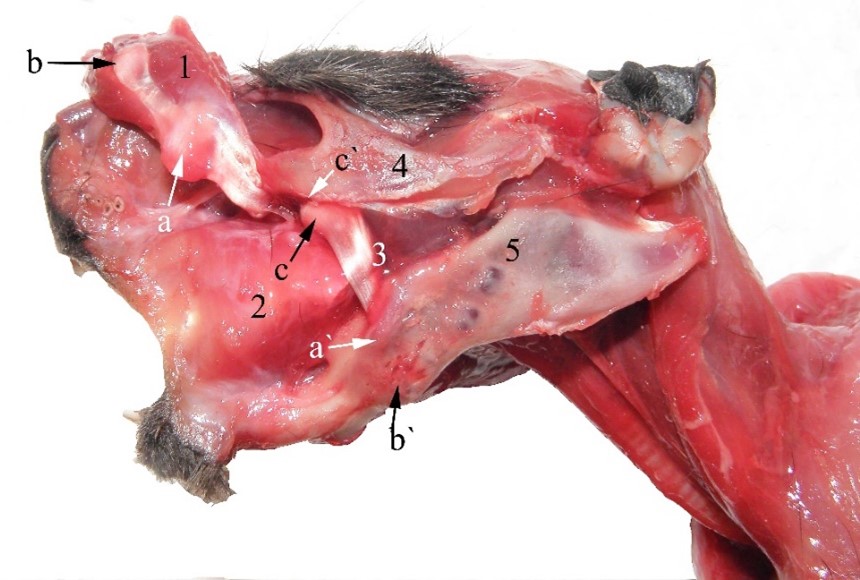
Figure 6 – Infraorbital part of the masseter muscle at guinea pig 1. m. masseter, pars superficialis (cut), a. cartilago proximalis (into the tendon of the superficial part of the masseter muscle), b. cartilago distalis (into the tendon of the superficial part of the masseter muscle), a`. tuberculum mandibularis dorsalis (surface over that the proximal cartilage slips), b`. tuberculum mandibularis ventralis (surface over that the distal cartilage slips), 2. m. buccinatorius, 3. m. masseter, pars infraorbitalis, c. cartilago tendinis infraorbitalis musculi masseter, 4. arcus zygomaticus, 5. mandibula
These aspects regarding the origin on a tubercle and its wide insertion with the oblique orientation of the fibers as well as the presence of the two cartilaginous nuclei, mean that the superficial part of the masseter muscle is involved both in mandible propulsion and its efficient and fast raising, considering that the temporal muscle is reduced (Anthwal and Tucker, 2012; Cox et al., 2012; Cox and Jeffery, 2011; Spataru et al., 2013).
The oblique position of fibers, the long tendon of origin and the presence of cartilaginous structures represent a factor of adaptation for grinding food (Álvarez et al., 2023), the contraction of the muscle permitting trituration for a long time, avoiding muscle fatigue.
The middle (zygomatic) part of the masseter muscle has similar tilting of fibers to other rodents. In guinea pigs, the origin is large, extending over the entire zygomatic arch, both on its lateral and medial faces. Fibres have a ventro-aboral oblique position, being crowded towards the dorsal edge of the angular process and the base of the mandibular condyle (Cooper and Schiller, 1975; Pereira et al., 2022). This plays a main role in the rapid and strong lifting of the mandible, due to the placement of the articular condyle at the level of the mandibular molar plate. The smaller deep (infraorbital) part of the masseter muscle originates on the infraorbital ridge. The muscle passes through the infraorbital foramen (which appears as a deep ditch), being inserted on an ovoid area of the masseter fossa, which is delimited by the tuberculum mandibularis dorsalis (Figure 6).
As in other species of rodents, the temporal muscle consists of two small parts (Álvarez and Pérez, 2019; Byrd, 1981; Cox and Jeffery, 2011; Kruska and Steffen, 2013). The rostral part is smaller having a triangular appearance (Figure 7) (Spataru et al., 2013). The origin occupies the spaces of the parietal bone, caudally to the orbit, and the insertion reaches the rostral edge of the coronoid process. The deep part (the aboral part) originates on the whole temporal fossa up to the external occipital protuberance. It has a fan-like shape; the fibers being inserted on top of the coronoid process (Figure 7).
Compared with other rodents, guinea pigs present a strongly developed digastricus (occipito-mandibularis) muscle, appearing as a cord of fibres (Figure 8) (Spataru et al., 2011). It originates on the paracondylar process (2–3 mm long in guinea pigs) and on the caudo-ventral edge of the tympanic bulla. The massive muscular belly is ventro-rostrally obliquely oriented to the caudal edge of the mandible body. The bellies of the digastricus muscles fuse into the intermandibular space, inserting together with the mylohyoideus and geniohyoideus (reduced) muscles on the mandible. The dorso-ventral position of fibers allows a large extension of the temporo-mandibular joint, as well as performing a strong retropulsion of the mandible.
The lateral (external) and medial (internal) pterygoid muscles arise from the entire surface of the pterygoid ridges of the basisphenoid and palatine bones, having a wide insertion on the mandible, throughout the pterygoid fossa (medial pterygoid muscle) and mandible pterygoid fovea and articular disc (lateral pterygoid muscle) (Woods, 1972; Constantinescu, 2018).
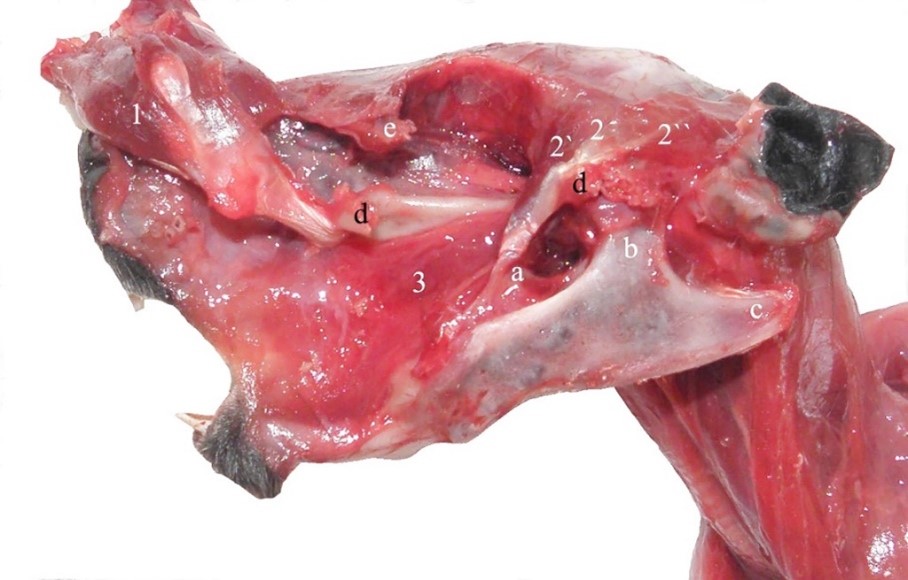
Figure 7 – Temporal muscle of the guinea pig 1. m. masseter, pars superficialis, 2. m. temporalis, 2’. m. temporalis, pars rostralis, 2’’. m. temporalis, pars aboralis, 3. m. buccinatorius, a. processus coronoideus, b. processus condilaris, c. processus angularis, d. arcus zygomaticus (cut), e. processus zygomaticus ossis frontalis (cut)
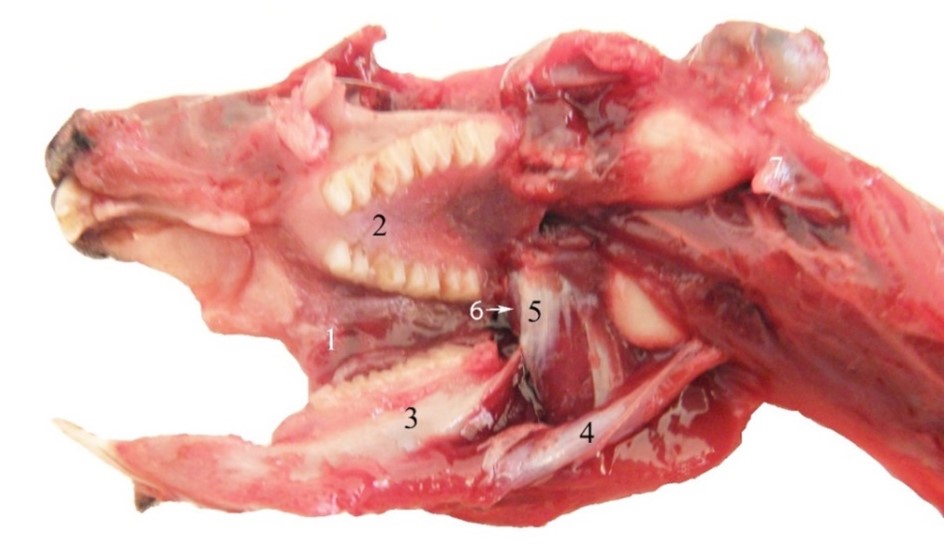
Figure 8 – Deep muscles of the head at guinea pig 1. Vestibulum oris, 2. palatum, 3. mandibula, 4. m. digastricus, 5. m. pterygoideus medialis , 6. m. pterygoideus lateralis, 7. processus paracondylaris (left)
Besides raising the mandible, the muscles produce the lateral movements which are very efficient in chewing food, an aspect shown by the ventro-medial obliquity of the occlusal surface of the lower molars. Some authors suggest that the lateral pterygoid muscle ensures long time and continuous contraction such as in retention of jaw position (Abe et al., 2008; Cox and Jeffery, 2011; Easton and Carlson, 1990; Spataru et al., 2013).
CONCLUSIONS
In guinea pigs, the mandible is impressive by its length,. The jointing condyle is placed in the plane of the mandibular molar level, similar to carnivores.
In guinea pigs, the oblique displacement of the molars and the slight mid-lateral obliquity of their abrasion surface show that, in addition to the propulsion and retropulsion movements of the mandible, lateral movements are also used for chewing food. In guineea pigs, the masseter muscle is predominant, being formed by three parts having its fibers distributed in different planes and angles. The superficial part is the most developed, two cartilaginous thickenings protecting the tendon when it passes over the ridges of the mandible. The oblique aspect of the deep part of the masseter muscle, the long tendon of origin and the presence of a cartilaginous structure represent a factor of adaptation for grinding food, the contraction of the muscle permitting trituration for a long time. The middle part has dorso-ventral disposition of fibers, it playing the role in the rapid and strong lifting of the mandible. The pterygoid muscles are the same developed producing the lateral movements of the mandible.
Author Contributions: MCS: conceptualization; MCS, CS: methodology; analysis; investigation; resources; data curation; writing, review; supervision; and so on. All authors declare that they have read and approved the publication of the manuscript in this present form.
Funding: There was no external funding for this study.
Conflicts of Interest: No conflicts of interest.
REFERENCES
Abe, S.; Hiroki, E.; Iwanuma, O.; Sakiyama, K.; Shirakura, Y.; Hirose, D.; Shimoo, Y.; Suzuki, M.; Ide, Y.; Yoshinari, M. Relationship between function of masticatory muscle in mouse and properties of muscle fibers. The Bulletin of Tokyo Dental College. 2008, 49, 53-8. https://doi.org/10.2209/tdcpublication.49.53.
Álvarez, A; Pérez, M.E. Deep changes in masticatory patterns and masseteric musculature configurations accompanied the eco-morphological evolution of cavioid rodents (Hystricognathi, Caviomorpha). Mammalian Biology. 2019, 96, 53-60. https://doi.org/10.1016/j.mambio.2019.03.009.
Álvarez, A.; Ercoli, M.D.; Boivin, M.; Ortiz Tejerina, A.M.; Ortiz Moyano, S.R. Head myology of wild cavies (Caviidae, Caviomorpha) and functional implications of hystricomorphous and hystricognathous configurations, Journal of Mammalian Evolution. 2023. https://doi.org/10.1007/s10914-023-09656-6.
Anthwal, N.; Heiko Peters, H.; Tucker, A.S. Species-specific modifications of mandible shape reveal independent mechanisms for growth and initiation of the coronoid. EvoDevo. 2015, 6, 35. https://doi.org/10.1186/s13227-015-0030-6.
Boivin, M.; Álvarez, A.; Ercoli, M.D. Integration patterns of cheek teeth and ecomorphological evolution in grinding herbivores: the case of caviine rodents (Caviomorpha: Caviidae). Zoological Journal of the Linnean Society. 2022, 196, 1094-1116. http://dx.doi.org/10.1093/zoolinnean/zlac005/6547390.
Byrd, K.E. Mandibular movement and muscle activity during mastication in the guinea pig (Cavia porcellus). Journal of Morphology. 1981, 170, 147-69. https://doi.org/10.1002/jmor.1051700203.
Butler, P.M. Functional aspects of the evolution of rodent molars. Palaeovertebrata Mém, Jubil. R. Lavocat. 1980, 249-262.
Butler, P.M. Homologies of Molar Cusps and Crests, and Their Bearing on Assessments of Rodent Phylogeny. Evolutionary Relationships among Rodents. 1985, 381-401
Carter, A.M. Animal models of human placentation – a review. Placenta. 2007, 28, S41-S47. https://doi.org/10.1016/j.placenta.2006.11.002.
Clarke, G.L.; Allen, A.M.; Small, J.D.; Lock, A. Subclinical Scurvy in the Guinea Pig. Veterinary Pathology. 1980, 17, 40-44. https://journals.sagepub.com/doi/pdf/10.1177/030098588001700104
Christiansen, P.; Adolfssen, J.S. Bite forces, canine strength and skull allometry in carnivores (Mammalia, Carnivora). Journal of Zoology. 2005, 266, 133-151. https://doi.org/10.1017/S0952836905006643.
Cooper, G.; Schiller, A.L. Anatomy of the Guinea Pig. Harvard University Press, 1975,Cambridge.
Constantinescu, G.M. Illustrated Veterinary Anatomical Nomenclature, 4th edition, 2018, Georg Thieme Verlag Stuttgart.Harkness, J.E., Murray, K.A., Wagner, J.E., Biology and diseases of guinea pigs. In: Fox, J.G., Anderson, L.C., Loew, F.M., Quimby, F.W. (Eds.), Laboratory Animal Medicine, second ed. Academic Press, San Diego, 2002, 203-246.
Cox, P.G.; Jeffery, N. Reviewing the morphology of the jaw-closing musculature in squirrels, rats, and guinea pigs with contrast-enhanced microCT. Anat Rec (Hoboken). 2011, 294, 1612. https://doi.org/10.1002/ar.21381.
Dharmadhikari, A.S.; Nardell, E.A. What animal models teach people about tuberculosis. American Journal of Respiratory Cell and Molecular Biology. 2008, 39, 503-508. https://doi.org/10.1165/rcmb.2008-0154TR.
Druzinsky, R.E. Functional anatomy of incisal biting in aplodontia rufa and sciuromorph rodents – Part 1: masticatory muscles, skull shape and digging. Cell, Tissues, Organs. 2010, 191, 510-522. https://doi.org/10.1159/000284931.
Easton, J.W.; Carlson, D.S. Adaptation of the lateral pterygoid and superficial masseter muscles to mandibular protrusion in the rat. American Journal of Orthodontics and Dentofacial Orthopedics. 1990, 97, 149-58. https://doi.org/10.1016/0889-5406(90)70088-t.
Graur, D.; Hide, W.A.; Li, W.H. Is the guinea-pig a rodent? Nature. 1991, 351, 649-652. https://doi.org/10.1038/351649a0.
Hautier, L.; Lebrun, R.; Saksiri, S., et al. Hystricognathy vs sciurognathy in the rodent jaw: a new morphometric assessment of hystricognathy applied to the living fossil Laonastes (Diatomyidae). PLoS ONE, 2011, 6, e18698. https://doi.org/10.1371/journal.pone.0018698.
Isotupa, K.; Rönning, O. Changes in the dentition of the guinea pig following total section of the inferior alveolar nerve and vessels. Acta Anatomica. 1977, 98, 71-76. https://doi.org/10.1159/000144780.
Kruska, D.C.T.; Steffen, K. Comparative allometric investigations on the skulls of wild cavies (Cavia aperea) versus domesticated guinea pigs (C. aperea f. porcellus) with comments on the domestication of this species. Mammalian Biology. 2013, 78, 178-186. https://doi.org/10.1016/j.mambio.2012.07.002.
Mitchell, B.F.; Taggart, M.J. Are animal models relevant to key aspects of human parturition? Regulatory, Integrative and Comparative Physiology. 2009, 297, R525-R545. https://doi.org/10.1152/ajpregu.00153.2009.
Myers, P.; Espinosa, R.; Parr, C.S.; Jones, T.; Hammond, G.S.; Dewey, T.A. The Animal Diversity Web (online). Accessed at https://animaldiversity.org.
Noguchi, T.; Fujiwara, S.; Hayashi, S.; Sakuraba, H. Is the guinea-pig (Cavia porcellus) a rodent?, Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1994, 107, 179-182. https://doi.org/10.1016/0305-0491(94)90037-X.
Obregon-Henao, A.; Shanley, C.; Bianco, M.V.; Cataldi, A.A.; Basaraba, R.J.; Orme, I.M.; Bigi, F. Vaccination of guinea pigs using mce operon mutants of Mycobacterium tuberculosis. Vaccine. 2011, 29, 4302-4307. https://doi.org/10.1016/j.vaccine.2011.04.027.
Padilla-Carlin, D.J.; McMurray, D.N.; Hickey, A.J. The Guinea Pig as a Model of Infectious Diseases. Comparative Medicine. 2008, 58, 324-340.
Pereira, F.M.A.M.; Bete, S.B.D.S.; Inamassu, L.R.; Mamprim, M.J.; Schimming, B.C. Anatomy of the skull in the capybara (Hydrochoerus hydrochaeris) using radiography and 3D computed tomography. Anatomia, Histologia, Embryologia. 2020, 49, 317-324. https://doi.org/10.1111/ahe.12531.
Popesko, P.; Rajtová, V.; Horák, J. A Colour Atlas of Anatomy of Small Laboratory Animals: Rabbit, the Guinea Pig. Vol. 1, Wolfe Publishing, Bratislava, 1992, 227.
Ricciardolo, F.L. et al. The guinea pig as an animal model for asthma. Current Drug Targets. 2008, 9, 452-465. https://doi.org/10.2174/138945008784533534.
Spataru, C.; Spataru, M.C.; Vulpe, V.; Lazar, M. The peculiarities of the masticator muscles in rodents. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2013, 65, 749-756. http://dx.doi.org/10.1590/S0102-09352013000300021.
Spataru, M.C.; Spataru C. Some aspects concerning the masticatory muscles in Guinea pig (Cavia porcelus) ”The 10th Meeting of the Young generation of Veterinary anatomists-YGVA2019”, 24-26 Jully, Bucuresti, Ed. EX TERRA AURUM, 2019.
Spataru, M.C. Comparative morphological peculiarities of the skulls at some rodents (rabbit, nutria, guineapig and squirrel). Journal “Scientific papers – Veterinary Medicine. 2016, 59, 503-509.
Spotorno, A.E.; Marín, J.C.; Manríquez, G.; Valladares, J.P.; Rico, E.; Rivas, C. Ancient and modern steps during domestication of guinea pigs (Cavia porcellus L.)”. Journal of Zoology. 2006, 270, 060606025751032. https://doi.org/10.1111/j.1469-7998.2006.00117.x.
Stan, F. Comparative morphological study of oral cavity in rabbits and guinea pigs. Scientific Works, Series C. Veterinary Medicine. 2014, LX (1) .
Vassallo, A.I.; Verzi, D.H. Skull pallerns and chewing modes in caviomorplh rodents (Rodentia, Caviomorpha) (in Spanish). Boletin de la Sociedad de Biología de Concepción. 2001, 72, 139-145.
Westropp, J.L.; Buffington, C.A. In vivo models of interstitial cystitis. The Journal of Urology. 2002, 167, 694-702. https://doi.org/10.1016/s0022-5347(01)69129-8.
West, K.L.; Fernandez M.L. Guinea pigs as models to study the hypocholesterolemic effects of drugs, Cardiovascular Drug Reviews. 2004, 22, 55-70. https://doi.org/10.1111/j.1527-3466.2004.tb00131.x.
Woods, C.A. Comparative myology of jaw, hyoid, and pectoral appendicular regions of New and Old World hystricomorph rodents. Bulletinoftheamericanmuseumo-Fnatural~History. 1972, 147, 115-198.
Academic Editor: Prof. Dr. Daniel Simeanu
Publisher Note: Regarding jurisdictional assertions in published maps and institutional affiliations ALSE maintain neutrality.



